Month: September 2023
Behaviour Of Gas Molecules
Behaviour Of Gas Molecules: The behavior of gas molecules is a fascinating topic in the realm of physics and chemistry.
To comprehend how gases behave under various conditions, scientists have developed the Kinetic Theory of Gases, which provides valuable insights into the movement, interactions, and properties of gas molecules.

Behaviour Of Gas Molecules
Key Assumptions of the Kinetic Theory of Gases:
- Gas Molecules are in Constant Motion: According to the kinetic theory, gas molecules are in a continuous state of motion. They move randomly and rapidly in all directions, colliding with each other and the walls of their container.
- Negligible Volume and Particle Size: Gas molecules are considered to have negligible volume compared to the total volume of the gas and are treated as point particles. The distances between gas molecules are relatively large compared to their sizes.
- Elastic Collisions: Gas molecules undergo perfectly elastic collisions with each other and the walls of the container. In an elastic collision, both kinetic energy and momentum are conserved.
- No Interactions Except During Collisions: Gas molecules do not exert attractive or repulsive forces on each other except during collisions. This is in contrast to liquids and solids, where intermolecular forces play a significant role.
- Average Kinetic Energy is Proportional to Temperature: The kinetic energy of gas molecules is directly proportional to the absolute temperature (in Kelvin) of the gas. This relationship is described by the equation:Where:
- is the average kinetic energy of gas molecules.
- is the Boltzmann constant.
- is the absolute temperature in Kelvin.
Consequences of the Kinetic Theory:
- Pressure: The pressure exerted by a gas on the walls of its container is a result of the constant collisions of gas molecules with those walls. More frequent and forceful collisions result in higher pressure.
- Temperature and Kinetic Energy: As the temperature of a gas increases, the average kinetic energy of its molecules also increases. This leads to faster molecular motion and more energetic collisions.
- Volume and Molecular Density: Changes in volume affect the density of gas molecules. If the volume of a gas is decreased while keeping the number of molecules constant (as in compression), the density of gas molecules increases, leading to higher pressure.
- Diffusion and Effusion: Gas molecules move from areas of high concentration to low concentration through diffusion. Effusion is the process by which gas molecules escape through a small opening. Both processes are explained by the random motion of gas molecules.
- Gas Laws: The kinetic theory provides a theoretical foundation for various gas laws, including Boyle’s Law, Charles’s Law, and Avogadro’s Law, which describe the relationships between pressure, volume, temperature, and the number of gas molecules.
In conclusion, the behavior of gas molecules is governed by the principles of the Kinetic Theory of Gases. Understanding this theory allows scientists and engineers to make predictions about the properties and behavior of gases under different conditions, which is essential in fields ranging from chemistry and physics to engineering and atmospheric science.
Read More
- Molar Mass Of Oxalic Acid
- Types Of Chemical Bonding
- Chemistry In Everyday Life
- Energy Stored In A Capacitor
- Compound Lenses Thin Lenses In Contact
Frequently Asked Question (FAQs) Behaviour Of Gas Molecules
-
What is the Kinetic Theory of Gases?
The Kinetic Theory of Gases is a scientific model that describes the behavior of gas molecules. It is based on several key assumptions, including that gas molecules are in constant motion, have negligible volume and particle size, undergo elastic collisions, and have average kinetic energy proportional to temperature.
-
What is the significance of gas molecules being in constant motion?
The constant motion of gas molecules explains many macroscopic properties of gases, such as pressure, temperature, and volume. It helps us understand how gases fill containers, exert pressure on their surroundings, and diffuse or mix with other gases.
-
How do gas molecules exert pressure?
Gas molecules exert pressure by colliding with the walls of their container. The force of these collisions per unit area is what we perceive as gas pressure. An increase in the frequency or force of collisions leads to higher pressure.
-
Why do gas molecules have negligible volume and size in the Kinetic Theory?
Considering gas molecules as having negligible volume and size simplifies calculations and is a reasonable approximation because the distances between gas molecules are much larger than their sizes.
