Month: July 2023
Electricity of Class 10 NCERT Science Chapter 11 Solutions
Discover comprehensive NCERT Science Electricity of Class 10 Chapter 12 Solutions containing well-explained answers to all the exercise questions found in your textbook. These solutions cover a wide range of topics, including electric cells, electric bulbs, electric circuits, switches, conductors, insulators, as well as examples of each.
You’ll encounter diverse question formats such as ‘Fill in the Blanks’, ‘True or False’, circuit diagrams, and descriptive answering questions, which will facilitate a deeper understanding of the concepts.
In this article, we will explore the very essence of electricity, from its historical roots and foundational principles to practical applications in our daily lives and cutting-edge technologies. Whether you’re just beginning your journey into the realm of electrical knowledge or seeking to reinforce your existing understanding, we have tailored this article to cater to all learning levels.
Electricity of Class 10 NCERT Science Chapter 11 Solutions
Electricity of Class 10
NCERT Electricity of Class 10 Science Chapter 11 Solutions
1. What does an electric circuit mean?
Answer:
An electric circuit refers to a seamless, unbroken loop comprising electric components, allowing the flow of an electric current. The fundamental elements of a basic circuit include:
(a) Conductors – These materials facilitate the easy movement of electric charges, enabling the flow of current within the circuit.
(b) Cell – The cell, acting as a power source, provides the necessary electric potential that propels the electrons to move through the circuit.
(c) Switch – A switch serves as a control device within the circuit, allowing us to open or close the path for the electric current, thus controlling its flow.
(d) Load – The load represents any device or component in the circuit that consumes electrical energy, converting it into various forms of useful output, such as light, heat, or motion.
2. Define the unit of current.
Answer:
The unit of current is known as the ampere. An ampere is defined as the rate of flow of one coulomb of electric charge per second.
3. Calculate the number of electrons constituting one coulomb of charge.
Answer:
The charge of an electron is determined to be 1.6 × 10^-19 C.
Based on the principle of charge quantization, the charge (Q) can be expressed as Q = n * qe, where ‘n’ represents the number of electrons, and ‘qe’ is the charge of an electron.
By substituting the given values into the above equation, we can calculate the number of electrons in one coulomb of charge as follows:
n = 1 C/1.6 * 10^-19 = 6.25 * 10^18
Consequently, the number of electrons comprising one coulomb of charge is found to be 6.25 × 10^18.
4. Name a device that helps to maintain a potential difference across a conductor.
Answer:
One of the devices responsible for sustaining a potential difference across a conductor is a battery, which comprises one or more electric cells.
5. What is meant by saying that the potential difference between two points is 1 V?
Answer:
The potential difference between two points is defined as 1 volt (V) when 1 joule (J) of work is expended to move a charge of 1 coulomb (C) from one point to another.
6. How much energy is given to each coulomb of charge passing through a 6 V battery?
Answer:
As per the potential difference equation:
V = W / Q, where:
V represents the potential difference between two points,
W is the work done in moving the charge from one point to another,
Q denotes the charge.
Using the above equation, we can determine the energy imparted to each coulomb:
W = V × Q
Upon substituting the given values into the equation, we find:
W = 6V × 1C = 6 J
Therefore, when passing through a 6 V battery, each coulomb of charge receives 6 joules of energy.
7. On what factors does the resistance of a conductor depend?
Answer:
The resistance of a conductor is influenced by the following factors:
a. Temperature of the conductor: The resistance tends to change with variations in the conductor’s temperature.
b. Cross-sectional area of the conductor: A larger cross-sectional area typically results in reduced resistance.
c. Length of the conductor: Longer conductors generally have higher resistance compared to shorter ones.
d. Nature of the material of the conductor: The resistance is determined by the specific material properties of the conductor. Different materials have different inherent resistances.
8. Will current flow more easily through a thick wire or a thin wire of the same material, when connected to the same source? Why?
Answer:
The resistance of a wire can be calculated using the formula:
R = ρ * l / A
where:
ρ represents the resistivity of the wire’s material,
l is the length of the wire, and
A denotes the cross-sectional area of the wire.
As we observe from the equation, the cross-sectional area of the wire is inversely proportional to its resistance. Consequently, a thinner wire exhibits higher resistance, while a thicker wire offers lower resistance. Therefore, current flows more effortlessly through a thick wire as compared to a thin wire.
9. Let the resistance of an electrical component remain constant while the potential difference across the two ends of the component decreases to half of its former value. What change will occur in the current through it?
Answer:
Ohm’s Law provides a means to calculate the change in the current flowing through an electrical component. According to Ohm’s Law, the current (I) can be determined by the equation:
I = V / R
Now, if we reduce the potential difference by half while keeping the resistance constant, we get:
New voltage: V’ = V / 2
New resistance: R’ = R
Let the new amount of current be denoted as I’.
We can determine the change in the current using Ohm’s Law as shown below:
NCERT Solutions for Class 10 Chapter 12 Image 2
Thus, the current flowing through the electrical component is reduced by half when the potential difference is halved while keeping the resistance constant.
10. Why are coils of electric toasters and electric irons made of an alloy rather than a pure metal?
Answer:
Alloys possess significantly higher melting points compared to pure metals due to their elevated resistivity. This inherent property allows alloys to resist melting easily at high temperatures.
As a result, alloys find extensive application in heating appliances, such as electric toasters and electric irons, where they can endure and maintain their structural integrity even when subjected to elevated temperatures.
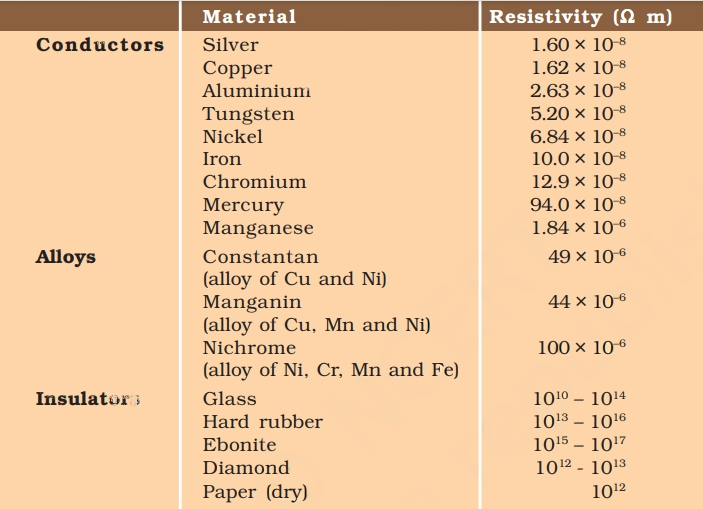
11. Use the data in the table given below and answer the following questions.
a. Which among iron and mercury is a better conductor?
b. Which material is the best conductor?
Answer
a. Iron exhibits better conductivity than mercury due to the lower resistivity of iron when compared to mercury.
b. Among all the materials listed in the table, silver stands out as the best conductor as it possesses the lowest resistivity of all, measuring at 1.60 × 10^-8.
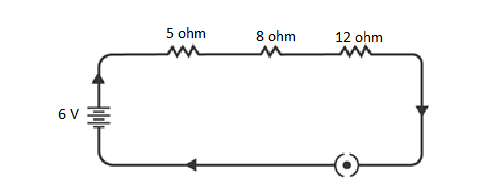
12. Draw a schematic diagram of a circuit consisting of a battery of three cells of 2 V each, a 5 Ω resistor, an 8 Ω resistor, and a 12 Ω resistor, and a plug key, all connected in series.
“A set of three cells, each having a voltage of 2 V, combines to form a battery with a total potential of 6 V. The circuit illustration provided depicts three resistors with resistances of 12 Ω, 8 Ω, and 5 Ω, connected in a series arrangement, along with the 6 V potential battery.”
Q 13. Redraw the circuit of Question 1, putting in an ammeter to measure the current through the resistors and a voltmeter to measure the potential difference across the 12 Ω resistor. What would be the readings in the ammeter and the voltmeter?
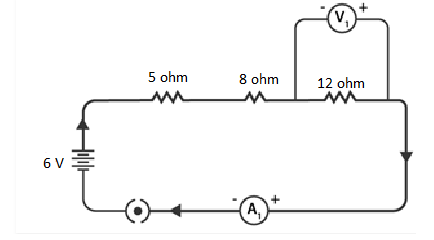
To measure current and potential difference in a circuit, it is important to connect the ammeter in series with the resistors and the voltmeter in parallel to the resistor, as depicted in the figure below.
NCERT Solutions for Class 10 Chapter 12 Image 4
By utilizing Ohm’s Law, the readings of the ammeter and the voltmeter can be determined.
The total resistance of the circuit is obtained by adding the individual resistances: 5 Ω + 8 Ω + 12 Ω = 25 Ω.
Given that the potential difference of the circuit is 6 V, the current flowing through the circuit (or the resistors) can be calculated as follows:
I = V / R = 6 V / 25 Ω = 0.24 A
Let the potential difference across the 12 Ω resistor be denoted as V1.
Using the obtained current, V1 can be calculated as follows:
V1 = I × 12 Ω = 0.24 A × 12 Ω = 2.88 V
Hence, the ammeter reading will be 0.24 A, and the voltmeter reading will be 2.88 V.
Q 14. Judge the equivalent resistance when the following are connected in parallel – (a) 1 Ω and 106 Ω, (b) 1 Ω, 103 Ω, and 106 Ω.
When 1 Ω and 106 are connected in parallel, the equivalent resistance is given by

Therefore, the equivalent resistance is 1 Ω.
(b) When 1 Ω, 103 Ω, and 106 Ω are connected in parallel, the equivalent resistance is given by

Therefore, the equivalent resistance is 0.999 Ω.
Q 15. An electric lamp of 100 Ω, a toaster of resistance 50 Ω, and a water filter of resistance 500 Ω are connected in parallel to a 220 V source. What is the resistance of an electric iron connected to the same source that takes as much current as all three appliances, and what is the current through it?
The circuit diagram illustrates the electric lamp, toaster, and water filter connected in parallel to a 220 V power source, as shown below:
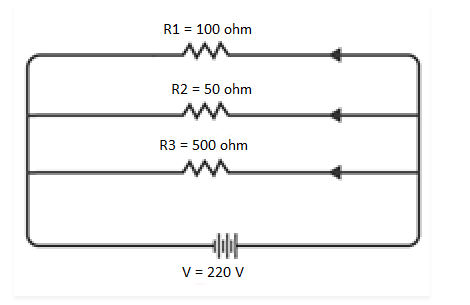
NCERT Solutions for Class 10 Chapter 12 Image 7
To determine the equivalent resistance of the resistors in the circuit, follow these calculations:

NCERT Solutions for Class 10 Chapter 12 Image 8
Furthermore, the resistance of the electric iron box is measured to be 31.25 Ω.
Q 16. What are the advantages of connecting electrical devices in parallel with the battery instead of connecting them in series?
When electrical devices are connected in parallel, the voltage across each appliance remains the same and is equal to the supply voltage. In this configuration, there is no division of voltage among the appliances. Each device receives the full potential difference of the source.
Additionally, connecting devices in parallel reduces the effective resistance of the circuit. The equivalent resistance of the parallel configuration is lower than the individual resistances of the devices. This leads to an increase in the total current flowing through the circuit, as the path for current is effectively widened by the parallel arrangement of appliances.
Q 17. How can three resistors of resistances 2 Ω, 3 Ω, and 6 Ω be connected to give a total resistance of (a) 4 Ω, (b) 1 Ω?
In the first circuit diagram shown below, three resistors are connected:

It can be observed that the resistors of 3 Ω and 6 Ω are connected in parallel. Their equivalent resistance can be calculated as follows:


The equivalent resistor of 2 Ω is then connected in series with another 2 Ω resistor. Consequently, the equivalent resistance of this combination can be calculated as follows:
Req = 2 Ω + 2 Ω = 4 Ω
Thus, the total resistance of the circuit is 4 Ω.
(b) In the second circuit diagram displayed below, three resistors are connected:
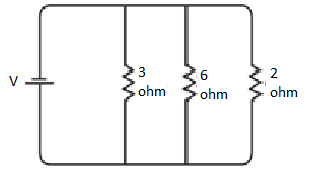
It is evident that all the resistors are connected in parallel. Therefore, their equivalent resistance can be calculated as follows:

The total resistance of the circuit is 1 Ω.
Q 18. What is (a) the highest, (b) the lowest total resistance that can be secured by combinations of four coils of resistance 4 Ω, 8 Ω, 12 Ω, 24 Ω?
(a) When the four resistors are connected in series, their total resistance will be the sum of their individual resistances, and it will be the highest. The total equivalent resistance of the resistors connected in series will be 4 Ω + 8 Ω + 12 Ω + 24 Ω = 48 Ω.
(b) If the resistors are connected in parallel, their equivalent resistance will be the lowest. The total equivalent resistance connected in parallel is:

Therefore, the lowest total resistance is 2 Ω.
Q 19. Why does the cord of an electric heater not glow while the heating element does?
The heating element in an electric heater is constructed using an alloy with high resistance. When electricity passes through the heating element, it generates significant heat, causing the element to glow red.
In contrast, the cord connecting the heater is typically made of materials like copper or aluminum, which have low resistance. As a result, the cord does not heat up enough to glow since the low resistance allows the electricity to flow through it smoothly without generating excessive heat.
Q 20. Compute the heat generated while transferring 96000 coulomb of charge in one hour through a potential difference of 50 V.
Given the following values:
Charge, Q = 96000 C
Time, t = 1 hr = 60 x 60 = 3600 s
Potential difference, V = 50 volts
Now, to calculate the current (I), we can use the formula:
I = Q / t
where Q is the charge, and t is the time.
Therefore, I = 96000 C / 3600 s = 80/3 A
Now, we can find the amount of heat generated (H) using Joule’s law:
H = V * I * t
H = 50 V * (80/3 A) * 3600 s = 4.8 x 10^6 J
Thus, the heat generated is approximately 4.8 x 10^6 joules.
Q 21. An electric iron of resistance 20 Ω takes a current of 5 A. Calculate the heat developed in 30 s.
The quantity of heat generated can be determined using Joule’s law of heating, given by the equation:
H = VIt
By substituting the given values into the equation, we find:
H = 100 × 5 × 30 = 1.5 × 10^4 J
Thus, the amount of heat developed by the electric iron in 30 seconds is 1.5 × 10^4 joules.
Q 22. What determines the rate at which energy is delivered by a current?
Electric power refers to the rate at which electrical energy is consumed by electric appliances. Consequently, it represents the rate at which energy is delivered by a current to the appliance.
Q 23. An electric motor takes 5 A from a 220 V line. Determine the power of the motor and the energy consumed in 2 h.
The power of the motor can be determined using the equation:
P = VI
By substituting the given values into the equation, we find:
P = 220 V × 5 A = 1100 W
Thus, the power of the motor is 1100 watts.
The energy consumed by the motor can be calculated using the equation:
E = P × T
By substituting the given values into the equation, we find:
E = 1100 W × 7200 s = 7.92 × 10^6 J
Therefore, the energy consumed by the motor in 2 hours is 7.92 × 10^6 joules.
Q 24. A piece of wire of resistance R is cut into five equal parts. These parts are then connected in parallel. If the equivalent resistance of this combination is R′, then the ratio R/R′ is _____.
(a) 1/25
(b) 1/5
(c) 5
(d) 25
Answer:
The original resistance is divided into five equal parts, which means each part has a resistance of R/5.
Since each part is connected to one another in parallel, the equivalent resistance (R’) can be calculated as shown below:

The ratio of R to R’ is 25.
Q 26. Which of the following does not represent electrical power in a circuit?
(a) I2R
(b) IR2
(c) VI
(d) V2/R
Answer:
Electrical power is expressed by the equation P = VI. (1)
According to Ohm’s law, V = IR.
By substituting the value of V in equation (1), we obtain:
P = (IR) × I
P = I^2R
Similarly, from Ohm’s law, I = V/R.
Substituting the value of I in equation (1), we get:
P = V × V/R = V^2/R
From this, it is evident that the expression IR^2 does not represent electrical power in a circuit. Instead, the correct representation is V^2/R.
Q 27. An electric bulb is rated 220 V and 100 W. When it is operated on 110 V, the power consumed will be _____.
(a) 100 W
(b) 75 W
(c) 50 W
(d) 25 W
The energy consumed by the appliance can be determined by the expression:
P = VI = V^2/R
The resistance of the light bulb can be calculated as follows:
R = V^2/P
By substituting the values, we find:
R = (220)^2 / 100 = 484 Ω
Even if the supply voltage is reduced, the resistance remains the same. Hence, the power consumed can be calculated as follows:
P = V^2 / R
Substituting the value, we get:
P = (110)^2 V / 484 Ω = 25 W
Thus, the power consumed when the electric bulb operates at 110 V is 25 W.
Q 28. Two conducting wires of the same material and of equal lengths and equal diameters are first connected in series and then parallel in a circuit across the same potential difference. The ratio of heat produced in series and parallel combinations would be _____.
(a) 1:2
(b) 2:1
(c) 1:4
(d) 4:1
Answer:
(c) 1:4
Q 29. How is a voltmeter connected in the circuit to measure the potential difference between two points?
To measure the voltage between any two points, the voltmeter should be connected in parallel across the two points.
Q 30. A copper wire has diameter 0.5 mm and resistivity of 1.6 × 10–8 Ω m. What will be the length of this wire to make its resistance 10 Ω? How much does the resistance change if the diameter is doubled?

Q 31. The values of current I flowing in a given resistor for the corresponding values of potential difference V across the resistor are given below –
| I (Ampere) | 0.5 | 1.0 | 2.0 | 3.0 | 4.0 |
| V (Volts) | 1.6 | 3.4 | 6.7 | 10.2 | 13.2 |
Plot a graph between V and I and calculate the resistance of that resistor.
The plot depicting the relationship between voltage and current is known as the IV characteristic. The current is represented on the y-axis, while the voltage is shown on the x-axis. The table provides various current values corresponding to different voltage values. The IV characteristic for the given resistor is illustrated below.
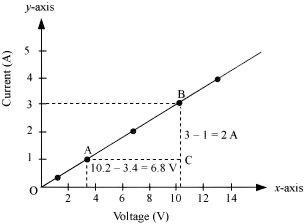
The value of resistance can be obtained from the slope of the line.
The slope is calculated as follows:
Slope = 1/R = BC/AC = 2/6.8
To calculate R:
R = 6.8/2 = 3.4 Ω
Thus, the resistance of the resistor is 3.4 Ω.
Q 32. When a 12 V battery is connected across an unknown resistor, there is a current of 2.5 mA in the circuit. Find the value of the resistance of the resistor.
The resistance (R) of a resistor is determined by Ohm’s law, which states V = IR.
To find R, we use the formula R = V/I, where:
Potential difference, V = 12 V
Current in the circuit, I = 2.5 mA = 2.5 x 10^-3 A
Therefore, the resistance of the resistor is 4.8 kΩ.
Q 33. A battery of 9 V is connected in series with resistors of 0.2 Ω, 0.3 Ω, 0.4 Ω, 0.5 Ω and 12 Ω, respectively. How much current would flow through the 12 Ω resistor?
In a series connection, there is no division of current, and the same current flows across all the resistors.
To calculate the amount of current flowing through the resistors, we can use Ohm’s law.
First, let’s determine the equivalent resistance of the series connection:
R = 0.2 Ω + 0.3 Ω + 0.4 Ω + 0.5 Ω + 12 Ω = 13.4 Ω
Now, applying Ohm’s law:

The current flowing through the 12 Ω resistor is 0.671 A.
Q 34. How many 176 Ω resistors (in parallel) are required to carry 5 A on a 220 V line?
Let’s assume that “n” 176 Ω resistors are connected in parallel.
The formula for calculating the equivalent resistance (Req) of “n” resistors connected in parallel is:
1/Req = 1/R₁ + 1/R₂ + 1/R₃ + … + 1/Rₙ
where R₁, R₂, R₃, …, Rₙ are the individual resistances.
Given that the current (I) is 5 A and the voltage (V) is 220 V, and we want to find the number of 176 Ω resistors (n).
First, let’s find the equivalent resistance (Req) using Ohm’s law:
V = I * Req
Req = V / I Req = 220 V / 5 A Req = 44 Ω
Now, we can calculate the number of resistors (n) using the equivalent resistance:
1/Req = 1/176 Ω + 1/176 Ω + … + 1/176 Ω (n times)
1/44 Ω = n/176 Ω
n = 4
Q 35. Show how you would connect three resistors, each of resistance 6 Ω, so that the combination has a resistance of (i) 9 Ω, (ii) 4 Ω.
If we connect the resistors in series, the equivalent resistance will be the sum of the resistors, i.e., 6 Ω + 6 Ω + 6 Ω = 18 Ω, which is not the desired outcome. If we connect them in parallel, the equivalent resistance will be 6/2 = 3 Ω, which is also not desired. Thus, we need to explore other combinations to achieve the desired total resistance.
(a) Two resistors in parallel:
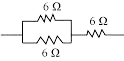
Two 6 Ω resistors are connected in parallel. Their equivalent resistance will be:
1/Req = 1/6 Ω + 1/6 Ω
1/Req = 2/6 Ω
Req = 6/2 = 3 Ω
The third 6 Ω resistor is in series with 3 Ω. Hence, the equivalent resistance of the circuit is 6 Ω + 3 Ω = 9 Ω.
(b) Two resistors in series:
![]()
Two 6 Ω resistors are in series. Their equivalent resistance will be the sum: 6 Ω + 6 Ω = 12 Ω.
The third 6 Ω resistor is in parallel with 12 Ω. Hence, the equivalent resistance will be:
1/Req = 1/6 Ω + 1/12 Ω
1/Req = 3/12 Ω
Req = 12/3 = 4 Ω
Therefore, the total resistance of the circuit is 4 Ω.
Q 36. Several electric bulbs designed to be used on a 220 V electric supply line, are rated 10 W. How many lamps can be connected in parallel with each other across the two wires of 220 V line if the maximum allowable current is 5 A?
The resistance (R1) of the bulb is given by the expression:
R1 = V^2 / P
Where:
Supply voltage, V = 220 V
Rating of the electric bulb, P = 10 watts
Since R = V^2 / P, we can substitute the given values to find R1.