-
What is an elastic collision, and why is it important in the Kinetic Theory of Gases?
An elastic collision is a collision in which both kinetic energy and momentum are conserved. In the context of the Kinetic Theory, elastic collisions between gas molecules and container walls help explain how gases exert pressure and how energy is transferred within the gas.
Asteroid And Comet Difference
Asteroid And Comet Difference: Asteroids and comets are two distinct types of celestial objects that orbit the Sun, and they differ in several ways, including their composition, appearance, and behavior. Here are the key differences between asteroids and comets:

Asteroid And Comet Difference
-
Composition:
- Asteroids: Asteroids are primarily composed of rocky and metallic materials. They are often referred to as “minor planets” because they are similar in composition to the terrestrial planets like Earth.
- Comets: Comets consist of a mixture of water ice, frozen gases (such as carbon dioxide, methane, and ammonia), dust, and rocky materials. They are often called “dirty snowballs” because of their icy nature.
-
Appearance:
- Asteroids: Asteroids typically have a solid and rocky appearance. They do not develop a visible coma (a cloud of gas and dust) or a tail, and they tend to reflect sunlight like rocky surfaces.
- Comets: Comets often display a distinctive appearance when they approach the Sun. As they heat up, their ices start to sublimate, releasing gas and dust, which form a glowing coma and a tail that points away from the Sun due to the solar wind and radiation pressure.
-
Orbital Characteristics:
- Asteroids: Asteroids usually have more circular or elliptical orbits, resembling the orbits of planets. They are found primarily in the asteroid belt, a region between the orbits of Mars and Jupiter.
- Comets: Comets often have highly elliptical orbits that can take them from the distant reaches of the solar system (Oort Cloud or Kuiper Belt) to much closer to the Sun during their active phases.
-
Location:
- Asteroids: Most asteroids are found within the asteroid belt, although some may have orbits that cross Earth’s path, making them potentially hazardous near-Earth objects.
- Comets: Comets can originate from various regions of the solar system, such as the Oort Cloud (comets with long orbital periods) or the Kuiper Belt (short-period comets), and they can approach the inner solar system from these distant locations.
-
Activity:
- Asteroids: Asteroids are generally considered inactive. They do not exhibit the kinds of outgassing and tail formation that comets do when they approach the Sun.
- Comets: Comets become active when they approach the Sun, and their ices start to vaporize and release gas and dust. This activity makes them visible from Earth.
-
Size:
- Asteroids: Asteroids can vary significantly in size, from a few meters to hundreds of kilometers in diameter. Some of the largest asteroids, like Ceres, are considered dwarf planets.
- Comets: Comets are typically smaller than asteroids, with nuclei ranging from a few hundred meters to a few kilometers in diameter.


In summary, while asteroids and comets are both celestial objects in our solar system, they differ in terms of composition, appearance, orbital characteristics, location, activity, and size.
Read More
- Molecular Weight Of Nitrogen
- Molecular Weight Of NaOH
- Difference Between Force And Pressure
- Difference Between Diode And Rectifier
- Difference Between AM And FM
Frequently Asked Question (FAQs) Asteroid And Comet Difference
-
What is the fundamental difference between asteroids and comets?
The fundamental difference lies in their composition and behavior. Asteroids are primarily composed of rocky and metallic materials and do not exhibit cometary activity, such as outgassing and tail formation. In contrast, comets are composed of ice, dust, and rocky materials and display visible tails and comas when they approach the Sun.
-
Where are most asteroids and comets located in the solar system?
Most asteroids are found in the asteroid belt, a region located between the orbits of Mars and Jupiter. Comets can originate from various regions, including the Oort Cloud (long-period comets) and the Kuiper Belt (short-period comets), which are much farther from the Sun.
-
What causes the tails of comets?
The tails of comets are caused by the sublimation (vaporization) of ices in the comet’s nucleus as it approaches the Sun. Solar radiation and the solar wind push the released gas and dust away from the comet, forming a glowing coma and a tail that points away from the Sun.