Q 37. A hot plate of an electric oven connected to a 220 V line has two resistance coils A and B, each of 24 Ω resistance, which may be used separately, in series, or in parallel. What are the currents in the three cases?
Case (i) When coils are used separately:
Using Ohm’s law, we can calculate the current flowing through each coil as follows:

When used separately, 9.166 A of current flows through each resistor.
Case (ii) When coils are connected in series:
The total resistance in the series circuit is 24 Ω + 24 Ω = 48 Ω
The current flowing through the series circuit is calculated as follows:

Therefore, a current of 4.58 A flows through the series circuit.
Case (iii) When coils are connected in parallel:
When the coils are connected in parallel, the equivalent resistance is calculated as follows:

The current in the parallel circuit is 18.33 A.
Q 38. Compare the power used in the 2 Ω resistor in each of the following circuits: (i) a 6 V battery in series with 1 Ω and 2 Ω resistors, and (ii) a 4 V battery in parallel with 12 Ω and 2 Ω resistors.
(i) When the potential difference is 6 V and the resistors 1 Ω and 2 Ω are connected in series, their equivalent resistance is given by 1 Ω + 2 Ω = 3 Ω. The current in the circuit can be calculated using Ohm’s law as follows:

Therefore, the power consumed by the 2 Ω resistor is 8 W.
(ii) When 12 Ω and 2 Ω resistors are connected in parallel, the voltage across the resistors remains the same. Knowing that the voltage across the 2 Ω resistor is 4 V, we can calculate the power consumed by the resistor as follows:

The power consumed by the 2 Ω resistor is 8 W.
Q 39. Two lamps, one rated 100 W at 220 V, and the other 60 W at 220 V, are connected in parallel to electric mains supply. What current is drawn from the line if the supply voltage is 220 V?
As both bulbs are connected in parallel, the voltage across each of them will be the same.
The current drawn by the bulb with a rating of 100 W can be calculated using the formula:
P = V × I
I = P / V
Substituting the given values, we get:
I = 100 W / 220 V = 100/220 A
Similarly, the current drawn by the bulb with a rating of 60 W can be calculated as follows:
I = 60 W / 220 V = 60/220 A
Therefore, the total current drawn from the line is:

Q 40. Which uses more energy, a 250 W TV set in 1 hr, or a 1200 W toaster in 10 minutes?
The energy consumed by electrical appliances is determined by the equation:
H = Pt, where P is the power of the appliance, and t is the time.
Using this formula, we can calculate the energy consumed by a TV with a power rating of 250 W as follows:
H = 250 W × 3600 seconds = 9 × 10^5 J
Similarly, the energy consumed by a toaster with a power rating of 1200 W is:
H = 1200 W × 600 s = 7.2 × 10^5 J
From the calculations, it can be observed that the energy consumed by the TV is greater than the toaster.
Q 41. An electric heater of resistance 8 Ω draws 15 A from the service mains 2 hours. Calculate the rate at which heat is developed in the heater.
The rate at which heat develops in the heater can be calculated using the following formula:
P = I^2 * R
By substituting the given values into the equation, we find:
P = (15A)^2 * 8 Ω = 1800 watts
Therefore, the electric heater produces heat at the rate of 1800 watts.
Q 42. Explain the following.
a. Why is the tungsten used almost exclusively for filament of electric lamps?
b. Why are the conductors of electric heating devices, such as bread-toasters and electric irons, made of an alloy rather than a pure metal?
c. Why is the series arrangement not used for domestic circuits?
d. How does the resistance of a wire vary with its area of cross-section?
e. Why copper and aluminium wires are usually employed for electricity transmission?
a. Tungsten is an ideal choice for the filament of electric lamps due to its high resistivity and melting point. These properties prevent it from burning readily when heated, making it suitable for operating at high temperatures in electric lamps.
b. Alloys are preferred as conductors for electric heating devices because of their high resistivity. Compared to pure metals, alloys have higher resistivity, leading to the generation of a substantial amount of heat when current passes through them, which is crucial for heating applications.
c. The series arrangement is not commonly used for domestic circuits due to the following reasons:
– The overall voltage gets divided in a series circuit, which may cause electric appliances not to receive their rated power for proper operation.
– All connected appliances in a series circuit cannot be operated independently. If one device is defective, the entire circuit will be affected.
– The total resistance increases in a series circuit, leading to reduced current flow, which may cause appliances to operate inefficiently.
d. Resistance is inversely proportional to the area of cross-section. When the area of the cross-section increases, the resistance decreases, and vice versa. This relationship between resistance and the cross-sectional area is an important factor to consider in electrical circuits and material design.
e. Copper and aluminum are widely used for electricity transmission due to their low resistivity and excellent conductivity. Their low resistivity results in significantly less power losses in the form of heat during the transmission of electricity, making them efficient choices for power transmission applications.
Electricity of Class 10 NCERT Science Chapter 11 Solutions
Chapter 11 – Electricity in Class 10 Science is an essential topic carrying at least 8 marks as per previous examination trends. However, the 2018 Class 10 Science exam had questions totalling up to 7 marks for this chapter. To strengthen your understanding of the key concepts in this chapter, make use of NCERT Solutions for Class 10.
The topics covered in the NCERT Solutions for Class 10 Science, Chapter 11 are as follows:
1. Ohm’s law
2. Resistivity and Resistance
3. Factors affecting the Resistance of a Conductor
4. Parallel and Series Combination of Resistors and their applications
5. Heating Effect of Electric Current and its Applications
6. Electric Power
7. The interrelation between P, V, I, and R
Electricity is a vital aspect of our society, shaping our civilization since the industrial revolution. It powers entire industries and businesses, and life without electricity would result in chaos, given its importance as a source of energy.
Through NCERT Solutions for Class 10 Science, you can explore how electricity works at the molecular level, understand crucial concepts, and discover its practical applications. The learning resources are designed to facilitate efficient learning.
Key Features of NCERT Solutions for Class 10 Science, Chapter 11 – Electricity:
– Content presented in an easy-to-understand language
– Solutions crafted by highly qualified teachers and industry experts
– Additional questions based on the latest prescribed syllabus
– Detailed explanations of challenging exam questions
– Access to additional learning resources like sample papers and previous year question papers
Read Also:
- NCERT Solutions Science Class 10 All Chapter
- Our Environment Class 10 Chapter 13 Solution for NCERT
- Chemical Equations and Reactions Class 10: Solution of Sci. Ch.1
Frequently Asked Question – FAQs on Electricity of Class 10 NCERT Science Chapter 11 Solutions
Q 1. What are the 4 types of electricity?
The four types of electricity are static electricity (buildup of charge due to friction), current electricity (flow of charge through conductors), direct current (DC, flows in one direction), and alternating current (AC, changes direction periodically, commonly used in household electricity).
Q 2. What is electricity in class 10?
In Class 10, electricity is a crucial topic in the Science curriculum. Students learn about the behavior of electric currents, Ohm’s law, resistance, and the heating effect of electric current. They also study the concepts of parallel and series combinations of resistors, as well as the relationship between power, voltage, current, and resistance.
Q 3. What is electricity and its formula?
Electricity is a form of energy resulting from the movement of electric charge. It involves the flow of electrons through conductive materials like wires. The formula for calculating electrical power (P) is P = VI, where V represents voltage (potential difference) and I denotes current flowing through the conductor.
History 10th Class Chapter-Wise Solutions for NCERT Students
The subject of History 10th class offers students a clear understanding of past events and their significance. Have you ever pondered how modern nations came into existence or how people began to identify themselves as part of a nation?
This sense of national identity evolved gradually over time. In the mid-18th century, people lived within kingdoms, small states, principalities, chiefdoms, and duchies, where the concept of a nation had not fully developed.
Class 10 History delves into the evolution of national sentiments and the development of a shared identity over time. Additionally, you will explore various other captivating topics in History.
To aid your studies, we have provided NCERT Solutions for Class 10 History, making your learning journey more accessible and enjoyable. You must visit at history of class 10th for chapter wise notes.
History 10th Class Chapter-Wise Solutions for NCERT Students

History 10th Class Chapter-Wise Solutions for NCERT Students
The NCERT history 10th class Solutions offer comprehensive answers to all the exercise questions, organized chapter-wise. If students face any difficulties in finding answers to the exercise questions, they can rely on the provided NCERT Solutions.
The table below presents solutions for all the chapters of the History textbook – “India and Contemporary World II.” These solutions serve as a valuable resource to aid students in their understanding and successful completion of the exercises.
| Chapter 1: The Rise of Nationalism in Europe |
| Chapter 2: Nationalism in India |
| Chapter 3: The Making of a Global World |
| Chapter 4: The Age of Industrialisation |
| Chapter 5: Print Culture and the Modern World |
Overview of History 10th Class Chapter-Wise Solutions for NCERT Students
Chapter 1: The Rise of Nationalism in Europe
“The Rise of Nationalism” chapter explores various aspects envisioned by Sorrieu and delves into the diverse processes that gave rise to nation-states and nationalism in nineteenth-century Europe. It covers topics such as the emergence of nationalism in Europe, the influence of the French Revolution, and the interplay between nationalism and imperialism. The 19th century is known as the age of nationalism in Europe, while the 20th century witnessed the proliferation of national movements across Asia and Africa.
Topics Covered in Class 10 History Chapter 1: The Rise of Nationalism in Europe
1. The French Revolution and the Idea of the Nation
2. The Making of Nationalism in Europe
3. The Age of Revolutions: 1830-1848
4. The Making of Germany and Italy
5. Visualizing the Nation
6. Nationalism and Imperialism
Chapter 2: Nationalism in India
“Nationalism in India” traces the origins of nationalism during the French Revolution and highlights its manifestation in India as a result of anti-colonialism. The chapter covers the Indian independence movement, with a focus on the Non-Cooperation and Civil Disobedience Movements that took place during the 1920s.
Topics Covered in Class 10 History Chapter 2: Nationalism in India
1. The First World War, Khilafat, and Non-Cooperation
2. Differing Strands within the Movement
3. Towards Civil Disobedience
4. The Sense of Collective Belonging
List of Map Items in Class 10 History Chapter 2: Nationalism in India
(1918 – 1930) for Locating and Labelling / Identification
1. Indian National Congress Sessions:
a. Calcutta (Sep. 1920)
b. Nagpur (Dec. 1920)
c. Madras (1927)
2. Important Centres of the Indian National Movement
a. Champaran (Bihar) – Movement of Indigo Planters
b. Kheda (Gujarat) – Peasant Satyagrah
c. Ahmedabad (Gujarat) – Cotton Mill Workers Satyagraha
d. Amritsar (Punjab) – Jallianwala Bagh Incident
e. Chauri Chaura (U.P.) – Calling off the Non-Cooperation Movement
f. Dandi (Gujarat) – Civil Disobedience Movement
Chapter 3: The Making of a Global World
“The Making of a Global World” chapter delves into the impact of globalization on the world and the Indian economy. It traces the history of globalization, identifying the causes behind social and economic transformations. The Industrial Revolution in the nineteenth century played a pivotal role in the history of globalization.
Topics Covered in Class 10 History Chapter 3: The Making of a Global World
1. The Pre-modern World
2. The Nineteenth Century (1815-1914)
3. The Inter-war Economy
4. Rebuilding a World Economy: The Post-War Era
Chapter 4: The Age of Industrialisation
“The Age of Industrialisation” chapter begins by examining the pre-Industrial Revolution era and its subsequent changes, including shifts in labor practices and the establishment of factories. It explores industrial growth, the market for goods, and the lives of workers during this transformative period.
Topics Covered in Class 10 History Chapter 4: The Age of Industrialisation
1. Before the Industrial Revolution
2. Hand Labour and Steam Power
3. Industrialisation in the Colonies
4. Factories Come Up
5. The Peculiarities of Industrial Growth
6. Market for Goods
Chapter 5: Print Culture and Modern World
“Print Culture and Modern World” discusses the development of print, from its origins in East Asia to its expansion in Europe and India. This chapter highlights the impact of print technology on social and cultural aspects, considering how it transformed lives and societies.
Topics Covered in Class 10 History Chapter 5: Print Culture and Modern World
1. The First Printed Books
2. Print Comes to Europe
3. The Print Revolution and its Impact
4. The Reading Mania
5. The Nineteenth Century
6. India and the World of Print
7. Religious Reform and Public Debates
8. New Forms of Publication
9. Print and Censorship
By studying these NCERT Solutions for Class 10 History, students can effectively prepare for their board exams and gain a deeper understanding of the subject.
Frequently Asked Questions History on 10th Class Chapter-Wise Solutions for NCERT Students
Q1. Is 10th History easy?
The difficulty level of Class 10 History may vary for different students based on their interests, study habits, and prior knowledge. Some students may find it easy, while others may find it challenging. To excel in the subject, consistent study, understanding of concepts, and practice are essential.
Q2. How can I pass the History exam?
To pass the History exam, follow these tips:
– Understand the syllabus and exam pattern.
– Take organized notes while studying.
– Create a study schedule and cover one topic at a time.
– Practice with previous years’ question papers and sample papers.
– Revise regularly and clarify doubts from teachers or study guides.
Q3. Which is the toughest chapter in Class 10 Social Science?
The perception of the toughest chapter may vary among students. Some common chapters that students often find challenging in Class 10 Social Science are “The Rise of Nationalism in Europe” and “The Making of a Global World.”
Q4. How to score full marks in SST Class 10?
Scoring full marks in Class 10 Social Science requires dedication and smart preparation:
– Thoroughly study each chapter, making sure to understand the concepts.
– Practice answering previous year’s question papers and sample papers.
– Focus on key events, dates, and important names.
– Use flow charts, diagrams, and mind maps to aid in better retention.
– Write clear and concise answers during exams.
Q5. How to complete the SST syllabus in 1 day?
Completing the entire SST syllabus in one day is a daunting task. However, if you have limited time, try to prioritize important topics from each chapter. Quickly go through the main concepts, key events, and dates. Focus on understanding the broader themes and connections between topics. It’s crucial to manage your time efficiently and make the most of the available study time. Keep in mind that deep learning and understanding may not be possible in one day, but a strategic overview can be helpful.
Q 6. How to study for exams?
To study for exams effectively, create a well-structured study schedule, break down the syllabus into manageable portions, and practice with past papers. Utilize active learning techniques like summarizing and making flashcards, and take regular breaks to avoid burnout. Find a quiet environment to study, stay focused, and minimize distractions. Ensure you get enough rest and sleep before the exam to be alert and perform at your best.
Q 7. How can I study hard?
Studying hard involves setting clear goals, maintaining consistency in your study routine, and staying organized. Challenge yourself with difficult concepts, seek help when needed, and maintain a positive attitude towards your studies. Believe in your abilities and be persistent in your efforts to achieve academic success.
Q 8. What is Chapter 2 in History Class 10?
Chapter 2 in Class 10 History is titled “Nationalism in India.” It explores the concept of nationalism, its connection to the French Revolution, and its manifestation in India during the anti-colonial movements. The chapter focuses on the Indian independence movement, highlighting the significance of the Non-Cooperation and Civil Disobedience Movements in the 1920s. These movements played a crucial role in shaping India’s struggle for independence against British colonial rule.
Class 10th How Do Organisms Reproduce Notes: Science Ch. 7
Class 10th How Do Organisms Reproduce Notes: All living beings have the ability to multiply or reproduce, giving rise to offspring of a similar nature. Reproduction is a fundamental process crucial for the survival of a species and the perpetuation of life.
In this chapter, we will delve into the fascinating world of reproduction, exploring the diverse methods employed by both unicellular and multicellular organisms. These include bacteria, algae, plants, animals, and humans. We shall explore the various reproductive structures they possess and the different modes of reproduction they engage in, such as cell division, vegetative reproduction, asexual reproduction, and sexual reproduction.
Class 10th How Do Organisms Reproduce Notes
Class 10th How Do Organisms Reproduce Notes
Reproduction
Reproduction is the natural mechanism through which every organism proliferates and enhances its population size
Asexual Reproduction
Asexual reproduction is a mode of reproduction wherein a single organism is solely responsible for generating two or more offspring. This process is observed in unicellular organisms, some multicellular organisms, and certain plant species.
Fission
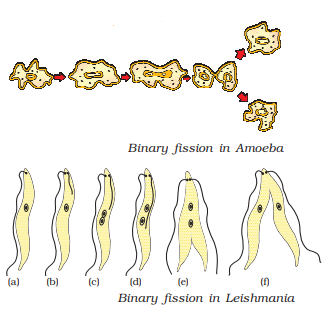
Fission is a prevalent form of asexual reproduction observed in many unicellular organisms. Depending on the outcome, it can be classified into two types:
- Binary fission: This type of fission results in the formation of two daughter cells. An example of an organism undergoing binary fission is paramecium.
- Multiple fission: In this case, fission leads to the production of multiple daughter cells. An example of an organism undergoing multiple fission is Plasmodium.
It’s important to note that the planes of fission may vary among different organisms.
Budding
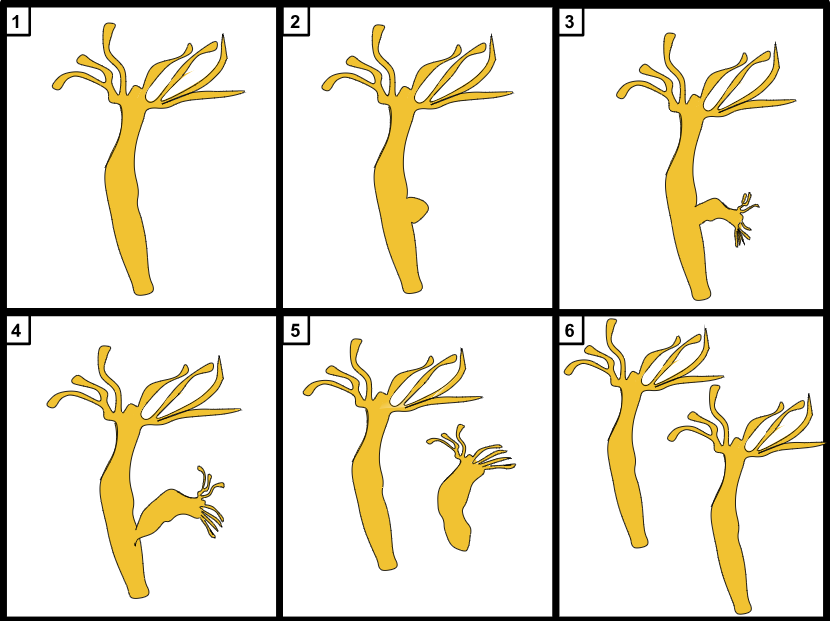
Budding is a form of asexual reproduction characterized by the development of a small cyst-like structure on the parent’s body, from which a new individual emerges.
The bud may either remain attached to the parent’s body, as observed in yeast, or it may eventually separate and become an independent new individual, as seen in hydra.
Regeneration and Fragmentation
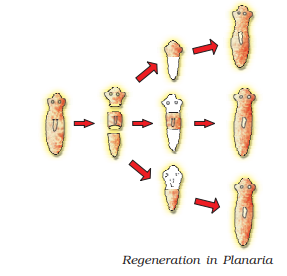
Regeneration is the remarkable process through which an organism, such as a lizard, can grow back a lost organ or body part.
On the other hand, fragmentation is a process in which an organism breaks into smaller pieces, and each fragment has the ability to develop into a complete new organism. Examples of organisms capable of fragmentation and subsequent regeneration include Planaria and Hydra.
Spore Formation
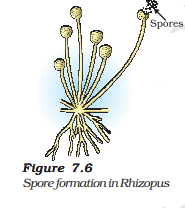
Certain organisms, like fungi, employ spores as a means of reproduction. These spores have the potential to develop into entirely new individuals once they are released from their fruiting bodies.
The sporangia are responsible for producing these spores, which are encased in a resilient outer layer, providing protection during challenging conditions.
Under favorable environmental circumstances, spores germinate, initiating their growth and development into new organisms.
Vegetative Propagation
Vegetative propagation is a form of asexual reproduction commonly observed in plants. In this process, various vegetative parts of the plant, such as leaves, stems, and roots, have the ability to give rise to new individual plants.
Natural vegetative propagation occurs through different means, including:
1. Leaves, as seen in plants like bryophyllum.
2. Stems, as observed in plants like turmeric and ginger.
3. Runners or stolons, as found in grass runners and strawberry plants.
4. Bulbs, exemplified by plants like onion and lily.
In addition to natural methods, artificial techniques of vegetative propagation are also practiced, including cutting, grafting, layering, and plant tissue culture. These methods allow for the controlled and efficient propagation of desired plant varieties.
Class 10th Sexual Reproduction Notes
The reproductive mode entails the involvement of two individuals – one male and one female – who produce specialized sex cells or gametes. These gametes then fuse together to give rise to a new organism.
Types of Cell Division
Eukaryotic organisms exhibit two distinct types of cell division:
1. Mitosis:
– Occurs in somatic cells (non-reproductive cells).
– Maintains the original chromosome number.
– Yields two diploid daughter cells (with the same number of chromosomes as the parent cell).
– Essential for asexual reproduction, development, growth, cell replacement, and regeneration.
2. Meiosis:
– Occurs in sex cells (reproductive cells).
– Reduces the number of chromosomes by half through two consecutive divisions.
– Produces four haploid daughter cells (with half the number of chromosomes as the parent cell).
– Crucial for sexual reproduction, specifically in the formation of gametes (sperm and egg cells).
The Reproductive System
Humans exhibit a significant contrast between the male and female reproductive systems. In males, the testes serve as the primary reproductive structure responsible for producing sperm, which are the male gametes.
On the other hand, the ovary is the key reproductive organ in females, where the production of ova (female gametes) takes place. Now, let us delve into a comprehensive exploration of the male and female reproductive systems in humans.
Male Reproductive System: Class 10th How Do Organisms Reproduce Notes
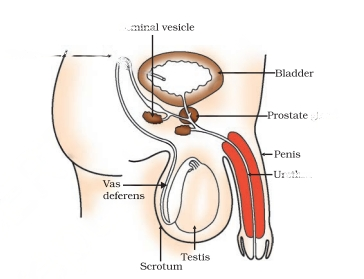
In males, the primary reproductive organs consist of a pair of testes. These testes play a crucial role in producing male sex cells known as sperm, as well as secreting the male sex hormone, testosterone.
Male Primary Reproductive Organ
In males, the primary reproductive organs are a pair of testes, situated within scrotal sacs outside the body. These testes consist of seminiferous tubules, which serve as the structural and functional units responsible for the production of male sex cells, sperms. The maturation of these sperms occurs in the epididymis.
Furthermore, the spaces between the seminiferous tubules contain Leydig cells or interstitial cells, which play a vital role in secreting the hormone testosterone.
Male Accessory Reproductive Organs
The reproductive process is supported by various accessory organs.
Within the reproductive system, the prostate gland and seminal vesicles function as glands responsible for producing semen and providing nourishment to the sperm.
Moreover, the penis, which accommodates the urethra, serves as a copulatory organ facilitating the passage of semen during sexual intercourse.
Male Ducts
Within males, the primary ducts involved in the reproductive process are the vas deferens and the urethra.
Each testis is connected to a single vas deferens, which serves as a conduit for transporting sperm from the testis to the urethra.
The urethra functions as a shared passageway for both semen and urine, facilitating the expulsion of semen during ejaculation and the elimination of urine from the body.
Female Reproductive System: Class 10th How Do Organisms Reproduce Notes
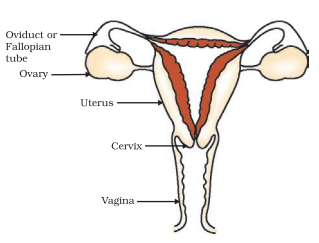
The female reproductive system in humans comprises a set of paired organs, including ovaries and fallopian tubes (oviducts), alongside accessory organs such as the uterus and vagina.
Female Primary Reproductive Organ
In females, the primary reproductive organs are a pair of ovaries. These ovaries play a pivotal role in producing female sex cells known as eggs or ova. Additionally, the ovaries are responsible for producing the female sex hormones, estrogen and progesterone.
Female Accessory Reproductive Organ
In human females, the uterus, oviducts, and vagina function as essential accessory reproductive organs.
The uterus serves as the site for fetal development during pregnancy, while the vagina receives sperm from the male during sexual intercourse.
Furthermore, a pair of oviducts are responsible for carrying the ovum from the ovaries to the uterus, facilitating fertilization and potential pregnancy.
Menstrual Cycle
Menstruation
Menstruation is a cyclical process where the ovum is released from the ovary and expelled from the body if fertilization does not occur.
During menstruation, the blood-rich endometrium lining of the uterus also breaks down and is shed along with the unfertilized ovum.
Various hormones play crucial roles in this process, including two pituitary hormones, LH and FSH, as well as two ovarian hormones, estrogen and progesterone.
In humans, this menstrual cycle typically repeats approximately every 28 days.
Fertilization
Human Reproduction
Humans reproduce sexually. The male produces sperms and the female produces eggs. When the sperm fuses with the egg, it forms a zygote that gives rise to a new progeny.
Contraceptive Methods

Reproductive Health
Reproductive health encompasses efforts aimed at preventing sexually transmitted diseases (STDs) and unwanted pregnancies. Moreover, fostering awareness and understanding of the reproductive system is an integral aspect of promoting reproductive health.
Contraceptives
Contraceptives are tools designed to prevent unwanted pregnancies and aid in the prevention of STDs.
These contraceptives come in various types, including mechanical barriers, hormonal/chemical methods, surgical methods, and more.
Their diverse range provides individuals with options to make informed choices regarding their reproductive health and family planning.
Coitus Interruptus
This method of contraception is highly unreliable and involves interrupting sexual intercourse before the male ejaculates inside the female reproductive tract.
Rhythm Method
Another unreliable method of contraception involves avoiding sexual intercourse when the female is fertile, and the chances of fertilization are significantly elevated.
Condoms:
Among the most effective contraception methods, condoms act as mechanical barriers, preventing semen from entering the female reproductive tract and thus avoiding pregnancy. Additionally, they provide protection against the transmission of STDs.
Diaphragms:
Diaphragms are barriers placed inside the female reproductive tract, effectively blocking semen entry and preventing pregnancy.
Contraceptive Pills:
These are chemical methods of contraception that modify hormone levels in the body, hindering the release of the ovum from the ovaries.
Emergency Pill:
Emergency pills can be taken after sexual intercourse to prevent pregnancy. They swiftly alter hormone levels, hindering successful implantation, even if fertilization has occurred.
IUD (Intrauterine Device):
IUDs are devices inserted into the uterus to alter its shape, effectively preventing the successful implantation of the zygote. They offer long-term contraception for several years.
Sterilization:
Sterilization is a permanent surgical method to achieve infertility. In males, it is called vasectomy, and in females, it is known as tubal ligation.
Reproduction in Plants
Plants employ both asexual and sexual methods for reproduction. Asexual reproduction is achieved through vegetative propagation. Now, let’s explore the process of sexual reproduction in plants.
Sexual Reproduction in Flowering Plants:
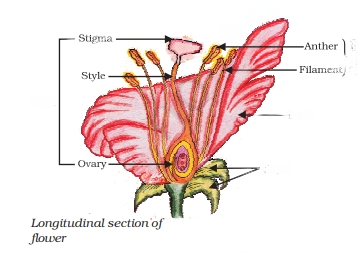
Sexual reproduction in plants occurs through flowers. The androecium and gynoecium are essential whorls of the flower involved in this process.
Non-Essential Parts of Flowers:
Apart from the essential whorls, flowers also have non-essential whorls, including sepals and petals. These parts do not directly participate in reproduction. Sepals protect the inner delicate parts during bud formation and, if green, perform photosynthesis. Petals, when colored, attract insects for pollination.
Essential Whorls of Flowers:
The androecium and gynoecium are considered the essential or reproductive whorls of a flower. The androecium produces pollen grains containing male gametes, while the gynoecium produces ovules, which are female gametes. Flowers may be bisexual (containing both whorls) or unisexual (containing either one).
Pollination:
Pollination is the transfer of pollen grains from anthers to the stigma of a flower, a necessary step for fertilization. It can occur through self-pollination (within the same flower or another flower of the same plant) or cross-pollination (between different flowers). Various agents, such as water, wind, insects, birds, and bats, play roles in cross-pollination.
Fertilization:
Fertilization involves the fusion of male and female gametes. After pollination, pollen grains germinate on the stigma surface, producing two male nuclei. The ovule contains an egg cell and two polar nuclei.
One male nucleus fuses with the polar nuclei to form a triploid endosperm. The other male nucleus fuses with the egg cell to form the zygote, which develops into the embryo and the future plant. After fertilization, the ovary transforms into a fruit, and the ovules develop into seeds, while other flower parts wither away.
Read More:
- Class 10 Notes for Science NCERT
- Chemical Reaction and Equation Notes Class 10 NCERT Sci. Ch.1
- Acids Bases and Salts Class 10 Notes of NCERT Science Ch. 2
- Metals and Non Metals Class 10 NCERT Science Chapter 3 Notes
- Life Process Notes Class 10 NCERT Science Chapter 5
- Control and Coordination Notes Class 10: NCERT Science Ch. 6
- Class 10th Heredity and Evolution Notes: NCERT Science Ch. 8
- Notes on Magnetic Effect of Electric Current Class 10 NCERT
- Human Eye and the Colourful World Notes Chapter 10 Science
- Class 10th Chapter 11 Science Notes for NCERT Students
- Notes of Our Environment Class 10: NCERT Science Chapter 13
Frequently Asked Questions – FAQs on Class 10th How Do Organisms Reproduce Notes
Q 1. What is an example of an organism reproduce?
One example of an organism that reproduces is the honeybee (Apis mellifera). Honeybees engage in sexual reproduction, where the male drone transfers sperm to the queen during mating.
The queen then stores the sperm in her spermatheca and uses it to fertilize eggs throughout her life. Honeybees also exhibit a unique form of asexual reproduction called parthenogenesis.
Unfertilized eggs laid by the queen develop into male drones, while fertilized eggs give rise to female worker bees or potential new queens. This diverse reproductive strategy ensures the continuation of the honeybee colony and enables them to adapt and thrive in various environments.
Q 2. Why do organisms reproduce?
Organisms reproduce to ensure the continuation of their species and the perpetuation of life. Reproduction is a fundamental biological process that allows organisms to pass on their genetic material to the next generation.
Through reproduction, organisms produce offspring with traits that enhance their survival and adaptation to changing environments. It also aids in maintaining biodiversity and ecological balance.
Reproduction is essential for the growth and development of populations, contributing to the stability and sustainability of ecosystems. It is a fundamental drive ingrained in the biological imperative of all living beings, ensuring the survival and success of their species over time.
Q 3. What are the 3 types of reproduction?
The three main types of reproduction are: a) Asexual reproduction: Involves a single parent, and offspring are genetically identical to the parent. b) Sexual reproduction: Involves two parents, and offspring inherit a combination of genetic traits from both parents. c) Parthenogenesis: A form of asexual reproduction where unfertilized eggs develop into offspring without involving males.
Q 4. According to Class 10th How Do Organisms Reproduce Notes why is reproduction important?
Reproduction is vital for the survival and continuity of life on Earth. It ensures the perpetuation of species, the maintenance of genetic diversity, and the adaptation to changing environments. Through reproduction, organisms pass on their genetic material to future generations, allowing them to adapt and evolve over time. It is essential for the growth of populations, the balance of ecosystems, and the functioning of biological communities.
Q 5. Is reproduction a life process?
Yes, reproduction is considered a life process. It is one of the fundamental characteristics of living organisms, distinguishing them from non-living entities. Reproduction is crucial for the continuation of life and is inherent in the biological imperative of all living beings. Without reproduction, species would cease to exist, and life as we know it would not be sustained.
Class 10th Heredity and Evolution Notes: NCERT Science Ch. 8
In this chapter “class 10th heredity and evolution notes“, we delve into the fascinating world of heredity and evolution. Heredity involves the transfer of characteristics from one generation to the next. While evolution encompasses the gradual progression from simple life forms to more complex organisms over multiple generations.
Here in class 10th heredity and evolution notes, we explore the mechanisms behind the creation of variations. Also, how the accumulation of these variations ultimately drives the process of evolution. Get ready to unravel the mysteries of life’s continuity and transformation.
Class 10th heredity and evolution notes of NCERT Science Ch. 8
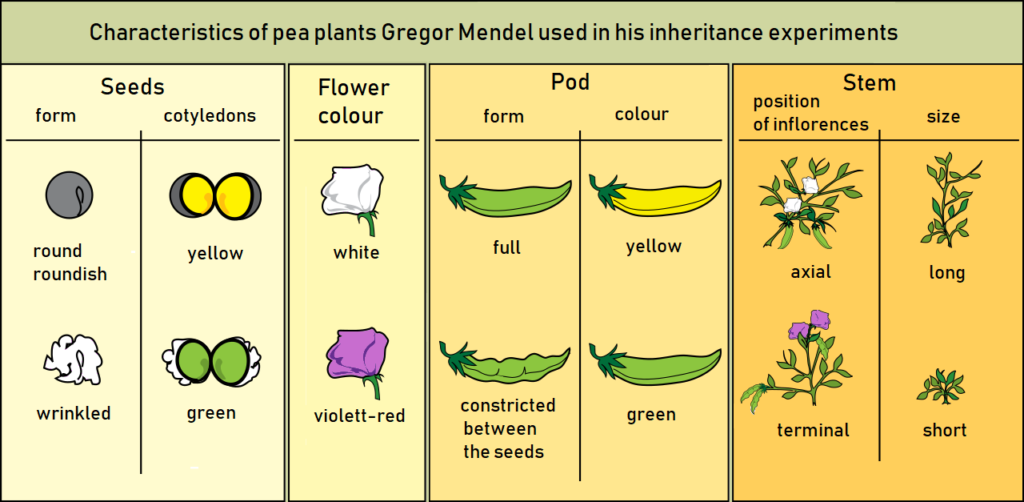
A cross that involves the consideration of only one character between two organisms is referred to as a monohybrid cross. The resulting ratio of characters in the F2 generation is known as the monohybrid ratio.
For example, when a tall plant (TT) is crossed with a dwarf plant (tt), the F2 generation yields a ratio of 3 tall plants to 1 short plant, which represents the monohybrid ratio of 3:1. In this cross, only the height of the plants is taken into account at a time, illustrating the principles of monohybrid inheritance.
Notes for Class 10th Chapter Heredity and Evolution
Dihybrid cross
A cross that involves the consideration of two characters between two organisms is termed a dihybrid cross. The resulting ratio of characters in the F2 generation is referred to as the dihybrid ratio.