-
Do asteroids ever have tails?
No, asteroids do not develop tails. They lack the icy compositions that lead to the outgassing and tail formation characteristic of comets.
-
Can comets become asteroids, or vice versa?
While both asteroids and comets can change orbits over time due to gravitational interactions, they typically retain their essential characteristics. For instance, a comet will still have its icy composition even if its orbit changes, and an asteroid will remain rocky and metallic.
Molar Mass Of Oxalic Acid
Molar Mass Of Oxalic Acid: The molar mass of oxalic acids, which has the chemical formula C2H2O4, can be calculated by summing the atomic masses of all the atoms in one mole of the compound. Here’s how you can calculate it:

Molar Mass Of Oxalic Acid
Calculating the Molar Mass of Oxalic Acid
To calculate the molar mass of oxalic acids (C2H2O4), we sum the atomic masses of its constituent elements:
- Carbon (C) has an atomic mass of approximately 12.01 g/mol.
- Hydrogen (H) has an atomic mass of approximately 1.01 g/mol.
- Oxygen (O) has an atomic mass of approximately 16.00 g/mol.
Now, let’s compute the molar mass of oxalic acid:
Molar Mass of Oxalic Acids (C2H2O4) = (2 × Atomic Mass of Carbon) + (2 × Atomic Mass of Hydrogen) + (4 × Atomic Mass of Oxygen)
Molar Mass of Oxalic Acids (C2H2O4) = (2 × 12.01 g/mol) + (2 × 1.01 g/mol) + (4 × 16.00 g/mol)
The Molar Mass of Oxalic Acids (C2H2O4) = 24.02 g/mol + 2.02 g/mol + 64.00 g/mol
Molar Mass of Oxalic Acids (C2H2O4) ≈ 90.04 g/mol
So, the molar mass of oxalic acids (C2H2O4) is approximately 90.04 grams per mole.
Significance of Oxalic Acid’s Molar Mass
Understanding the molar mass of oxalic acids is essential for various reasons:
- Chemical Reactions: Molar mass plays a pivotal role in stoichiometry, helping chemists determine the quantity of substances needed and produced in chemical reactions involving oxalic acids.
- Laboratory Procedures: In laboratories, chemists use molar mass to measure and prepare precise amounts of substances, ensuring accurate experiments and analyses.
- Solution Concentrations: Molar mass aids in calculating the concentration of oxalic acids solutions, which is crucial in fields like analytical chemistry and titration experiments.
- Industrial Applications: Oxalic acids finds application in various industries, including textile, metal polishing, and pharmaceuticals. Knowing its molar mass is essential for quality control and production processes.
- Safety Precautions: Understanding the molar mass of oxalic acids is vital for safety considerations in handling, storage, and transportation, as it helps assess potential hazards.
Conclusion
The molar mass of oxalic acids, approximately 90.04 g/mol, may seem like a mere numerical value, but it holds great importance in the realm of chemistry and scientific endeavors.
Read More
- ypes Of Chemical Bonding
- Chemistry In Everyday Life
- Energy Stored In A Capacitor
- Compound Lenses Thin Lenses In Contact
- 12th Class English Book Question Answer Pdf Download
Frequently Asked Questions (FAQs) Molar Mass Of Oxalic Acid
-
What is the molar mass of oxalic acid (C2H2O4)?
The molar mass of oxalic acids is approximately 90.04 grams per mole (g/mol).
-
Why is knowing the molar mass of oxalic acid important?
Knowing the molar mass of oxalic acids is crucial for various chemical calculations, including stoichiometry, determining solution concentrations, and conducting laboratory experiment. It is also essential for quality control in industrial applications involving oxalic acids.
-
How is the molar mass of oxalic acid calculated?
To calculate the molar mass of oxalic acids, sum the atomic masses of its constituent elements: carbon (C), hydrogen (H), and oxygen (O). Use the atomic masses of these elements and multiply them by the number of atoms of each element in the compound (as indicated by the chemical formula).