For example, if a plant with round and green peas is crossed with a plant having wrinkled and yellow peas, the first-generation plants would all exhibit round and green peas. In the subsequent F2 generation cross, we would observe four combinations of characters in the ratio of 9:3:3:1.
Thus, the dihybrid ratio is represented as 9:3:3:1. IT is indicating the occurrence of different combinations of traits resulting from the dihybrid cross between the two organisms.
Inheritance
In Biology, inheritance refers to the transmission of traits from one generation to the next.
Laws of Mendel as define in Heredity and Evolution class 10th
The Law of Dominance states that a gene possesses two contrasting alleles, and one of them always expresses itself in the organism. This allele is known as the dominant gene, and it can manifest in any possible combination.
According to the Law of Segregation, traits are completely separated during gamete formation, with no mixing of alleles. Each gamete carries only one allele for a particular trait.
The Law of Independent Assortment reveals that traits can segregate independently of different characters during gamete formation. This means that the inheritance of one trait does not influence the inheritance of another trait. It is allowing for diverse combinations of traits in offspring.
Class 10th Heredity and Evolution Notes
Sex Determination
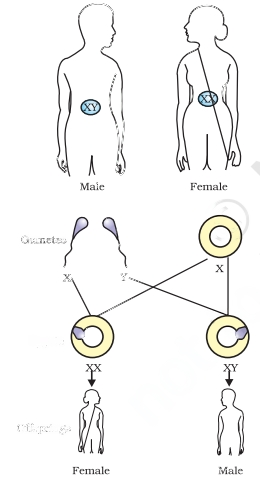
Sex determination is the process of identifying an individual’s sex based on their genetic material composition. In various animals, the sex of an embryo is determined by diverse factors. In humans, sex determination occurs through the presence or absence of the Y chromosome.
A female human is represented by XX chromosomes, while a male is represented by XY chromosomes. An ovum always carries an X chromosome. When the ovum fuses with a sperm carrying a Y chromosome, it results in the birth of a male child. On the other hand, when it fuses with a sperm carrying an X chromosome, it leads to the birth of a female child.
This process of sex determination plays a vital role in defining an individual’s gender. It is governed by specific genetic factors that shape human development and reproduction.
What is Traits in Class 10th Heredity and Evolution
Traits are distinguishing characteristics of an organism, evident either in its visible physical form or in its physiological aspects.
Acquired Characters
Acquired characteristics refer to the traits acquired by an organism during its lifetime. However, these characteristics are not transmitted to the DNA of germ cells and, consequently, are not inherited by the next generation. For instance, traits like muscle loss and reduced weight due to starvation, loss of limbs or tails from injuries, and similar acquired features are not passed on to future generations.
Class 10th Heredity and Evolution Notes
Inherited Characters
Inherited characters refer to the traits passed down from parents to their offspring. These traits are consistently transmitted to the next generation, but their expression depends on whether they are dominant or recessive.
For instance, characteristics like height, skin color, and eye color are examples of inherited traits. While they are reliably inherited, their manifestation in the offspring can vary based on whether the alleles governing these traits are dominant or recessive. The interplay of dominant and recessive alleles determines the observable expression of these inherited characters in the offspring.
Variation
Variation represents the extent of dissimilarity among individuals within the same species. Offspring are not identical to their parents, exhibiting distinct variations. Each member of a population differs from others due to the influence of recombination and mutation. Which serve as the primary causes of these variations.
Sexually reproducing organisms display considerable diversity among individuals of a species, and the long-term accumulation of variations plays a pivotal role in the process of evolution. Environmental factors play a crucial part in evolutionary processes as they select advantageous variants, driving the progression of evolution.
Genetic Variations
Genetic variations refer to the dissimilarities in DNA sequences among organisms, contributing to the diverse gene pool. These variations result in distinct physical characteristics or biochemical pathways, showcasing the unique traits exhibited by each individual.
Natural Selection
Natural selection is the process through which a favorable trait within a species’ population is chosen. Changing environmental conditions exert equal pressure on all existing species. The species or organisms that demonstrate better adaptation to the altering conditions survive and reproduce, signifying their selection by nature.
Conversely, species or organisms that struggle to adapt face elimination, being rejected by nature. Natural selection thus acts as a driving force, shaping the survival and evolution of species in response to dynamic environmental changes.
Class 10th Heredity and Evolution Notes
Speciation
Genetic Drift
In the determination of traits that endure within a population, natural selection assumes a significant role. Nevertheless, random fluctuations in gene variants can be observed on various occasions, which is termed genetic drift. Genetic drift denotes a change in the frequency of an existing allele within a small population.
As a consequence of genetic drift, a gene variant may diminish and eventually disappear from the population, leading to a reduction in genetic variation. This phenomenon highlights the influence of chance events on the genetic makeup of small populations, and it has implications for the evolutionary trajectory of species over time.
Speciation
Speciation is the transformative process through which new species emerge from existing ones, driven by various evolutionary forces such as genetic drift, population isolation, and natural selection. This intricate process gives rise to diversity within ecosystems, and this diversity, in turn, fuels the continued evolution of life forms.
As species adapt to their unique environments and encounter diverse ecological challenges, they undergo changes that set them apart from their ancestral populations. Over time, these modifications accumulate, leading to the formation of distinct species with specialized characteristics. The diverse array of species contributes to the complexity and richness of ecosystems, driving the ongoing process of evolution and perpetuating the fascinating tapestry of life on Earth.
Gene Flow: Class 10th Heredity and Evolution Notes
Gene flow involves the movement of genes from one population to another, often facilitated by migration or the introduction of organisms to new populations. As genes are transferred between populations, the frequencies of certain genes change, leading to alterations in the genetic makeup of the receiving population.
This dynamic process of gene flow helps in the exchange and dissemination of genetic traits among different groups, contributing to genetic diversity and evolution within and across species.
Population
A population refers to a community or a collective of animals, plants, or any living organism capable of reproducing with one another and producing fertile, viable offspring.
Charles Darwin
Charles Darwin, renowned as the “Father of Evolution,” was an English naturalist and biologist. His groundbreaking theory of evolution was shaped during a five-year expedition aboard the HMS Beagle to the Galapagos Islands. This remarkable journey provided him with invaluable insights into the diversity of life and the processes of adaptation and natural selection.
In 1859, Darwin unveiled his comprehensive theory of evolution in his influential book titled “On the Origin of Species.” In this seminal work, he expounded on the concept of natural selection and its role in shaping the development and diversity of species over time. Darwin’s pioneering contributions revolutionized the field of biology and laid the foundation for our modern understanding of the evolutionary processes that govern life on Earth.
Evolution and Fossils
Evolution represents a measurable transformation in the heritable traits of a population across multiple generations. These alterations can lead to the emergence of a new species or enable existing species to adapt and thrive in their environment more effectively.
Class 10th Heredity and Evolution Notes on Origin of Species
Following a fruitful expedition aboard HMS Beagle, Charles Darwin penned a book detailing his observations from the Galapagos Islands. Titled ‘The Origin of Species,’ the book presented a comprehensive theory of evolution, primarily founded on the concept of Natural Selection.
Origin of Life – Haldane’s Theory
JBS Haldane, a distinguished British scientist, postulated the idea that life originated from organic and lifeless matter. This hypothesis was later substantiated by Urey and Miller’s experiment, confirming the validity of his theory, which became known as the theory of abiogenesis.
Evolutionary Evidence – Fossils
Numerous pieces of evidence strongly support the theory of evolution, with fossils being among the most compelling. Fossils are the well-preserved remnants of ancient animals or plants that existed millions of years ago.
These remarkable relics provide invaluable insights into the anatomy and physiology of these organisms, enabling us to comprehend the mechanisms of evolution and how they culminated in the development of the diverse life forms we encounter today.
Fossils serve as crucial time capsules, shedding light on the fascinating history of life on Earth and bolstering our understanding of the gradual transformation and diversification of species over time.
Formation of Fossils
Fossils play a crucial role in providing evidence for evolution and are created through the following steps:
1. Organisms perish and become buried in mud and silt.
2. Soft tissues decompose rapidly, leaving behind the durable bones or shells.
3. Over time, sediment accumulates and solidifies into rock.
4. As the bones decay, minerals gradually infiltrate and replace the cell contents, a process known as petrification.
5. If the bones decompose entirely, they leave behind the impression or cast of the original animal, preserving its form in the fossil record.
Evolutionary Relationships: Class 10th Heredity and Evolution Notes
Studying homologous and analogous organs offers insights into the evolutionary relationships among animals.
Homologous organs exhibit similar structures but serve different functions:
– For instance, the wings of birds and the forelimbs of mammals share similar structures, but they have been modified to suit different functions.
– Similarly, the tendril of a pea plant and the spine of a barberry plant are both modified leaves, yet they perform distinct functions.
Analogous organs, on the other hand, serve similar functions but have different structures and origins:
– The wings of bats, birds, and insects, for example, are all used for flying, but their structures vary significantly.
– Likewise, the leaves of opuntia and peepal both perform photosynthesis, but while the leaves of opuntia are modified stems, the peepal leaves are typical leaves.
Evolution by Stage
It is a gradual and extended process, unfolding over time rather than occurring abruptly. Nearly all present-day animals have undergone several stages of evolution in their journey to their current forms.
The complexities of organisms do not arise suddenly but progress incrementally, with certain traits serving limited purposes during specific stages.
This gradual progression in the evolution of species is known as “evolution by stages.” It involves a step-by-step development, where small changes accumulate over generations, leading to the emergence of new traits and characteristics.
Evolutionary transformations unfold over extended periods, encompassing numerous intermediary stages, reflecting the intricate and fascinating journey that shapes the diversity of life on Earth.
Artificial Selection
Artificial selection can lead to the evolution of multiple distinct species from a single ancestral species. A prime example is the cabbage family, where a common ancestor gave rise to several diverse species through the deliberate selection of various traits.
Through human intervention and cultivation practices, specific desirable traits were favored and perpetuated. Resulting in the emergence of distinct cabbage species with unique characteristics and adaptations. Artificial selection, driven by human influence, demonstrates the remarkable capacity to shape the course of evolution and contribute to the diversity and specialization observed in the natural world.
Molecular Phylogeny
Phylogeny refers to the evolutionary connections between various biological species, giving rise to an evolutionary tree. In molecular phylogeny, these relationships are explored at the molecular level, primarily through the examination of DNA sequences. This entails the analysis of DNA composition and gene comparisons among different species.
By studying the hereditary molecular traits, such as DNA sequences, scientists can unravel the intricate evolutionary history and relationships among diverse organisms. Molecular phylogeny offers valuable insights into the common ancestry and evolutionary divergence of species, shedding light on the dynamic and interconnected nature of life’s evolutionary journey.
Human Evolution
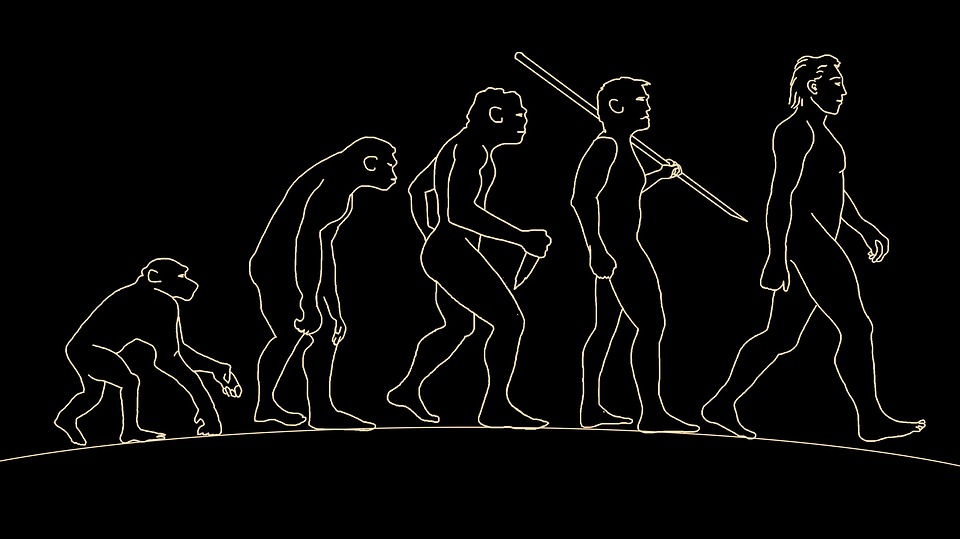
Human Evolution
Humans are categorized within the primate family, and their genetic connection to chimps and other primates is remarkably close. Although the full evolutionary journey from primates to humans remains a mystery, a broader understanding of human evolution has emerged.
The ancestry of humans includes various predecessors like Dryopithecus, Ramapithecus, Australopithecus, Homo erectus, Homo sapiens neanderthalensis, Cro-magnon man, and ultimately, Homo sapiens – us.
The study of these ancestors and their characteristics provides valuable insights into the fascinating path of human evolution. While some aspects of our evolutionary history may remain elusive, the evidence gathered from these ancestral relatives paints a vivid picture of the transformation that led to the emergence of modern humans.
Read Also:
- Class 10 Notes for Science NCERT
- Chemical Reaction and Equation Notes Class 10 NCERT Sci. Ch.1
- Acids Bases and Salts Class 10 Notes of NCERT Science Ch. 2
- Metals and Non Metals Class 10 NCERT Science Chapter 3 Notes
- Life Process Notes Class 10 NCERT Science Chapter 5
- Control and Coordination Notes Class 10: NCERT Science Ch. 6
- Class 10th How Do Organisms Reproduce Notes: Science Ch. 7
- Class 10th Heredity and Evolution Notes: NCERT Science Ch. 8
- Notes on Magnetic Effect of Electric Current Class 10 NCERT
- Human Eye and the Colourful World Notes Chapter 10 Science
- Class 10th Chapter 11 Science Notes for NCERT Students
- Notes of Our Environment Class 10: NCERT Science Chapter 13
Frequently Asked Questions on Class 10th Heredity and Evolution Notes
Q 1. what are the sources of genetic diversity within a population?
The sources of genetic diversity within a population include mutations, which introduce new variations in the DNA sequence. Genetic recombination during sexual reproduction, creating unique combinations of alleles. Gene flow, through migration or interbreeding, brings in new genes from other populations. Natural selection favors advantageous traits, increasing their frequency, while genetic drift, in small populations, causes random changes in gene frequencies. These processes collectively contribute to the variability of traits in a population, enabling adaptation to changing environments and promoting species survival and evolution.
Q 2. From Class 10th Heredity and Evolution Notes How you can explain the evolution diversity of life on Earth?
The Evolution explains the diversity of life on Earth through the gradual process of genetic change and natural selection. Over millions of years, genetic variations arise within populations due to mutations and genetic recombination during reproduction. Natural selection then acts on these variations, favoring advantageous traits that increase an organism’s chances of survival and reproduction in specific environments. As a result, organisms with beneficial traits pass on their genes to future generations, leading to the accumulation of adaptive characteristics and the diversification of species. This continuous process of genetic change and selection has resulted in the vast array of life forms and their adaptations to different ecological niches on Earth.
Q 3. What is the role of adaptation in the survival and success of organisms in various environments?
Adaptation plays a crucial role in the survival and success of organisms in various environments. It refers to the process by which organisms develop advantageous traits that enable them to better suit their surroundings. Organisms with beneficial adaptations have increased chances of survival, reproduction, and passing on their advantageous genes to the next generation. In different environments, such as extreme temperatures, limited resources, or predators, adaptations can provide a competitive advantage. Over time, these advantageous traits become more prevalent in the population, leading to the specialization and diversification of species, ultimately enhancing their ability to thrive and persist in their respective habitats.
Q 4. What is the significance of studying heredity and evolution in fields like medicine and agriculture?
Studying heredity and evolution holds immense significance in fields like medicine and agriculture. In medicine, understanding heredity helps identify genetic factors responsible for various diseases, enabling personalized treatments and genetic counseling. Knowledge of evolution aids in comprehending the origin of diseases and their potential spread. In agriculture, studying heredity assists in breeding programs to develop crops with desired traits, such as higher yield or resistance to pests and diseases. Understanding evolutionary principles aids in combating evolving pests and diseases, improving crop resilience, and developing sustainable agricultural practices. Both fields benefit from the insights gained through the study of heredity and evolution, leading to advancements and better strategies for human health and food security.
Q 5. What is heredity, and how does it influence the transmission of traits from one generation to another?
Heredity is the process by which traits or characteristics are passed from parents to their offspring through genetic information encoded in DNA. It influences the transmission of traits from one generation to another by the inheritance of genes. Offspring inherit a combination of genes from their parents, determining their physical and biochemical traits. Genes come in different forms called alleles. The combination of alleles inherited from each parent influences the expression of traits in the offspring. Some traits may be dominant, and their alleles are expressed over recessive traits. Heredity ensures continuity and variation in populations, allowing the transfer of genetic information and shaping the diversity of life on Earth.
Chemical Reaction and Equation Notes Class 10 NCERT Sci. Ch.1
Chemical Reaction and Equation Notes Class 10: Explore the fascinating world of Science, where we discover how things work and learn about chemical reactions that bring changes around us. From metal rusting to things breaking down, chemical reactions show us how tiny particles interact and create transformations. In Class 10 Science, Chapter 1 explains how substances change their form.
In the article “Chemical Reaction and Equation Notes Class 10” you can learn more about CBSE science class 10 chapter 1 Chemical Reaction and Equation. These notes are carefully made to give you the right amount of information without overwhelming you. They will help you prepare for exams smoothly. Understand how substances change and be ready to explore the amazing world of science. Start this educational journey now and discover the wonders of chemistry.
CBSE Science Chapter 1: Chemical Reaction and Equation Notes Class 10
Chemical Reactions
In a chemical reaction, one or more reactants change into one or more products. During this process, the atoms of the reactants get rearranged, leading to the creation of different substances as the end products.
Chemical reactions are transformations in which reactants change into products by forming or breaking bonds between different atoms.
During a chemical reaction, one set of chemical components undergoes a change to become another. These changes involve the movement of electrons in the formation and breaking of chemical bonds between atoms, with no alteration in the nuclei. Chemical equations are used to describe these reactions. The rate at which chemical reactions occur can be predicted at a given temperature and chemical concentration. Generally, reaction rates increase with higher temperatures since more thermal energy is available to reach the activation energy required to break bonds between atoms.
Types of Chemical Reactions Described in Chemical Reaction and Equation Notes Class 10
Considering various factors, chemical reactions can be categorized into multiple groups. Here are a few examples:
- Combination
- Decomposition
- Single Displacement
- Double displacement
- Redox
- Endothermic
- Exothermic
- Precipitation
- Neutralisation
Combination Reaction
A combination reaction is a type of chemical reaction where two or more substances react to form a single new compound. In this reaction, elements or compounds come together to create a more complex compound as the product.
Let’s take the example of the reaction between iron (Fe) and sulfur (S) to form iron(II) sulfide (FeS):
Fe + S → FeS
In this combination reaction, solid iron and solid sulfur combine to produce iron(II) sulfide, a new compound. This type of reaction can occur under appropriate conditions, such as when iron and sulfur are heated together. The atoms of iron and sulfur rearrange to form the compound FeS.
Combination reactions are essential in various chemical processes, ranging from the formation of simple compounds like water to more complex ones found in industrial applications and biological systems.
Decomposition Reaction
Decomposition reactions involve the breakdown of one compound into two or more compounds or elements. They are the opposite of combination reactions.
A typical decomposition reaction is represented as follows:
AB → A + B
Examples:
1. When hydrogen peroxide (H2O2) decomposes, it forms water (H2O) and oxygen gas (O2).
2H2O2(l) → 2H2O(l) + O2(g)
Hydrogen peroxide → Water + Oxygen gas
2. Silver chloride (AgCl) decomposes upon exposure to light, forming silver (Ag) and chlorine gas (Cl2).
2AgCl(s) + light → 2Ag(s) + Cl2(g)
Silver chloride + Light → Silver + Chlorine gas
Decomposition reactions are significant in various fields, such as chemistry, biology, and environmental science. They are involved in processes ranging from the breakdown of compounds in nature to chemical reactions in industrial applications.
a. Decomposition reactions that require heat are known as thermolytic decomposition or thermolysis.
Example:
Thermal decomposition of HgO:
2HgO(s) → 2Hg(l) + O2(g)
b. Decomposition reactions that require light are called photolytic decomposition or photolysis.
Example:
Photolytic decomposition of H2O2:
2H2O2(l) + light → 2H2O(l) + O2(g)
c. Decomposition reactions that require electricity are referred to as electrolytic decomposition or electrolysis.
Example:
Electrolytic decomposition of water:
2H2O(l) → 2H2(g) + O2(g)
In these specific types of decomposition reactions, the input of heat, light, or electricity triggers the breakdown of compounds into their constituent elements or simpler compounds.
Displacement Reaction
Displacement reactions, also known as Substitution Reactions or Single Displacement/Replacement Reactions, occur when a more reactive element displaces a less reactive element from a compound.
A typical displacement reaction can be expressed using a chemical equation as follows:
A + BC → AC + B
For a displacement reaction to occur, element ‘A’ must be more reactive than element ‘B’. If ‘B’ is more reactive than ‘A’, then ‘A’ will not displace ‘C’ from ‘BC’, and the reaction will not take place.
Examples:
1. When zinc reacts with hydrochloric acid, it produces hydrogen gas and zinc chloride.
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
2. When zinc reacts with copper sulfate, it forms zinc sulfate and copper metal.
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
In these examples, zinc, being more reactive than hydrogen in hydrochloric acid and copper in copper sulfate, displaces them from the compounds, resulting in the formation of new products. Displacement reactions play a significant role in various chemical processes and have practical applications in different industries.
Double Displacement Reaction or Precipitation Reaction
Double displacement reactions, also known as Double Replacement Reactions, involve the exchange of ions between two reactants, resulting in the formation of new compounds.
The general representation of a double displacement reaction is:
AB + CD → AC + BD
Examples:
1. When a solution of barium chloride reacts with a solution of sodium sulphate, a white precipitate of barium sulphate is formed, along with sodium chloride.
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) (Precipitate) + 2NaCl(aq)
2. When sodium hydroxide (a base) reacts with hydrochloric acid, sodium chloride and water are formed.
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Note: A double displacement reaction that forms a precipitate is also referred to as a precipitation reaction. Neutralization reactions are also examples of double displacement reactions, where an acid and a base react to form a salt and water. Double displacement reactions play a crucial role in various chemical processes and are encountered in different applications, such as in the synthesis of salts and precipitation reactions.
Redox Reaction
A redox reaction occurs when the oxidation states of the substances involved change. Oxidation refers to the loss of electrons or an increase in the oxidation state of a chemical or its atoms, while reduction refers to the gain of electrons or a decrease in the oxidation state of a chemical or its atoms.
During a redox reaction, both oxidation and reduction processes happen simultaneously. In oxidation, a substance loses electrons, gains oxygen, or loses hydrogen. On the other hand, in reduction, a substance gains electrons, loses oxygen, or gains hydrogen.
An oxidizing agent is a substance that causes another substance to undergo oxidation while it itself gets reduced. Conversely, a reducing agent is a substance that causes another substance to undergo reduction while it itself gets oxidized.
Redox reactions play a crucial role in various chemical processes, including combustion, corrosion, and energy production. They are essential for the transfer of electrons between substances, leading to significant transformations in the properties and behavior of chemicals.

Exothermic reaction
During a reaction, heat is released. Many combination reactions are exothermic, meaning they release heat.
Examples:
1. Al + Fe2O3 → Al2O3 + Fe + heat
In this reaction, when aluminum reacts with iron(III) oxide, it forms aluminum oxide and iron, releasing heat in the process.
2. CH4 + 2O2 → CO2 + 2H2O + heat
When methane reacts with oxygen, it produces carbon dioxide and water, along with the release of heat.
In both these combination reactions, the formation of new compounds leads to the release of heat energy, making them exothermic reactions. Exothermic reactions are common in nature and industry and play a significant role in various chemical processes, providing valuable energy and driving various natural phenomena.
Endothermic
Energy in the form of heat is necessary to drive the reaction.
6CO2 + 6H2O + Sunlight → C6H12O6 + 6O2
Glucose
In this reaction, glucose is produced from carbon dioxide and water in the presence of sunlight, and oxygen is released. The reaction requires the input of heat energy from sunlight to proceed.
Most of the decomposition reactions are endothermic, meaning they absorb heat from the surroundings to proceed. In these reactions, energy is required to break down a compound into its constituent elements or simpler compounds.
Chemical Reaction and Equation Notes Class 10
Corrosion
The gradual deterioration of a material, often a metal, due to the influence of moisture, air, or chemicals in the surrounding environment is known as corrosion.
Rusting:
4Fe(s) + 3O2(from air) + xH2O(moisture) → 2Fe2O3.xH2O(rust)
Corrosion of copper:
Cu(s) + H2O(moisture) + CO2(from air) → CuCO3.Cu(OH)2(green)
Corrosion of silver:
Ag(s) + H2S (from air) → Ag2S(black) + H2(g)
In these examples, rusting, copper corrosion, and silver corrosion all occur due to the interaction with elements in the environment, such as moisture, air, and chemicals. These reactions lead to the formation of new compounds on the surface of the materials, causing them to deteriorate over time. Corrosion is a common phenomenon that affects various materials, and it is a significant concern in various industries and everyday applications.
Rancidity
Food undergoes a process called rancidity when fats and oils in it undergo oxidation over an extended period. This results in the food developing a foul smell and unpleasant taste. Consuming rancid food can lead to stomach infections and discomfort.
Prevention methods include:
(i) Using air-tight containers to minimize exposure to air and oxygen.
(ii) Packaging food with nitrogen to create a protective atmosphere.
(iii) Refrigeration to slow down the oxidation process.
(iv) Adding antioxidants or preservatives to inhibit the oxidation of fats and oils.
By employing these prevention measures, we can prolong the freshness and quality of food, ensuring safer and more enjoyable consumption.
Word Equation
A word equation represents a chemical reaction using words instead of chemical formulas, making it easier to identify the reactants and products involved.
In this shorthand representation, the names of the reactants appear on the left side of the word equation. If there is more than one reactant, their names are separated by a plus sign (+). Similarly, the products are listed on the right side of the word equation, with their names separated by a plus sign (+) if there is more than one product.
For instance,
Sodium + Chlorine → Sodium chloride
The equation above can be read as: “Sodium reacts with chlorine to form sodium chloride.
Symbols of Elements and Their Valencies used in Chemical Reaction and Equation Notes Class 10
A symbol serves as a chemical code for an element. Every element is represented by a one or two-letter atomic symbol, typically an abbreviation of its name.
Valency refers to the combining capacity of an element. It represents the number of electrons that an atom gains, loses, or shares when it combines with another atom to form a molecule.
Writing Chemical Equations
A chemical equation is the representation of a chemical reaction using symbols and chemical formulas for the reactants and products.
In chemical equations:
- “(s)” is used for solids.
- “(l)” is used for liquids.
- “(g)” is used for gases.
- “(aq)” is used for aqueous solutions.
- “(↑)” is used to indicate a gas produced in the reaction.
- “(↓)” is used to indicate a precipitate formed in the reaction.
Ex: Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) ↑
Physical and Chemical Changes
Chemical change happens when one or more new substances are created, and these substances have different physical and chemical properties compared to the original ones.
In a physical change, there is a modification in color or state, but no new substance is produced. For instance, when water is boiled and changes into steam, no new substance is formed (even though water and steam appear different, they react in the same way when exposed to sodium, producing identical products). This change solely involves a shift in the state of matter, from liquid to vapor.
Observations that Help Determine a Chemical Reaction
To identify a chemical reaction, you can make use of the following observations:
a) The release of a gas
b) A change in temperature
c) The formation of a precipitate
d) A change in color
e) A change in state
Balancing of a Chemical Reaction
Law of Conservation of Mass
The Law of Conservation of Mass states that in a chemical reaction, atoms cannot be created or destroyed. Therefore, the total number of atoms of each element on the reactants side must be equal to the total number of atoms on the products side.
In simpler terms, the total mass of the products formed during a chemical reaction is always equal to the total mass of the reactants involved in the reaction. Mass is conserved throughout the entire process.
Balanced chemical equation
A chemical equation is considered balanced when the number of atoms for each element on the reactants side is equal to the number of atoms on the products side. Such an equation is referred to as a balanced chemical equation.
Steps for Balancing Chemical Equations
A chemical equation represents the changes that occur during a chemical reaction.
Reactants → Products
For a chemical equation to be balanced, the equilibrium must be maintained, meaning the number of each type of atom must be the same on both sides of the arrow.
Scientists use coefficients to balance chemical equations. Coefficients are numerical values added in front of chemical symbols or formulas, indicating the number of atoms or molecules involved.
Place coefficients as needed to achieve a balanced chemical equation, ensuring the same number of each type of atom appears in both reactants and products.
For instance:
Zn + HCl → ZnCl2 + H2
The balanced equation is:
Zn + 2HCl → ZnCl2 + H2
To balance the equation, you may use the hit and trial method, adjusting the coefficients until the number of atoms of each element is the same on both sides of the chemical equation.
Read Also:
- Acids Bases and Salts Class 10 Notes of NCERT Science Ch. 2
- Life Process Notes Class 10 NCERT Science Chapter 5
- Control and Coordination Notes Class 10: NCERT Science Ch. 6
- Class 10th How Do Organisms Reproduce Notes: Science Ch. 7
- Class 10th Heredity and Evolution Notes: NCERT Science Ch. 8
- Class 10th Chapter 11 Science Notes for NCERT Students
- Notes of Our Environment Class 10: NCERT Science Chapter 13
Frequently Asked Questions on Chemical Reaction and Equation Notes Class 10
Q 1. How can you identify a chemical reaction?
Several observable changes and indicators can identify a chemical reaction. During a chemical reaction, it forms new substances with different properties than the original reactants.
These changes can be evident through alterations in color, the evolution of gas, the formation of a precipitate, and variations in temperature. Some reactions emit light or produce distinct odors, providing further evidence of chemical change.
Additionally, the irreversibility of most chemical reactions sets them apart from physical changes. Observing effervescence or the rapid release of gas in the form of bubbles is also a common clue. Scientists rely on these recognizable signs to study and understand chemical processes across various disciplines.
Identifying chemical reactions is essential for advancing research, developing new materials, and comprehending the transformations that occur in the world around us.
Q 2. Why chemical equation balancing important?
In chemistry, the balancing of chemical equations is of paramount importance as it upholds the fundamental principle of the Law of Conservation of Mass. This law states that the total mass of the reactants in a chemical reaction must be equal to the total mass of the products. By balancing the equation, the same number of atoms of each element appears on both sides of the reaction, guaranteeing the preservation of mass.
Balanced chemical equations accurately represent the stoichiometry of a reaction, illustrating the correct proportions in which the reactants combine and the products are formed. This information is crucial for understanding the quantitative aspects of the reaction and for predicting the amounts of reactants and products needed or obtained.
Furthermore, chemical equation balancing is essential for performing stoichiometric calculations in laboratories and industries, allowing efficient synthesis and production of substances. Furthermore, accurate reproducibility and standardized study of the reaction require clear communication among scientists and researchers.
In summary, chemical equation balancing is the backbone of chemical understanding and enables precise calculations, ensuring the consistent application of chemical principles in research, industry, and education.
Q 3. How do chemical reactions impact our daily lives and the environment?
Chemical reactions have a profound impact on our daily lives and the environment. In our daily routines, chemical reactions are integral to numerous processes, such as cooking, digestion, and combustion in vehicles and appliances. They provide us with essential products like medicines, plastics, and cleaning agents. Chemical reactions in food preservation and fermentation play a vital role in food industry practices.
However, certain chemical reactions also pose environmental challenges. Combustion of fossil fuels releases carbon dioxide and other greenhouse gases, contributing to climate change. Industrial processes and emissions generate air and water pollutants, leading to environmental degradation and health concerns. Chemical reactions in fertilizers and pesticides can harm ecosystems and disrupt biodiversity.
Balancing these effects is crucial. To minimize the negative environmental impact of chemical reactions, we employ sustainable practices, green chemistry, and waste reduction. Understanding and managing chemical reactions contribute to finding solutions for environmental sustainability and improving our overall quality of life.
Q 4. How do chemical reactions play a role in food preparation and cooking?
Chemical reactions play a crucial role in food preparation and cooking, transforming raw ingredients into delicious and nutritious dishes. The Maillard reaction is responsible for the browning and development of savory flavors in grilled meats, roasted vegetables, and toasted bread. Caramelization of sugars produces a rich and sweet taste in dishes like caramelized onions and desserts. Fermentation, driven by yeast and bacteria, enhances the taste and texture of foods like bread, cheese, and pickles.
Chemical leavening agents like baking powder and baking soda release carbon dioxide, making dough and batters rise, resulting in light and fluffy cakes and bread. Denaturation of proteins through heat and acids changes their structure and texture in foods like eggs and meat.
Understanding these chemical processes empowers cooks and chefs to manipulate ingredients and cooking techniques, creating a wide array of flavors, textures, and appearances in culinary delights. Chemical reactions, when harnessed skillfully, are the key to transforming raw ingredients into delectable and visually appealing dishes that tantalize our taste buds and nourish our bodies.
Q 5. What are some safety precautions to consider when conducting chemical reactions in the laboratory?
Safety is of utmost importance when conducting chemical reactions in the laboratory to prevent accidents, injuries, and exposure to hazardous substances. Here are some essential safety precautions to consider:
1. Wear Appropriate Personal Protective Equipment (PPE): Always wear safety goggles, lab coats, gloves, and closed-toe shoes to protect eyes, skin, and clothing from chemical splashes and spills.
2. Work in a Well-Ventilated Area: Ensure proper ventilation to minimize exposure to fumes and gases. Use fume hoods when handling volatile or toxic substances.
3. Label and Store Chemicals Properly: Clearly label all chemicals and store them in designated, secure locations, following safety data sheet guidelines.
4. Know Emergency Procedures: Familiarize yourself with emergency protocols, including eyewash stations, safety showers, and fire extinguishers.
5. Do Not Taste or Smell Chemicals: Avoid tasting or smelling chemicals, as some substances can be toxic, corrosive, or harmful.
By observing these safety precautions and practicing responsible laboratory conduct, researchers can minimize risks and create a safe working environment for themselves and others.
Chemical Equations and Reactions Class 10: Solution of Sci. Ch.1
Welcome to our comprehensive guide on mastering chemical equations and reactions for Class 10 students. In this article, we delve into the fascinating world of chemistry, specifically focusing on the essential topic of “chemical equations and reactions class 10.”

Chemical equations and reactions are fundamental concepts in chemistry that form the basis for understanding various chemical processes and transformations. As you progress through your Class 10 studies, this topic becomes increasingly important and lays the foundation for more advanced chemistry topics in higher grades.
In this guide, we will take you through the key aspects of chemical equations and reactions, including how to write and balance chemical equations, identifying different types of reactions, and understanding the underlying principles behind each reaction type.
By the end of this article, you will gain a thorough understanding of chemical equations and reactions, enabling you to confidently tackle related questions and problems in your Class 10 examinations and beyond. Let’s embark on this exciting journey into the world of chemical reactions and equations! For other chapter’s solution, visit NCERT Solutions Science Class 10 All Chapter
NCERT Chapter 1 Chemical Equations and Reactions Class 10 Solution
1. Why should a magnesium ribbon be cleaned before burning in the air?
Solution:
Before burning in air, it is crucial to clean the magnesium ribbon since it readily reacts with atmospheric oxygen, leading to the formation of a highly stable compound known as Magnesium Oxide (MgO). To prevent any subsequent reactions with oxygen, it becomes imperative to cleanse the ribbon and remove the MgO layer.
2. Write a balanced equation for the following chemical reactions.
i) Hydrogen + Chloride —-> Hydrogen chloride
ii) Barium chloride + Aluminium sulphate —-> Barium sulphate + Aluminium chloride
iii) Sodium + Water —-> Sodium hydroxide + Hydrogen
Solution:
i) H2 + Cl2 → 2HCl
ii) 3BaCl2 + Al2(SO4)3 →3BaSO4 + 2AlCl3
iii) 2Na + 2H2O → 2NaOH + H2
3. Write a balanced chemical equation with state symbols for the following reactions
i) Solutions of Barium chloride and Sodium sulphate in water react to give insoluble Barium sulphate and solution of Sodium chloride.
ii) Sodium hydroxide solution in water reacts with the hydrochloric acid solution to produce Sodium chloride solution and water.
Solution:
i) BaCl2 + Na2SO4 → BaSO4 + 2NaCl
ii) NaOH + HCl → NaCl + H2O
Chemical Equations and Reactions Class 10: Set 2, Page number – 10
1. A solution of a substance, ‘X,’ is used for whitewashing.
(i) Name the substance ‘X’ and write its formula.
(ii) Write the reaction of the substance ‘X’ named in (i) above with water.
Solution:
i) The substance ‘X’ which is used in whitewashing is quick lime or Calcium Oxide and its formula is CaO.
ii) CaO + H2O → Ca(OH)2
2. Why is the amount of gas collected in one of the test tubes in Activity 1.7 double the amount collected in the other? Name this gas.
Solution:
In activity 1.7, there is a notable difference in the amount of gas collected between two test tubes. This variance occurs because water undergoes hydrolysis, resulting in the release of H2 and O2 gases. During electrolysis, two molecules of hydrogen and one molecule of oxygen gas are generated. It explains why the quantity of hydrogen collected is twice that of oxygen.
Chemical Equations and Reactions Class 10: Set 3, Page number – 13
1. Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Solution:
When immersing an iron nail in the copper sulphate solution, a displacement reaction occurs wherein iron displaces copper from the copper sulphate compound due to iron’s higher reactivity compared to copper. Consequently, the color of the copper sulphate solution changes. The reaction can be represented as:
Fe + CuSO4 → FeSO4 + Cu
2. Give an example of a double displacement reaction other than the one given in Activity 1.10.
Solution:
The interaction between silver nitrate (AgNO3) and sodium chloride (NaCl) exemplifies a double displacement reaction. In this reaction, the negative and positive ions exchange places, leading to the creation of a white precipitate called silver chloride (AgCl). The chemical equation representing this process is as follows:
Ag+ + NO3– + Na+ + Cl– → AgCl + Na+ + NO3–
3. Identify the substances that are oxidised and that are reduced in the following equation.
i) 4Na(s) + O2(g) → 2Na2O(s)
ii) CuO(s) + H2(g) → Cu(s) + H2O(l)
Solution:
In the first equation, Sodium (Na) undergoes oxidation upon reacting with Oxygen (O2), while in the second equation, Copper (Cu) gets reduced as a result of its reaction with Hydrogen (H2).
Chemical Equations and Reactions Class 10: Exercise Questions (Q 1 to Q 5)
1. Which of the statements about the reaction below are incorrect?
2PbO(s) + C(s) → 2Pb(s) + CO2(g)
(a) Lead is getting reduced
(b) Carbon Dioxide is getting oxidised
(c) Carbon is getting oxidised
(d) Lead oxide is getting reduced
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) all the above
Solution:
(i) (a) and (b)
2. Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a
- Combination reaction
- Double displacement reaction
- Decomposition reaction
- Displacement reaction
Answer:
4. Displacement reaction.
3. What happens when dilute hydrochloric acid is added to iron fillings? Tick the correct answer.
- Hydrogen gas and Iron chloride are produced.
- Chlorine gas and Iron hydroxide are produced.
- No reaction takes place.
- Iron salt and water are produced.
Answer: 1. Hydrogen gas and Iron chloride are produced.
4. What is a balanced chemical equation? Why should a chemical equation be balanced?
Answer:
A balanced equation ensures that the count of distinct atoms on both the reactant and product sides is identical. Balancing chemical equations is very important to follow the Law of Conservation of Mass. According to this law matter can’t be created or destroyed during a chemical reaction. Balancing equations doesn’t have a set way to do it, so people try different methods until they find the right one that works.
5. Translate the following statements into chemical equations and balance them.
(a) Hydrogen gas combines with nitrogen to form ammonia.
(b) Hydrogen sulphide gas burns in the air to give water and sulphur dioxide.
(c) Barium chloride reacts with aluminium sulphate to give Aluminium chloride and a precipitate of barium sulphate.
(d) Potassium metal reacts with water to give potassium hydroxide and Hydrogen gas.
Solution:
(a) Unbalanced: H2 + N2 → NH3
Balanced: 3H2 + N2 → 2NH3
(b) Unbalanced: H2S + O2 → H2O + SO2
Balanced: 2H2S + 3O2 → 2H2O + 2SO2
(c) Unbalanced:
BaCl2 + Al2(SO4)3 → AlCl3 + BaSO4
Balanced: 3BaCl2 + Al2(SO4)3 → 2AlCl3 + 3BaSO4
(d) Unbalanced: K + H2O → KOH + H2
Balanced: 2K + 2H2O → 2KOH + H2
Chemical Equations and Reactions Class 10: Exercise Questions (Q 6 to Q 10)
6. Balance the following chemical equations.
(a) HNO3 + Ca(OH)2 → Ca(NO3)2 + H2O
(b) NaOH + H2SO4 → Na2SO4 + H2O
(c) NaCl + AgNO3 → AgCl + NaNO3
(d) BaCl2 + H2SO4 → BaSO4 + HCl
Solution:
(a) 2HNO3 + Ca(OH)2 → Ca(NO3)2 + 2H2O
(b) 2NaOH + H2SO4 → Na2SO4 + 2H2O
(c) NaCl + AgNO3 → AgCl + NaNO3
(d) BaCl2 + H2SO4 → BaSO4 + 2HCl
7. Write the balanced chemical equation for the following reactions.
Calcium hydroxide + Carbon dioxide —-> Calcium carbonate + Water
Zinc + Silver nitrate —-> Zinc nitrate + Silver
Aluminium + Copper chloride —-> Aluminium chloride + Copper
Barium chloride + Potassium sulphate —-> Barium sulphate + Potassium chloride
Solution:
2Ca(OH)2 + 2CO2 → 2CaCO3 + 2H2O
Zn + 2AgNO3 → Zn(NO3)2 + 2Ag
2Al + 3CuCl2 → 2AlCl3 + 3Cu
BaCl2 + K2SO4 → BaSO4 + 2KCl
8. Write a balanced chemical equation for the following and identify the type of reaction of each case.
KBr + BaI2 → KI + BaBr2
ZnCO3 → ZnO + CO2
H2 + Cl → HCl
Mg + HCl → MgCl2 + H2
Solution:
2KBr + BaI2 → 2KI + BaBr2 (Double Displacement Reaction)
ZnCO3 → ZnO + CO2 (Decomposition Reaction)
H2 + Cl → 2HCl (Combination Reaction)
Mg + 2HCl → MgCl2 + H2 (Displacement Reaction)
9. What is meant by exothermic and endothermic reactions? Give examples.
Answer:
An endothermic reaction takes place when the system absorbs energy from the surroundings, primarily in the form of heat. Examples of endothermic reactions include processes like photosynthesis, melting of ice, and evaporation.
On the other hand, an exothermic reaction is characterized by the release of energy from the system into the surroundings. Instances of exothermic reactions encompass events such as explosions, concrete setting, and nuclear fission and fusion.
10. Why is respiration considered to be an exothermic reaction?
Answer:
Energy is essential for the sustenance of life, and we acquire it from the food we consume. During digestion, the food molecules are broken down into simpler substances, such as glucose.
When these substances interact with the oxygen present in our body cells, they produce carbon dioxide and water, along with a certain amount of energy (a process known as respiration).
Since this energy is in the form of heat, which helps maintain our body temperature, respiration is classified as an exothermic reaction. The equation is describe as:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
Chemical Equations and Reactions Class 10: Exercise Questions (Q 11 to Q 15)
11. Why are decomposition reactions called the opposite of Combination reactions? Write equations for decomposition reactions.
Answer:
A combination reaction involves the interaction of two or more molecules to create a larger molecule, whereas a decomposition reaction refers to the breakdown of larger molecules into two or more smaller molecules. Essentially, the decomposition reaction stands as the reverse process of a combination reaction.
In lots of cases, the decomposition reaction needs heat from the surroundings or added heat to break the bonds of the larger molecule. This makes it an endothermic reaction. A few examples of decomposition reactions include: [Here you can list the examples of decomposition reactions]
ZnCO3 → ZnO + CO2
CaCO3 + Energy → CaO + CO2
2HgO → 2Hg + O2
12. Write one equation each for decomposition reactions in which energy is supplied in the form of heat, light or electricity.
Answer:
(a) Thermal decomposition reaction (Thermolysis)
2KClO3 + Heat → 2KCl + 3O2
(b) Electrolytic decomposition reaction (Electrolysis)
2NaCl → 2Na + Cl2
(c) Photodecomposition reaction (Photolysis)
2H2O2 → 2H2O
13. What is the difference between displacement and double displacement reactions? Write relevant equations for the above.
Solution:
A displacement reaction occurs when a more reactive substance displaces a less reactive one from its salt solution, whereas a double displacement reaction involves a mutual exchange of ions between two compounds.
In a displacement reaction, a single displacement occurs, whereas in the double displacement reaction, as the name suggests, two displacements take place between the molecules.
Examples:
Displacement reaction:
Mg + 2HCl → MgCl2 + H2
Double displacement reaction:
2KBr + BaI2 → 2KI + BaBr2
14. In the refining of Silver, the recovery of silver from Silver nitrate solution involves displacement reaction by Copper metal. Write down the reaction involved.
Solution:
Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
15. What do you mean by a precipitation reaction? Explain by giving examples.
Solution:
When we mix two solutions that have dissolvable salts, a special reaction called double displacement happens. In this reaction, the ions in the compounds switch places with each other. If one of the compounds formed in this reaction is in a solid form and insoluble in the aqueous solution, it settles down at the bottom of the container. Here are a few examples of precipitation reactions:
Example 1:
CdSO4(aq) + K2S(aq) → CdS(s) + K2SO4(aq)
Example 2:
2NaOH(aq) + MgCl2(aq) → 2NaCl(aq) + Mg(OH)2(s)
Chemical Equations and Reactions Class 10: Exercise Questions (Q 16 to Q 20)
16. Explain the following in terms of the gain of oxygen with two examples each.
(a) Oxidation
(b) Reduction
Solution:
(a) In a chemical reaction, when oxygen combines with an element to form its corresponding oxide, the element is undergoing oxidation. Examples:
1. 4Na(s) + O2(g) → 2Na2O(s)
In this reaction, sodium (Na) is being oxidized as it combines with oxygen (O2) to form sodium oxide (Na2O).
2. H2S + O2 → H2O + SO2
Here, hydrogen sulfide (H2S) is oxidized by oxygen (O2) to produce water (H2O) and sulfur dioxide (SO2).
(b) In a chemical reaction, when oxygen is removed from a compound, it is considered to be reduced. Examples:
1. CuO(s) + H2(g) → Cu(s) + H2O(l)
In this reaction, copper oxide (CuO) is reduced as it loses oxygen, and hydrogen gas (H2) is oxidized to form water (H2O).
2. 2HgO → 2Hg + O2
In this case, mercury oxide (HgO) is reduced as it gives up oxygen, resulting in the production of mercury (Hg), and the released oxygen (O2) becomes a separate gas.
17. A shiny brown coloured element ‘X’ on heating in the air becomes black in colour. Name the element ‘X’ and the black-coloured compound formed.
Solution:
The lustrous, brown-colored element is Copper metal (Cu). When we heat this metal while it is near air, it reacts with the oxygen in the air. This reaction makes copper oxide. Therefore, the black-colored compound obtained is copper oxide.
The chemical equation representing the reaction is:
2Cu(s) + O2(g) → 2CuO(s)
18) Why do we apply paint on iron articles?
Solution:
Iron particles are coated with paint to safeguard them from rusting. When left unpainted, the metal surface comes into contact with atmospheric oxygen and, in the presence of moisture, forms Iron(III) oxide, commonly known as rust. But when we paint the surface, it stays protected from water and air. This stops rusting from happening and keeps the object safe.
19) Oil and Fat containing food items are flushed with Nitrogen. Why?
Solution:
The primary objective of flushing Nitrogen into food packets containing oil and fat items is to inhibit rancidity. Rancidity occurs when the oil or fat reacts with oxygen, resulting in an unpleasant smell and taste. When we put Nitrogen into the packets, it creates a special environment where things don’t react much. This stops rancidity from happening and keeps the items fresh for a longer time.
20) Explain the following terms with one example each.
(a) Corrosion
(b) Rancidity
Solution:
(a) Corrosion is a natural process where a refined metal undergoes oxidation upon exposure to atmospheric oxygen, resulting in the formation of more stable compounds like oxides. Over time, the metal gradually degrades during the corrosion process. A common example of corrosion is the rusting of iron, which happens when iron turns into iron oxide. Preventing rusting is very important because it can cause damage to bridges and monuments, leading to millions of dollars spent each year to protect them from harm.
(b) Rancidity refers to the condition caused by the aerial oxidation of oil and fat present in food materials, leading to an unpleasant taste and smell. To slow down this process of degradation, you can put the food in the refrigerator. The cold temperature stops the reaction that causes the unpleasant taste and smell, keeping the food fresh and good to eat.
NCERT Solutions for Science Chapter 1 Chemical Equations and Reactions Class 10
“Chemical Reactions and Equations” is a foundational chapter that imparts fundamental knowledge about chemical reactions and equations. It is essential for students to grasp the concepts in this chapter as it forms the basis for understanding more advanced topics in chemistry. If you want to study science, especially in higher secondary school, it’s highly recommended to become very familiar with this chapter. The concepts learned here will hold great importance and relevance throughout their academic journey.
Features of NCERT Solutions for Chapter 1 Science Chemical Equations and Reactions Class 10
By engaging in comprehensive practice, students can refine their skills in balancing various types of equations.
NCERT Solutions play a crucial role in guiding students to write chemical equations correctly, offering valuable assistance.
Thoroughly working through NCERT Solutions, which involve solving questions of different levels of difficulty, can effectively prepare students for the CBSE examination.
Chemical reactions are integral to our lives. It is observed in the rusting of iron, curdling of milk, respiration, digestion, and growth.
To do well in the CBSE Class 10 examination, students should focus on practicing the NCERT Solutions for Chemical Equations and Reactions Class 10, which are available here.
Frequently Asked Question – FAQs on Chemical Equations and Reactions Class 10
Q 1. What are 10 chemical reactions examples?
Certainly! Here are 10 chemical reaction examples:
- Combustion of methane: CH4 + 2O2 → CO2 + 2H2O
- Photosynthesis: 6CO2 + 6H2O + light energy → C6H12O6 + 6O2
- Rusting of iron: 4Fe + 3O2 + 6H2O → 4Fe(OH)3
- Decomposition of hydrogen peroxide: 2H2O2 → 2H2O + O2
- Formation of table salt (sodium chloride): Na + Cl2 → 2NaCl
- Acid-base neutralization: HCl + NaOH → NaCl + H2O
- Fermentation of glucose: C6H12O6 → 2C2H5OH + 2CO2
- Synthesis of water: 2H2 + O2 → 2H2O
- Precipitation reaction: AgNO3 + NaCl → AgCl + NaNO3
- Single displacement reaction: Zn + CuSO4 → ZnSO4 + Cu
Keep in mind that these are simplified representations of the reactions, and in practice, various factors and conditions can influence the reactions.
Q 2. What are the 4 types of chemical equations?
The four main types of chemical equations are:
1. Combination (Synthesis) Reactions: In these reactions, two or more substances combine to form a single product. The general form of a combination reaction is:
A + B → AB
2. Decomposition Reactions: In decomposition reactions, a single compound breaks down into two or more simpler substances. The general form of a decomposition reaction is:
AB → A + B
3. Single Displacement (Replacement) Reactions: In single displacement reactions, an element replaces another element in a compound, leading to the formation of a new compound and a different element. The general form of a single displacement reaction is:
A + BC → AC + B
4. Double Displacement (Metathesis) Reactions: In double displacement reactions, the positive and negative ions of two compounds exchange places, leading to the formation of two new compounds. The general form of a double displacement reaction is:
AB + CD → AD + CB
These types of chemical equations represent the various ways in which chemical reactions can occur and are essential for understanding the behavior of substances during chemical transformations.
Q 3. How many chemical formulas are there in Chemical Equations and Reactions Class 10?
The number of chemical formulas is virtually infinite because there are countless possible combinations of elements that can form different compounds. Each unique chemical compound has its own distinct chemical formula, which represents the types and proportions of atoms present in the compound.
Chemical formulas are a concise and standardized way to represent chemical compounds, and they follow specific rules to convey information about the elements and their relative ratios in the compound. As scientists continue to discover and synthesize new compounds, the number of chemical formulas continues to grow. While it is not possible to determine an exact count of all possible chemical formulas, the diversity of compounds is a fundamental aspect of the vast and intricate field of chemistry.
Q 4. Which is the best example of a chemical reaction?
One of the best and most well-known examples of a chemical reaction is the combustion of methane (CH4) with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O). The reaction complete combustion of methane represented by the following chemical equation:
CH4 + 2O2 → CO2 + 2H2O
In this reaction, methane (a hydrocarbon) reacts with oxygen gas in the presence of heat or a flame. The result is the complete transformation of the reactants (methane and oxygen) into the products (carbon dioxide and water). This reaction releases a significant amount of energy in the form of heat and light, making it an essential process for various applications, including cooking, heating, and power generation.
The combustion of methane is a fundamental example of a chemical reaction that showcases the rearrangement of atoms and the formation of new substances with distinct properties from the original reactants.
Q 5. Use of chemical reaction in daily life according to Chemical Equations and Reactions Class 10?
Chemical reactions are an integral part of our daily lives, impacting various processes and activities. Combustion, such as burning fuels for cooking and transportation, releases heat and light through chemical reactions.
Photosynthesis in plants converts carbon dioxide and water into glucose and oxygen, sustaining life. Respiration in our bodies utilizes chemical reactions to produce energy and expel carbon dioxide. During digestion, chemical reactions break down food for nutrient absorption. Rusting occurs as metals react with oxygen and moisture. Baking involves reactions between ingredients, causing dough or batter to rise.
Fermentation creates products like yogurt and alcoholic beverages through microbial reactions. Cleaning products work by chemical reactions to remove dirt and stains. Batteries operate through chemical reactions to generate electrical energy. Additionally, pharmaceuticals use chemical reactions to produce medicines for treating diseases. These examples illustrate how chemical reactions play a vital role in our daily activities and advancements in modern life.
10th Class Geography: NCERT Chapter-Wise Solutions
Welcome to our comprehensive guide to NCERT 10th Class Geography! If you’re a student seeking chapter-wise solutions and in-depth explanations for your Geography course, you’ve come to the right place. We understand the importance of acing your 10th Class Geography exams. That’s why we have curated a collection of high-quality, chapter-wise solutions to help you excel in your studies.
In this article, you will find well-structured and easy-to-understand solutions to all the chapters of the 10th Class Geography textbook. Our expert educators have crafted these solutions to provide you with a clear understanding of the concepts, making your learning experience enjoyable and productive.

Overview of 10th Class Geography Chapters
The Class 10 Geography book comprises a total of seven insightful chapters. In this article, you’ll find a concise overview and description of each of these chapters, providing you with valuable insights into the topics covered. Let’s take a closer look at what each chapter has to offer!
Chapter 1: Resources and Development
In Class 10 Geography Chapter 1, we embark on an exploration of resources and development. The chapter commences by defining resources and delving into their classification, considering factors such as origin, exhaustibility, ownership, and potential for development. The focus then shifts to the development of resources and the crucial aspect of resource planning.
The central theme revolves around land resources, where we delve into topics like land utilization, land use patterns in India, land degradation, and the measures for conservation. Another significant aspect covered in-depth is soil as a resource, including a detailed classification of soils, soil erosion, and methods of soil conservation.
10th Class Geography covered the following topics:
1. Concept of Resources
2. Development of Resources
3. Resource Planning in India
4. Conservation of Resources
5. Land Resources
6. Land Utilisation
7. Land Use Pattern in India
8. Land Degradation and Conservation Measures
9. Soil as a Resource
10. Classification of Soils
11. Soil Erosion and Soil Conservation (excluding Box Information on State of India’s Environment)
Through this chapter, students gain a profound understanding of the significance of resources and their sustainable development for a thriving future.
Chapter 2: Forest and Wildlife Resources
Class 10 Geography Chapter 2 offers valuable insights into the fascinating world of flora and fauna, encompassing their diversity and the factors contributing to their depletion. Delving deeper, the chapter explores the conservation and distribution of forests and wildlife in India, shedding light on the crucial efforts to safeguard these precious resources.
The topics covered in this enriching chapter include:
1. Conservation of Forest and Wildlife in India: Understanding the measures taken to preserve and protect the invaluable forest and wildlife resources of India.
2. Types and Distribution of Forests and Wildlife Resources: An exploration of the different types of forests and wildlife found across India, along with their distribution patterns.
3. Community and Conservation: Learning about the roles played by communities in conservation efforts, recognizing the importance of collective action in safeguarding our natural heritage.
Through this chapter, students gain a deeper appreciation for the significance of preserving our forests and wildlife and are inspired by the efforts of common people in ensuring the sustainable coexistence of humans and nature.
Chapter 3: Water Resources
In Class 10 Geography Chapter 3, students embark on a comprehensive exploration of the world’s most precious resource – water. The chapter commences by illuminating the availability of fresh water on the Earth’s surface. It delves into the causes of water scarcity, emphasizing the urgent need for conserving and effectively managing water resources.
Key topics covered in this insightful chapter include:
1. Water Scarcity and the Need for Water Conservation and Management: Understanding the reasons behind water scarcity and recognizing the importance of conservation and efficient management of water resources.
2. Multi-Purpose River Projects and Integrated Water Resources Management: Learning about multi-purpose river projects and the concept of integrated water resources management, which aim to optimize water usage and promote sustainable development.
3. Rainwater Harvesting: Exploring the practice of rainwater harvesting, a vital technique for conserving water and replenishing groundwater sources.
Additionally, the chapter includes a list of map items related to various dams, such as Salal, Bhakra Nangal, Tehri, Rana Pratap Sagar, Sardar Sarovar, Hirakud, Nagarjuna Sagar, and Tungabhadra, providing students with a comprehensive understanding of water resources management and its implementation in different regions.
Chapter 4: Agriculture
Delving into the heart of India’s agricultural heritage, Class 10 Geography Chapter 4 unravels the intricate tapestry of farming practices that have thrived in the country for centuries. As an agricultural nation, India’s farming legacy spans diverse types of farming systems, cropping patterns, and a plethora of major crops cultivated across its fertile landscapes.
Key topics covered in this enriching chapter include:
1. Types of Farming: Students gain insights into the three prominent types of farming in India, including Primitive Subsistence, Intensive Subsistence, and Commercial farming.
2. Cropping Pattern: Exploring the fascinating mosaic of crops, the chapter illuminates the major crops produced in India, encompassing food crops other than grains, non-food crops, and the role of technological and institutional reforms in shaping the agricultural landscape.
3. Food Security: The chapter underscores the importance of food security and its relationship with agriculture, while excluding the impact of globalization on this vital sector.
The chapter also features a list of map items, offering students the opportunity to identify and comprehend the major areas of rice and wheat cultivation and the largest/major producer states of sugarcane, tea, coffee, rubber, cotton, and jute, thereby enriching their understanding of India’s diverse agricultural geography.
Chapter 5: Minerals and Energy Resources
Embarking on a fascinating journey beneath the Earth’s surface, Class 10 Geography Chapter 5 uncovers the mesmerizing world of minerals and energy resources. Delving into the very foundation of the planet’s crust, the chapter unravels the mysteries of minerals, their occurrence, and their diverse classifications.
The chapter unfolds with a comprehensive exploration of minerals, shedding light on their definition and the mode of their occurrence. Students are introduced to a wide array of minerals, including ferrous minerals, non-ferrous minerals, nonmetallic minerals, and rock minerals, each explained in meticulous detail.
Furthermore, the chapter addresses the crucial topic of mineral conservation, emphasizing the significance of preserving these invaluable resources for future generations.
In addition to minerals, the chapter also delves into the captivating realm of energy resources. It acquaints students with conventional sources of energy and non-conventional sources of energy, providing a holistic understanding of the world’s energy landscape.
The significance of conserving energy resources is underscored, highlighting the need to harness and utilize energy in a sustainable manner.
To enhance geographical knowledge, the chapter features a list of map items, enabling students to identify key mineral locations. It includes iron ore mines in Mayurbhanj, Durg, Bailadila, Bellary, and Kudremukh, coal mines in Raniganj, Bokaro, Talcher, and Neyveli, and oil fields in Digboi, Naharkatia, Mumbai High, Bassein, Kalol, and Ankleshwar. It also includes the location of power plants, both thermal and nuclear.
By unraveling the intricate web of minerals and energy resources, this chapter equips students with a deeper understanding of the Earth’s hidden treasures and their importance in sustaining our world.
Chapter 6: Manufacturing Industries
Unlocking the gateway to development, the manufacturing sector stands as a cornerstone of progress. Nestled within the pages of NCERT Geography, Chapter 6 unveils the significance of manufacturing industries and their vital contribution to the national economy. With a focus on enlightening students, the chapter unravels the intricacies of industrial locations and the factors influencing them.
Venturing further, the chapter delves into a captivating exploration of agro-based industries, mineral-based industries, chemical industries, and more. Each industry is intricately explained, providing students with a comprehensive understanding of their functions and significance.
Moreover, the chapter delves into a critical topic – industrial pollution and its repercussions on the environment. Students are made aware of the detrimental impact of industrial activities on the ecosystem and the measures undertaken to prevent environmental degradation.
To enhance geographical knowledge, the chapter includes a list of map items. It enables students to locate and label various manufacturing industries. It includes Software Technology Parks in Noida, Gandhinagar, Mumbai, Pune, Hyderabad, Bengaluru, Chennai, and Thiruvananthapuram.
By journeying through the realms of manufacturing industries, students gain insights into the foundation of development, the role of industries in the economy, and the imperative to balance progress with environmental preservation.
Chapter 7: Lifelines of National Economy
Embarking on a voyage of connectivity, Chapter 7 of NCERT Geography unveils the lifelines of our nation’s economy – modern means of transport and communication. Within its pages, students will uncover the intricate web of roadways, railways, pipelines, waterways, seaports, and airways. That link humanity to the world, fostering significant growth in India’s economy.
The chapter begins by delving into the vast network of roadways, railways, and pipelines. Which facilitate the smooth flow of goods and people across the country. Students will traverse the waterways and major seaports that play a pivotal role in bolstering trade and commerce.
Venturing into the skies, the chapter sheds light on the expansive airways that have shrunk distances and intensified international trade. Moreover, students will gain insights into the essential realm of communication in India. The pivotal role it plays in connecting individuals and communities.
To further enrich their geographical acumen, the chapter also sheds light on international trade and the economic potential of tourism.
For a comprehensive learning experience, the chapter includes a list of map items, inviting students to locate and label major ports. Such as Kandla, Mumbai, Chennai, and international airports like Delhi (Indira Gandhi), Mumbai (Chhatrapati Shivaji), and Hyderabad (Rajiv Gandhi).
As students navigate through the lifelines of national economy, they will develop a profound appreciation for the intricate mechanisms that bind the nation and foster economic prosperity.
Frequently Asked Question – FAQs on Class 10 Geography
Q1. Which chapter in Geography class 10?
In the Class 10 Geography NCERT textbook (India and Contemporary World-II), the various chapters are as follows:
- Chapter 1: Resources and Development
- Chapter 2: Forest and Wildlife Resources
- Chapter 3: Water Resources
- Chapter 4: Agriculture
- Chapter 5: Minerals and Energy Resources
- Chapter 6: Manufacturing Industries
- Chapter 7: Lifelines of National Economy
Each chapter covers different aspects of geography, such as natural resources, agriculture, industries, transport, and communication. They providing students with a comprehensive understanding of the subject.
Q 2. Who is the father of geography?
The ancient Greek scholar Eratosthenes is often referred to as the “Father of Geography.” He was a mathematician, astronomer, and librarian at the Library of Alexandria during the third century BCE. Eratosthenes made significant contributions to geography and cartography during his time.
Egypt has one of his most famous achievements. At there two locations, it accurately calculating the circumference of the Earth using the angles of shadows. His calculation was remarkably close to the actual circumference, considering the limited technology available in his era.
Additionally, Eratosthenes compiled one of the earliest known world maps, incorporating information from travelers, traders, and scholars of his time. His work laid the foundation for the development of geography as a discipline and greatly influenced later geographers and explorers.
Q 3. Which chapter is most important in Geography class 10 cbse?
The importance of chapters in Geography Class 10 CBSE may vary for different students based on their interests and future career paths. However, some chapters are generally considered important as they cover fundamental concepts and provide a strong foundation in the subject. Here are a few chapters that are often considered significant:
- Chapter 1: Resources and Development
- Chapter 2: Forest and Wildlife Resources
- Chapter 5: Minerals and Energy Resources
- Chapter 7: Lifelines of National Economy
While these chapters are important, it is essential to study all the chapters in the syllabus to have a comprehensive understanding of Geography and excel in the subject.
Power Sharing Notes Class 10th of NCERT Civic Chapter 1
Welcome to our definitive guide: “Power Sharing Notes Class 10th – Unlocking Success.” If you’re a Class 10th student striving for academic excellence, understanding the intricacies of power sharing is paramount. Mastering this essential topic not only enriches your knowledge of political science but also cultivates your sense of responsibility as an informed citizen.
Within this article, we have curated a comprehensive set of power sharing notes exclusively designed to cater to the needs of Class 10th students. Crafted by subject matter experts, these notes offer a clear and concise grasp of the subject matter. Whether you’re preparing for exams or seeking a deeper understanding, these notes will become your ultimate study companion.
In this article, we will delve into the intriguing narratives of Belgium and Sri Lanka, exploring their unique stories. We’ll also examine the concept of Majoritarianism in Sri Lanka, shedding light on its implications. Additionally, we’ll explore the reasons why power sharing is considered desirable and the various forms it can take. Get ready to embark on a captivating journey through the histories of Belgium and Sri Lanka, while gaining valuable insights into the significance of power sharing in governance.
Important terms Used in Power Sharing Notes Class 10th
Definition of Power Sharing
Power sharing refers to a mutually agreed-upon policy between political parties or within a coalition to collectively shoulder responsibility for decision-making and political actions.
The significance of power sharing becomes evident when it is practiced among the three organs of the state, namely the Legislature, Executive, and Judiciary. Such an arrangement is vital for ensuring the proper functioning of a democratic system.
Ethnic
An ethnic group is formed through a social division based on a shared culture. Its members believe in a common descent, either due to similarities in physical characteristics, cultural practices, or both. It is important to note that members of an ethnic group may not necessarily share the same religion or nationality.
Community Government
Community government is a decentralized governance system in linguistically diverse countries. It allows linguistic or cultural groups to have autonomy over cultural, educational, and language-related matters. For example, in Belgium, separate community governments represent Dutch-speaking, French-speaking, and German-speaking communities, promoting their unique interests while contributing to national unity. These governments work alongside central and regional authorities, ensuring a balance between local autonomy and national cohesion.
Majoritarianism
Majoritarianism refers to the ideology that grants the majority community the unrestricted power to govern a country as it pleases, even if it means neglecting the desires and interests of the minority groups. For instance, Sri Lanka adopted a majoritarian approach, where the majority Sinhala community holds authority over the governance of the country.
Civil War
Civil war is a type of armed conflict that occurs within the borders of a single country or state, usually between different factions or groups within the same nation. Unlike international wars, civil wars are internal conflicts where the parties involved are from the same country. The reasons for civil wars can vary and may include political, ethnic, religious, or economic factors, among others.
Power Sharing Notes Class 10th
Story of Belgium
Belgium, a compact federal state located in Western Europe, possesses a land area smaller than the Indian state of Haryana. The country’s population comprises a diverse and intricate ethnic composition. Situated at a strategic geographical position, Belgium shares its borders with neighboring countries, including the Netherlands, France, Germany, and Luxembourg.
In Belgium, there existed a minority French-speaking community (40%) that held considerable wealth and influence, while the Dutch-speaking community (59%) experienced delayed access to development and education. The capital city of Belgium is Brussels, where 80% of the population speaks French, while the remaining 20% speaks Dutch. Within this dynamic, the minority French-speaking community enjoyed relative affluence and power.
Accommodation in Belgium
Brussels, the capital of Belgium, is predominantly occupied by French-speaking residents. Over time, power has predominantly rested in the hands of the French-speaking population, granting them advantages in terms of economic development and education.
As a result, the French-speaking community exhibited higher levels of education and qualification, with access to greater resources compared to the native Dutch population. Additionally, the French-speaking individuals formed a well-established aristocracy of tradespeople, contributing to their financial strength and influence in the region.
During the 1950s and 1960s, the social disparities between the Dutch-speaking and French-speaking communities gave rise to tensions.
Causes of Conflict in Belgium
The French-speaking minority community enjoyed relative wealth and influence, which caused resentment among the Dutch-speaking community. The latter received the benefits of economic development and education at a later stage, leading to tensions between the two language groups.
Steps taken to Remove Tensions in Belgium
The central government consists of an equal number of Dutch and French-speaking ministers. State governments hold extensive powers and are not subordinate to the central government.
In Brussels, a separate government operates with equal representation for both language communities. Additionally, a “community government” is elected by each particular language-speaking community, which holds authority over cultural, educational, and language-related matters.
Concept of ‘Community government’ of Belgium
The concept of ‘Community government’ in Belgium refers to a form of decentralized governance that recognizes the linguistic and cultural diversity within the country. Belgium is composed of distinct linguistic communities, primarily Dutch-speaking (Flemish), French-speaking (Walloon), and German-speaking.
To address the interests and needs of these communities, separate ‘Community governments’ were established. Each community government has authority over cultural, educational, and language-related matters for its respective linguistic group, empowering them to promote and preserve their unique identity while contributing to the overall unity of Belgium.
Power Sharing Notes Class 10th
Story of Sri Lanka
Sri Lanka, officially known as the Democratic Socialist Republic of Sri Lanka, is an island country situated in South Asia. It is located in the Indian Ocean, to the southwest of the Bay of Bengal and to the southeast of the Arabian Sea. The island is renowned for its rich history, diverse culture, and stunning natural beauty.
Colombo serves as the capital and largest city of Sri Lanka. The country boasts a fascinating heritage, with ancient cities, temples, and historical sites, as well as breathtaking landscapes, including tropical beaches, lush forests, and tea plantations. Sri Lanka is a popular destination for tourists seeking a blend of cultural exploration and natural wonders.
In Sri Lanka, the major social groups are comprised of Sinhala speakers (74%) and Tamil speakers (18%). The Tamil community further divides into two subgroups: “Sri Lankan Tamils” and “Indian Tamils.” For a visual representation of the population distribution of these diverse communities, please refer to the map below.
Sri Lankan Tamils
The Tamil natives of Sri Lanka are known as Sri Lankan Tamils, comprising 13% of the population and primarily residing in the Northern and Eastern regions of the country. The majority of Sinhala speakers follow Buddhism, whereas most Tamils practice Hinduism or Islam.
Indian Tamils
Indian Tamils in Sri Lanka refer to Tamil individuals whose ancestors migrated from India as plantation workers during the colonial era and settled in the country. They make up approximately 5% of the population.
Majoritarianism in Sri Lanka
The Sinhala community in Sri Lanka held dominance over the minority Tamil population and implemented majoritarian policies.
In 1956, an Act was passed, declaring Sinhala as the sole official language. The government also adopted preferential policies that favored Sinhala applicants for university positions and government employment.
Additionally, the state protected and promoted Buddhism, further exacerbating the sense of alienation among Sri Lankan Tamils. Over time, these measures contributed to a growing feeling of marginalization among the Tamil community.
The Sri Lankan Tamils voiced their demands for regional autonomy, equal opportunities in education and employment, and recognition of Tamil as an official language.
However, their pleas were consistently rejected, leading to the rise of various political organizations like the LTTE (Liberation Tigers of Tamil Eelam). As tensions escalated, the situation eventually escalated into a full-fledged civil war during the 1980s.
Factors Led to A Civil War in Sri Lanka
The civil war in Sri Lanka was a complex and multi-faceted conflict with various factors contributing to its outbreak. Some of the key factors that led to the civil war include:
- Ethnic and Linguistic Tensions: Deep-rooted ethnic and linguistic divisions between the Sinhalese majority and Tamil minority communities fueled tensions and grievances.
- Discriminatory Policies: The implementation of policies favoring the Sinhala community in areas such as language, education, and employment created a sense of marginalization and inequality among the Tamil population.
- Regional Autonomy Demand: The Sri Lankan Tamils demanded greater autonomy and recognition of their distinct cultural identity, which was met with resistance and denial by the government.
- Insurgency and Militancy: The formation of militant groups, such as the Liberation Tigers of Tamil Eelam (LTTE), emerged in response to perceived injustices and sought to achieve Tamil separatism through armed struggle.
- Failed Negotiations: Repeated failure of negotiations between the government and Tamil representatives to address the root issues and find a peaceful resolution contributed to the escalation of the conflict.
- International Involvement: The involvement of external actors and support to various parties in the conflict further complicated the situation and prolonged the war.
- Escalating Violence: Escalation of violence and atrocities committed by both sides deepened animosity and further polarized communities.
Overall, the combination of historical grievances, discriminatory policies, political complexities, and armed resistance culminated in a prolonged and devastating civil war in Sri Lanka that lasted for decades.
Form of Power Sharing in Class 10th Political Science
You might have thought that sharing power means dividing it, which could weaken the country. Many people used to believe this in the past. They thought that all the power of the government should be in the hands of one person or a group in one place. This way, they believed, decisions could be made quickly and enforced effectively. However, things have changed with the rise of democracy!
In a democratic system, the people themselves have the power. We rule ourselves through self-governing institutions. Every single one of us has a say in shaping the policies that affect our lives. That’s what makes democracy special! So, in a democratic country, it’s important to distribute political power among all citizens. This way, everyone gets to participate and have a say in how the country is run. It’s about fairness, representation, and making sure everyone’s voice is heard.
Remember, democracy is all about inclusivity and making sure that each of us has a part in shaping our nation’s future. By sharing power among citizens, we create a stronger and more united country, where everyone’s rights and perspectives matter. So, let’s embrace democracy and celebrate the power we all have as citizens to create a better and more inclusive society!
Power Sharing Notes Class 10th Political Science
1. Power is shared among different organs of government, such as the legislature, executive and judiciary
We call this type of power distribution “horizontal distribution” because it allows different branches of the government, placed at the same level, to have distinct powers. This separation ensures that no single branch can have absolute power. Instead, each branch acts as a check on the others, creating a system of checks and balances. This arrangement promotes accountability and prevents any one branch from becoming too dominant, safeguarding the rights and interests of the people.
2. Power can be shared among governments at different levels
In this system, there is a central government that governs the entire country, alongside governments at the provincial or regional level, collectively known as the federal government. This arrangement allows for a division of responsibilities and authority between the central and regional authorities, ensuring effective governance at both levels. The federal government is responsible for matters that affect the entire nation. While provincial or regional governments handle issues specific to their respective areas, catering to the diverse needs of different regions within the country.
3. Power shared among different social groups
This type of arrangement is designed to provide representation and participation in the government. Also, administration for diverse social groups that might otherwise feel marginalized and excluded. It aims to give minority communities a fair share of power and influence in decision-making processes. An example of this approach can be seen in India, where reserved constituencies exist in the assemblies and Parliament.
Another excellent example of this type of power-sharing is the Community Government system in Belgium. This arrangement empowers linguistic and cultural communities, granting them autonomy over specific matters related to their identity and heritage. By fostering inclusivity and acknowledging the diversity of its population, Belgium promotes unity and ensures the equitable distribution of power among its communities.
4. Power shared among political parties, pressure groups and moments
In a democracy, citizens have the freedom to choose from a range of contenders vying for power. This competition is vital as it prevents power from becoming concentrated in the hands of a single entity. Over time, power is shared among diverse political parties, each representing distinct ideologies and social groups. This form of government is known as a “Coalition Government.”
Moreover, various interest groups, such as traders, businessmen, farmers, and industrial workers, also have a stake in governmental power. They participate in the decision-making process and exert influence, ensuring that the government considers a wide array of perspectives and interests.
In essence, democracy thrives on the principles of inclusivity and power-sharing, granting citizens a voice in shaping their nation’s future and fostering a dynamic and responsive governance system.
What do we learn from the two stories of Belgium and Srilanka?
The stories of Belgium and Sri Lanka offer essential lessons in governance and managing diversity. Belgium’s power-sharing model, with federalism allowing linguistic communities to have regional autonomy, has fostered social cohesion and political stability. By recognizing and respecting the unique identities of different communities, Belgium has strengthened national unity.
In contrast, Sri Lanka’s experience warns against the perils of majoritarianism. The dominance of the Sinhala community over the Tamil minority, coupled with discriminatory policies, led to tensions and alienation among Sri Lankan Tamils. This resulted in a prolonged civil war, emphasizing the need for equitable power-sharing and respect for the rights of all communities.
Both stories underscore the significance of cultural recognition. Embracing diverse identities promotes stronger social bonds and national pride, while denying linguistic and cultural rights can fuel division and grievances.
Peaceful resolution emerges as a crucial aspect of conflict management. Open dialogue, negotiation, and compromise are essential in addressing grievances and finding lasting solutions. International involvement can also facilitate peace-building efforts.
In conclusion, the stories of Belgium and Sri Lanka highlight the value of power sharing, inclusivity, and respect for cultural identities in governance. By learning from these experiences, societies can strive towards building inclusive and harmonious nations, where citizens’ rights are upheld, and conflicts are resolved through peaceful means. Emphasizing the importance of cultural recognition and dialogue can pave the way for a more united and stable future.
Summery of Power Sharing Notes Class 10th
In Power sharing notes class 10th article we have read about Belgium and Sri Lanka. Belgium, a compact federal state in Western Europe, exemplifies the strength of inclusive governance despite its small land area. Its diverse ethnic composition and strategic geographical location near neighboring countries like the Netherlands, France, Germany, and Luxembourg underscore its significance.
Within Belgium, the imbalance between the French-speaking minority (40%) enjoying wealth and influence and the Dutch-speaking community (59%) facing development and education challenges led to tensions. To address these disparities, Belgium adopted power-sharing measures. The central government ensured equal representation of Dutch and French-speaking ministers, while state governments gained autonomy from the central authority. Brussels, with its mixed linguistic population, had a separate government to cater to both communities. Additionally, a unique “community government” granted linguistic autonomy, preserving identities while promoting national unity.
In contrast, Sri Lanka’s history serves as a cautionary tale of majoritarianism. The dominance of the Sinhala community over the Tamil minority and preferential policies led to alienation and a civil war. Both stories offer crucial lessons on governance and diversity management. Which emphasizing the value of power sharing for inclusivity, accountability, and citizen participation in decision-making. Through equitable distribution of power among various entities, societies can build harmonious nations, respecting diverse identities and resolving conflicts peacefully. Power sharing remains the key to creating a united and stable future where every citizen’s voice shapes the country’s destiny.
Read Also:
- Class 10th Political Parties Notes of NCERT Civics Chapter 4
- Outcomes of Democracy Class 10 Notes of NCERT Civics Ch. 7
Frequently Asked Question – FAQ on Power Sharing Notes Class 10th
Q 1. What kind of power sharing problems were faced by Belgians and Sri Lankans?
The Belgians faced power sharing problems related to linguistic and cultural diversity. The country is divided between Dutch-speaking (Flemish) and French-speaking (Walloon) communities. Each of them were seeking recognition and representation. Struggles for regional autonomy, language rights, and fair representation in government posed significant challenges.
Similarly, Sri Lanka encountered power sharing issues primarily centered around ethnic tensions. The conflict arose between the Sinhalese majority and Tamil minority communities, demanding equality, regional autonomy, and recognition of their cultural identity. The denial of these demands led to prolonged civil strife and violence, highlighting the complexities of power sharing in a diverse society.
Q 2. Why power sharing is desirable?
Power sharing is desirable because it prevents the concentration of power. It reducing the risk of tyranny and abuse. It fosters social stability by accommodating diverse interests and ensuring representation of various communities in decision-making.
Power sharing mechanisms, like checks and balances, promote accountability and prevent the dominance of any single authority. It can also resolve conflicts and promote reconciliation in divided societies.
Encouraging cooperation and compromise, power sharing enhances the legitimacy of the government and fosters inclusivity, making it an essential aspect of democratic governance.
Q 3. What are the system various democratic rules?
In a democratic system, various rules are crucial for ensuring fair and effective governance. These include the rule of law, which holds everyone accountable to the law.
The separation of powers, dividing authority among different branches to prevent concentration of power. Checks and balances, enabling each branch to limit the powers of others.
Freedom of speech and expression; freedom of the press; protection of minority rights; and respect for human rights. These democratic principles collectively promote transparency, accountability, inclusivity, and protection of individual liberties within the framework of democratic governance.
Outcomes of Democracy Class 10 Notes of NCERT Civics Ch. 7
Welcome to our exclusive and comprehensive guide on “Outcomes of Democracy Class 10 Notes.” This chapter delves into the fascinating world of democracy. Explore its profound impact on societies and the diverse outcomes it generates. Democracy, as a system of governance, holds a special place in modern societies. It offers citizens the power to participate in decision-making and ensuring accountability of the ruling authorities.
In this article, we aim to provide you with a well-rounded understanding of the outcomes that emerge from democratic processes. Democracy plays a pivotal role in shaping the course of nations. Fostering political stability, social harmony, and economic development, promoting individual rights. Also, in freedom of expression, and equitable distribution of resources.
Our meticulously curated notes present complex concepts in a clear and concise manner, making it an invaluable resource for Class 10 students. Say goodbye to overwhelming textbooks and disjointed online resources; our user-friendly approach ensures a seamless learning experience.
Join us on this enlightening journey as we explore the multifaceted outcomes that democracy brings to the world. Happy learning!
NCERT Chapter 7 Outcomes of Democracy Class 10 Notes
Political Science Chapter 7 Outcomes of Democracy Class 10 Notes
How Do We Assess Democracy’s Outcomes?
In comparing democracy to dictatorship, the advantages of democracy become evident:
1. Fosters Equality: Democracy promotes equality among citizens, ensuring that every individual’s voice and opinion hold significance.
2. Upholds Dignity: The democratic system enhances the dignity of each individual, empowering them to participate in the decision-making process.
3. Enhances Decision-Making: With diverse perspectives and opinions taken into account, democracy leads to more informed and inclusive decision-making.
4. Conflict Resolution: Democratic governance provides a structured method to resolve conflicts peacefully through dialogue and consensus-building.
5. Allows Learning from Mistakes: The democratic setup allows for corrections and improvements as citizens can hold their representatives accountable.
These attributes make democracy a preferred form of government. It priorities the rights and involvement of citizens, fostering a just and progressive society.
Democracy Produces Accountable, Responsive and Legitimate Government
In a democracy, people are bestowed with the right to elect their rulers, granting them control over the governance process. This empowerment enables citizens to actively participate in decision-making, fostering a government that remains accountable and responsive to their needs and expectations.
One of the key strengths of democracy lies in its adherence to norms and procedures during decision-making. This transparency allows citizens to access information about how decisions were made, ensuring a fair and just process. Moreover, in a democratic setup, citizens have the opportunity to engage in decision-making whenever they desire, making their voices heard in shaping policies and governance.
In Democracy, fundamentally, is the people’s government, where representatives are chosen through elections by the very individuals they will govern. This ensures that the government truly represents the will of the people. Leading to a system that reflects the collective aspirations of the nation.
Economic Growth and Development
Economic development is influenced by a multitude of factors, such as the country’s population size, the global economic situation, international cooperation, and the economic priorities adopted by the nation. Notably, there exists a substantial disparity in economic growth rates between countries governed under dictatorship and those under democracy. In light of these observations, democracy emerges as the preferred form of governance, as it leads to several positive outcomes in terms of economic progress and prosperity.
Economic Outcomes of Democracy
The relationship between democracy and economic growth, as well as economic inequalities, can be outlined as follows:
1. Dictatorial regimes have shown a slightly better economic growth record. However, when considering only poor countries, there is virtually no difference in their economic performance compared to democracies.
2. Democracies can also exhibit a high degree of inequality, both in terms of income and wealth distribution.
3. Opportunities available to the poorer sections of society are often unequal, leading to disparities in accessing essential resources and services.
In summary, while economic growth may vary between dictatorships and democracies, both systems can experience economic inequalities, and the distribution of opportunities may remain unequal in democratic setups as well.
Reduction of Inequality and Poverty
In a democratic setup, all individuals have equal weight in electing representatives, aiming to bring them into the political arena on an equal footing. However, despite these intentions, we observe growing economic inequalities in society:
1. A small minority of ultra-rich individuals holds a significantly disproportionate share of wealth and incomes. Over time, their portion of the total national income has been increasing, accentuating income disparities.
2. Conversely, those at the bottom strata of society face limited economic opportunities and have very little to depend upon. Their incomes have been declining, exacerbating their financial challenges.
In practice, democracies have not been entirely successful in effectively reducing economic inequalities, highlighting the complexity of addressing this issue within the democratic framework. Despite the principle of equal representation, societal disparities persist and require ongoing attention and efforts to achieve more equitable outcomes.
Accommodation of Social Diversity
While no society can entirely eliminate conflicts among diverse groups, the key lies in learning to respect these differences and developing mechanisms to negotiate them peacefully. Democracy emerges as the most suitable system to achieve this outcome. The strength of democratic regimes lies in their ability to handle social differences, divisions, and conflicts effectively. However, for democracy to successfully attain this goal, two vital conditions must be met:
- The majority must collaborate with the minority to ensure that governments represent the collective views and interests of the entire populace.
- Rule by the majority implies that different individuals and groups may form majorities during various decisions and elections, fostering a system where varying perspectives are considered and accommodated.
Dignity and Freedom of the Citizens
Democracy stands as the most exemplary form of government, championing the dignity and freedom of each individual. The essence of democracy lies in its unwavering commitment to respect and liberty. One significant aspect where democracy has made remarkable strides is in upholding the dignity of women. The tireless efforts of women’s movements have instilled a profound awareness that respect and equal treatment for women are indispensable pillars of a democratic society. Similarly, democracy has played a pivotal role in challenging caste inequalities, empowering disadvantaged and discriminated castes to demand equal status and opportunities.
In the democratic framework, people firmly believe that their votes hold the power to shape the government’s functioning and impact their own well-being. The process of democratic evaluation is perpetual, continuously setting new standards and aspirations. As individuals experience the benefits of democracy, their desire for greater progress grows, fueling a collective quest to refine and strengthen democracy further. The fact that people voice their concerns and expectations is a testament to democracy’s success in fostering an environment where citizen participation and continuous improvement are valued and embraced.
Read ALso:
- Power Sharing Notes Class 10th of NCERT Civic Chapter 1
- Class 10th Political Parties Notes of NCERT Civics Chapter 4
Frequently Asked Question – FAQs on Outcomes of Democracy Class 10 Notes
Q 1. What are the five outcomes of democracy for Class 10?
five outcomes of democracy are
- Equality: Democracy promotes equality among citizens by giving each individual an equal say in decision-making regardless of their social status or background.
- Dignity and Freedom: Democracy upholds the dignity and freedom of individuals, safeguarding their fundamental rights and liberties.
- Quality Decision-Making: Democracy enhances the quality of decision-making by following transparent norms and procedures and allowing citizens access to information and active participation.
- Conflict Resolution: Democracy provides a peaceful mechanism to resolve conflicts, recognizing that differences and diverse interests exist in society.
- Empowerment of Marginalized Groups: Democracy empowers marginalized groups by giving them a voice in political processes, promoting social justice and equal opportunities for all.
Q 2. What is the importance of Democracy?
Democracy holds immense importance in shaping a just and inclusive society. Here are the key reasons why democracy is essential:
- Protection of Rights and Freedoms: Democracy ensures the protection of individual rights and freedoms. Citizens have the right to express their opinions, assemble peacefully, and participate in decision-making, fostering an environment of tolerance and respect.
- Government Accountability: In a democratic system, the government is accountable to the people. Regular elections allow citizens to hold their representatives responsible for their actions, promoting transparency and reducing corruption.
- Inclusive Governance: Democracy promotes inclusive governance, where diverse voices and perspectives are considered in policy-making. It gives a platform to marginalized groups and minorities, safeguarding their interests.
- Peaceful Conflict Resolution: Democratic societies provide peaceful mechanisms to resolve conflicts through negotiation and dialogue, preventing the escalation of tensions into violence.
- Economic Development: Democracy encourages economic growth and development. Political stability, protection of property rights, and rule of law attract investments and foster entrepreneurship.
- Social Progress: Democracy supports social progress by addressing inequalities, promoting education, and ensuring access to healthcare and social welfare.
- Global Peace and Cooperation: Democracies tend to maintain peaceful relations with other democracies, fostering global cooperation and reducing the likelihood of armed conflicts.
- Human Dignity: Democracy upholds human dignity by recognizing the inherent value and rights of every individual, irrespective of their background or social status.
In conclusion, democracy is essential for building a just, progressive, and peaceful society that empowers its citizens and upholds their rights and freedoms. It is a system that allows for continuous growth, social cohesion, and a collective pursuit of common goals.
Q 3. How democracy offers freedom and dignity to citizens?
Democracy empowers citizens with freedom and dignity through active participation in the decision-making process, expressing their opinions, and electing representatives. It upholds the values of equality and safeguards individual rights, ensuring that every citizen is treated with respect and impartiality. In democratic societies, the rule of law ensures that everyone receives fair treatment and that the government remains answerable to the people. By encouraging political engagement and protecting human rights, democracy fosters a sense of dignity and ownership among individuals, empowering them to influence their society’s development.
Money and Credit Class 10 Solution of NCERT Economics Ch. 3
Welcome to our comprehensive guide on the “Solution of Money and Credit of Class 10.” If you’re a Class 10 student seeking a deeper understanding of the intriguing world of money, credit, and financial systems, you’ve come to the right place. In this article, we present a well-structured and detailed solution to the chapter “Money and Credit” from the Class 10 curriculum.
Explore the various concepts and mechanisms related to money. Its role in an economy, the functioning of banks, and the significance of credit systems. With our expertly crafted explanations and step-by-step solutions, you’ll grasp the fundamentals and complexities of the financial world with ease. Let’s dive in and unlock the secrets of “Money and Credit of Class 10.
The fascinating chapter on “Money and Its Evolution” has been thoughtfully included in the Class 10 Economics curriculum. This chapter delves into the intriguing history of money, exploring its various forms and usage throughout different periods. Additionally, students will gain valuable insights into the interconnection between modern forms of money and the intricate banking system. As a comprehensive resource, this chapter concludes with exercise questions to test and reinforce students’ understanding of the subject matter. Prepare to embark on an enlightening journey through the evolution of money and its profound impact on economies worldwide.
Solution of NCERT Chapter 3 Economics Money and Credit of Class 10

Money and Credit of Class 10 Question and Solutions
Q 1. In situations with high risks, credit might create further problems for the borrower. Explain.
In situations with high risks, credit can indeed create further problems for the borrower. When a borrower takes on credit in a high-risk scenario, several potential issues may arise:
1. Increased Debt Burden: If the borrower faces difficulties in repaying the borrowed amount due to the high-risk nature of the venture, it could lead to an increased debt burden. This may result in mounting interest payments and the risk of defaulting on the loan, leading to further financial distress.
2. Financial Stress: High-risk situations are often associated with uncertain outcomes, such as in speculative investments or ventures with an uncertain future. If the borrower’s investment or venture does not yield expected returns, they may struggle to meet their credit obligations, leading to financial stress and potential insolvency.
3. Limited Access to Credit: High-risk borrowers may find it challenging to access credit in the future, especially if they have a history of defaulting on loans. Lenders may view them as risky borrowers and be hesitant to extend credit. It make difficult for them to meet their financial needs in the future.
4. Higher Interest Rates: Lenders typically charge higher interest rates to compensate for the increased risk they take when lending to high-risk borrowers. The higher interest rates can further strain the borrower’s financial situation, making it more challenging to repay the loan.
5. Adverse Impact on Credit Score: Failure to repay credit in a high-risk situation can adversely affect the borrower’s credit score. A poor credit score can hinder their ability to access credit in the future and may lead to limited financial opportunities.
Q 2. How does money solve the problem of double coincidence of wants? Explain with an example of your own.
Money solves the problem of double coincidence of wants by acting as a medium of exchange. In a barter system, where goods and services are directly exchanged for other goods and services, there is a requirement for a perfect match of wants between two parties involved in the exchange. However, this can be challenging and inefficient, as finding someone who has what you need and needs what you have can be quite difficult.
With the introduction of money, individuals no longer need to find a direct coincidence of wants. They can exchange their goods and services for money, which is universally accepted as a medium of exchange. Money acts as an intermediary, enabling transactions to occur smoothly and efficiently.
For example, let’s consider a scenario in a barter system where Alice, who is a baker, needs a pair of shoes, and Bob, who is a cobbler, needs bread. In a barter system, Alice and Bob would have to find each other and directly exchange bread for shoes. However, this may not always be possible, and they may not come across each other at the right time.
Now, let’s introduce money into the scenario. Instead of directly exchanging bread for shoes, Alice and Bob can use money as an intermediary. Alice sells her bread to a customer for money, and Bob sells his shoes to the same customer for money. Then, Alice can use the money she earned to purchase the shoes from Bob. In this way, money facilitates the exchange between Alice and Bob, even though they do not have a direct coincidence of wants.
By eliminating the need for a double coincidence of wants, money greatly simplifies and accelerates economic transactions, making the exchange of goods and services much more efficient and convenient for everyone involved.
Q 3. How do banks mediate between those who have surplus money and those who need money?
Banks play a crucial role in mediating between those who have surplus money and those who need money. They act as financial intermediaries, facilitating the flow of funds between savers (those with surplus money) and borrowers (those in need of money). Here’s how banks perform this important function:
- Deposits and Savings: Individuals and businesses with surplus money deposit their funds in banks. These deposits can be in the form of savings accounts, fixed deposits, or other types of accounts. By depositing their money in banks, savers earn interest on their deposits, which encourages them to save and invest.
- Loan and Credit Facilities: On the other hand, banks offer various loan and credit facilities to individuals, businesses, and other borrowers who need money for various purposes, such as starting a business, buying a home, or financing their education. When borrowers take loans from banks, they agree to pay back the principal amount along with interest over a specific period.
- Intermediation: Banks act as intermediaries by using the deposits they receive from savers to extend loans and credit to borrowers. They channel funds from the surplus units (savers) to the deficit units (borrowers), effectively allocating capital to where it is needed most. This process ensures that the money is put to productive use and contributes to economic growth and development.
Overall, banks act as crucial financial intermediaries in the economy, bringing together those who have surplus money and those who need money, thereby supporting economic activities and promoting growth and development.
Q 4. Look at a 10 rupee note. What is written on top? Can you explain this statement?
At the top of a 10 rupee note, you will find the inscriptions “Reserve Bank of India” and “Guaranteed by the Central Government.” These statements hold significant importance as they indicate the authority and credibility behind the currency in India.
The Reserve Bank of India (RBI) serves as the central bank of the country and is responsible for issuing and regulating the currency. As the central banking institution, the RBI plays a crucial role in formulating monetary policies, managing the money supply, and maintaining the stability of the Indian rupee.
The statement “Guaranteed by the Central Government” signifies that the currency note is backed by the full faith and credit of the Indian government. This guarantee ensures the trust and confidence of the public in the value and acceptability of the currency for transactions within the country.
Both the Reserve Bank of India and the Central Government are the sole authorities responsible for issuing and managing currency in India. By inscribing these statements on the currency note, it is established that the note is officially authorized, and its value is supported by the combined authority of the central bank and the government.
This ensures the smooth functioning of the monetary system, instills public confidence in the currency’s stability, and upholds the integrity of the Indian rupee as a reliable medium of exchange for economic transactions.
Q 5. Why do we need to expand formal sources of credit in India?
Expanding formal sources of credit in India is essential for several reasons:
1. Financial Inclusion: Expanding formal credit sources ensures that a larger section of the population, including the unbanked and financially marginalized, has access to financial services. This promotes financial inclusion and empowers individuals and businesses with opportunities to participate in the formal economy.
2. Reduced Dependence on Informal Credit: In India, a significant portion of the population relies on informal sources of credit, such as moneylenders and local lenders, which often charge high-interest rates. By expanding formal credit channels, people can access credit at more reasonable and regulated interest rates, reducing their dependency on exploitative informal sources.
3. Boost to Economic Growth: Adequate and accessible credit facilities fuel economic growth by promoting investments, entrepreneurship, and business expansion. Formal credit institutions can cater to the financial needs of small and medium-sized enterprises, which play a crucial role in driving economic development.
4. Employment Generation: When businesses have access to formal credit, they can expand their operations, invest in technology, and create more job opportunities. This, in turn, leads to improved livelihoods and increased economic activity.
5. Financial Stability: Formal credit institutions are regulated and supervised by the central bank. It ensures prudent lending practices and risk management. This contributes to financial stability and reduces the likelihood of systemic risks in the economy.
By expanding formal sources of credit, India can achieve broader financial inclusion, sustainable economic growth, and equitable development, benefitting individuals, businesses, and the nation as a whole.
Q 6. What is the basic idea behind the SHGs for the poor? Explain in your own words.
The basic idea behind Self-Help Groups (SHGs) for the poor is to empower marginalized individuals, particularly women, by bringing them together to form a collective and supportive platform. SHGs are small voluntary associations formed at the community level, comprising members from similar socio-economic backgrounds.
The primary objective of SHGs is to promote financial inclusion, social cohesion, and economic empowerment among its members. Members contribute small amounts of savings regularly to create a common fund. This fund is then utilized to provide microcredit and loans to the group members for various income-generating activities and personal needs.
SHGs enable the poor to access financial services, build a savings habit, and access credit without relying on traditional banks or moneylenders. By pooling their resources, SHG members can collectively address their financial requirements and uplift their socio-economic status.
Moreover, SHGs also serve as platforms for capacity building, skill development, and knowledge-sharing among members. They encourage leadership, decision-making, and entrepreneurship skills, fostering a sense of ownership and responsibility among the participants.
Furthermore, SHGs promote social solidarity and support systems among the members. They create a sense of belonging and encourage mutual trust and cooperation. Members share their experiences, offer emotional support, and collectively address various social and economic challenges faced by their community.
In summary, SHGs for the poor aim to promote self-reliance, financial independence, and socio-economic empowerment among marginalized individuals by providing them with access to credit, financial literacy, and a supportive community. Through these groups, the poor can break the cycle of poverty, improve their livelihoods, and lead a dignified life.
Q 7. What are the reasons why banks might not be willing to lend to certain borrowers?
Banks might not be willing to lend to certain borrowers due to various reasons, including:
1. Poor Credit History: If a borrower has a history of defaulting on previous loans or has a low credit score, banks may consider them as high-risk borrowers and may be reluctant to lend to them.
2. Insufficient Income: Banks assess a borrower’s ability to repay the loan. If the borrower’s income is not sufficient to cover the loan repayments, the bank may deny the loan application.
3. Lack of Collateral: Some loans, especially large ones, may require collateral as security. If a borrower does not have sufficient assets to offer as collateral, the bank may be hesitant to approve the loan.
4. Unstable Employment: Banks prefer borrowers with stable employment history as it indicates a steady source of income. Borrowers with irregular or uncertain employment may face difficulties in obtaining loans.
5. High Debt-to-Income Ratio: If a borrower already has significant existing debts in relation to their income, the bank may be concerned about their ability to manage additional loan repayments.
6. Industry Risk: Banks may avoid lending to borrowers in industries that are considered high-risk or volatile.
7. Lack of Documentation: Insufficient or incomplete documentation can lead to loan rejection as banks require proper verification of a borrower’s identity, income, and other relevant information.
8. Age of the Borrower: Some banks have age restrictions for borrowers, especially for long-term loans, as older borrowers may be perceived as having higher repayment risks.
It is important for borrowers to understand these factors and maintain a good financial profile to increase their chances of obtaining loans from banks and other financial institutions.
Q 8. In what ways does the Reserve Bank of India supervise the functioning of banks? Why is this necessary?
The Reserve Bank of India (RBI) supervises the functioning of banks in several ways to ensure financial stability and maintain the integrity of the banking system. It employs both on-site and off-site inspections to assess banks’ financial health, risk management practices, compliance with regulations, and governance standards.
RBI also sets prudential norms and guidelines for capital adequacy, asset quality, and liquidity to safeguard depositors’ interests and maintain the overall health of the banking sector.
This supervision is crucial to prevent risks, detect potential issues early on, and take corrective measures to protect the interests of depositors, maintain financial stability, and foster a sound and secure banking environment in the country.
Q 9. Analyse the role of credit for development.
Credit plays a vital role in driving economic development by providing financial resources to individuals, businesses, and governments. It fuels investment, fosters entrepreneurship, and promotes job creation, leading to increased productivity and overall growth.
Access to credit empowers the poor, promotes financial inclusion, and supports poverty alleviation efforts. Moreover, credit facilitates the development of critical infrastructure, technological advancements, and industrial growth.
However, proper supervision and regulation by institutions like the Reserve Bank of India are essential to prevent misuse and ensure financial stability. By balancing credit availability and risk management, economies can harness its potential to drive sustainable development and improve living standards.
Q 10. Manav needs a loan to set up a small business. On what basis will Manav decide whether to borrow from the bank or the moneylender? Discuss.
Manav’s decision to borrow from a bank or a moneylender will depend on several factors. If Manav can meet the bank’s eligibility criteria and has a good credit history, borrowing from the bank may offer several advantages. Banks generally provide loans at lower interest rates, flexible repayment terms, and are regulated by authorities, ensuring fair practices.
On the other hand, if Manav faces challenges in meeting the bank’s requirements or has a poor credit history, the moneylender might be more accessible. Moneylenders may offer quick loans without strict eligibility checks, but often charge higher interest rates.
Manav should carefully consider the terms, interest rates, and the reputation of the lender before making an informed decision to ensure financial stability and avoid potential debt traps.
Q 11 (a). Why might banks be unwilling to lend to small farmers?
Banks might be unwilling to lend to small farmers due to various reasons:
1. Lack of Collateral: Small farmers often lack substantial assets or collateral to secure loans, making banks hesitant to extend credit, as they face higher risks of default.
2. Uneven Income and Cash Flows: Agricultural income is seasonal and dependent on factors like weather and crop yield. Banks may perceive this income variability as a risk, leading to reluctance in lending.
3. Limited Financial Literacy: Some small farmers may have limited financial literacy and struggle with documentation and loan procedures Thus it is challenging for banks to assess their creditworthiness.
4. High Administrative Costs: Providing small loans to numerous farmers can result in high administrative costs for banks, making it less profitable for them.
5. Previous Loan Defaults: If small farmers have a history of loan defaults, banks may become cautious about lending to them in the future.
To address these challenges, governments and financial institutions can work on providing targeted support. Also, promoting financial literacy among farmers, and offering loan products tailored to their specific needs.
Q 11 (b). What are the other sources from which the small farmers can borrow?
Banks might be unwilling to lend to small farmers due to several reasons. Firstly, small farmers often lack proper collateral or credit history, making them riskier borrowers in the eyes of banks.
Additionally, the administrative costs associated with processing small loans may deter banks from lending to them. Moreover, unpredictable weather conditions and the vulnerability of agriculture as an economic activity can affect the repayment capacity of small farmers.
As a result, banks may prefer larger and more established borrowers with higher creditworthiness. To address this issue, the government and financial institutions need to implement measures to support and incentivize banks to provide affordable credit to small farmers, ensuring their financial inclusion and agricultural development.
Q 11 (c). Explain with an example of how the terms of credit can be unfavourable for the small farmer.
Let’s take the example of a small farmer named Ramesh who needs a loan to purchase seeds and fertilizers for the upcoming crop season. He approaches a local moneylender as he faces difficulty obtaining credit from a formal financial institution.
The moneylender agrees to lend Ramesh the required amount but imposes a high-interest rate of 24% per annum, significantly above the prevailing market rate. Additionally, the moneylender demands that Ramesh repay the loan within three months, leaving him with a short repayment period.
This unfavorable term puts immense financial pressure on Ramesh, making it challenging for him to repay the loan on time. Leading to a cycle of debt and financial vulnerability for the small farmer.
Q 11 (d). Suggest some ways by which small farmers can get cheap credit.
Small farmers face challenges in accessing credit from formal financial institutions due to various reasons. Banks may be unwilling to lend to small farmers because of their limited financial resources. Also due to lack of collateral, and higher credit risk associated with agriculture.
Small farmers often operate on small landholdings, making it difficult for banks to recover loans in case of default. Additionally, the seasonal and unpredictable nature of agriculture poses risks to loan repayment. As a result, banks may perceive lending to small farmers as less profitable and may prefer lending to more established and creditworthy borrowers.
To address this, governments and financial institutions must design tailored credit schemes and improve financial inclusion to support the needs of small farmers.
Q 12. Fill in the blanks:
- Majority of the credit needs of the _________________households are met from informal sources. (Answer: Poor)
- ___________________costs of borrowing increase the debt-burden. (Answer: High)
- __________________ issues currency notes on behalf of the Central Government. (Answer: Reserve Bank of India)
- Banks charge a higher interest rate on loans than what they offer on __________. (Answer: Deposits)
- _______________ is an asset that the borrower owns and uses as a guarantee until the loan is repaid to the lender. (Answer: Collateral)
Q 13. Choose the most appropriate answer.
1. In an SHG, most of the decisions regarding savings and loan activities are taken by
-
-
- Bank
- Members
- Non-government organisation
-
Answer: 2. Members
2. Formal sources of credit do not include
-
-
- Banks
- Cooperatives
- Employers
-
Answer: 3. Employers
Money and Credit of Class 10 Summery
“Money and Credit” is a crucial chapter in Class 10 Economics that explores the concepts of money, credit, and their significance in the economy. The chapter delves into the history of money, the evolution of different forms of money, and how modern forms of money are linked to the banking system.
It also highlights the role of banks in mediating between surplus money and those in need of credit. The chapter discusses the importance of formal sources of credit and the challenges faced by small farmers in accessing credit.
It further explains the functioning of Self-Help Groups (SHGs) for the poor and the need for expanding formal sources of credit in India. Overall, “Money and Credit” offers valuable insights into the functioning of the monetary and credit systems, providing students with a comprehensive understanding of these vital economic aspects.
Read Also:
Frequently Asked Questions – FAQs on Money and Credit Class 10
Q 1. Importance of money and credit for class 10 students?
The chapter on “Money and Credit” holds immense importance for Class 10 students. It provides a fundamental understanding of the crucial role of money and credit in an economy.
By studying this chapter, students grasp the concept of money as a medium of exchange, unit of account, and store of value. They learn about various forms of money used historically and the evolution of modern banking systems. Understanding credit becomes essential as it enables students to comprehend the financial needs of different sections of society, especially small farmers and the poor.
Overall, this chapter equips students with essential knowledge about the functioning of monetary systems. Also, about significance of credit in fostering economic development and financial inclusion.
Q 2. What are the various Sources of Credit for Rural Households?
Rural households in India have access to various sources of credit to meet their financial needs. Some of the significant sources of credit for rural households include:
1. Formal Financial Institutions: These include banks, cooperative societies, and regional rural banks. They offer agricultural loans and other financial services to farmers and rural households.
2. Self-Help Groups (SHGs): SHGs are community-based organizations. It provide micro-credit and other financial services to their members, primarily women, in rural areas.
3. Moneylenders: Despite the formal financial institutions, moneylenders still play a role in rural credit. Especially in remote areas where formal credit is not easily accessible.
4. Microfinance Institutions: Microfinance institutions offer small loans and financial services to low-income individuals, including rural households, to support their livelihood activities.
5. Government Schemes: Various government schemes provide credit support to rural households for agricultural and entrepreneurial activities. Also for promoting rural development and financial inclusion.
Rural households often rely on a combination of these sources to fulfill their credit requirements and improve their economic well-being.
Q 3. What are the various modern form of money?
- Cash
- Deposits in Bank
- Check