-
What is oxalic acid used for in industry?
Oxalic acids has various industrial applications. It is used in metal cleaning and polishing, textile processing, and as a reducing agent in chemical processes. It is also employed in pharmaceutical and research applications.
-
Is oxalic acid safe to handle?
Oxalic acids should be handled with care as it is toxic. Proper safety precautions, including the use of protective equipment and handling procedures, should be followed when working with oxalic acids to minimize health risks.
Molecular Weight Of Nitrogen
Molecular Weight Of Nitrogen: Nitrogen, a fundamental element found abundantly in our planet’s atmosphere, plays a pivotal role in various aspects of science and industry.
Its molecular weight, a key property, provides valuable insights into its behavior and applications. In this article, we’ll explore the molecular weight of nitrogen and its significance in diverse fields.

Molecular Weight Of Nitrogen:
Molecular Weight: A Fundamental Concept
Before delving into nitrogen’s molecular weight, let’s grasp the concept itself. Molecular weight, also known as molar mass, signifies the sum of the atomic weights of all the atoms present in a molecule. This numerical value is usually expressed in atomic mass units (amu) or grams per mole (g/mol). Molecular weight serves as a foundational parameter in chemical calculations, enabling scientists to understand the properties and interactions of substances at the molecular level.
Calculating the Molecular Weight of Nitrogen
Nitrogen primarily exists as a diatomic molecule in its natural state, with two nitrogen atoms bonded together as N2. To determine its molecular weight, we simply add the atomic weights of these constituent atoms. The atomic weight of a single nitrogen atom (N) is approximately 14.00674 amu. As nitrogen exists as N2, we multiply this value by 2 to obtain the molecular weights:
Molecular Weight of Nitrogen (N2) = 2 × Atomic Weight of Nitrogen (N)
Molecular Weight of Nitrogen (N2) = 2 × 14.00674 amu ≈ 28.01348 amu
Therefore, the molecular weights of nitrogen, when it exists as N2, is approximately 28.01348 amu or 28.01348 g/mol.
Implications of Nitrogen’s Molecular Weight
Understanding the molecular weights of nitrogen is essential for comprehending its behavior and applications:
- Gas Properties: Nitrogen, with a molecular weight close to 28 g/mol, is lighter than many other gases in the atmosphere, making up about 78% of the air we breathe. Its relatively low molecular weight allows it to remain close to the Earth’s surface, playing a vital role in sustaining life.
- Chemical Reactivity: Nitrogen’s molecular weight influences its chemical reactivity. As N2, it possesses a robust and stable triple bond between its nitrogen atoms, making it unreactive under normal conditions. This stability is critical for maintaining the composition of Earth’s atmosphere.
- Industrial Applications: Nitrogen’s low molecular weight and inert nature have led to various industrial applications, including its use in food packaging, preventing oxidation in electronics manufacturing, and as a key component in ammonia production for fertilizers.
- Biological Significance: In biology, nitrogen’s molecular weight is pivotal. It determines the mass of nitrogen-containing biomolecules such as proteins and nucleic acids (DNA and RNA). Understanding nitrogen’s molecular weight aids in quantifying and analyzing these essential biomolecules, which are fundamental to life.
Conclusion
The molecular weight of nitrogen, approximately 28.01348 g/mol when existing as N2, is a critical parameter that influences its behavior and applications in numerous fields.
Read More
- Molecular Weight Of NaOH
- Difference Between Force And Pressure
- Difference Between Diode And Rectifier
- Difference Between AM And FM
- Dielectric Material And Dipole Moment
Frequently Asked Question (FAQs) Molecular Weight Of Nitrogen
-
What is the molecular weight of nitrogen?
The molecular weights of nitrogen, when it exists as a diatomic molecule (N2), is approximately 28.01348 atomic mass units (amu) or 28.01348 grams per mole (g/mol).
-
Why is nitrogen’s molecular weight important?
Nitrogen’s molecular weight is crucial because it influences its behavior as a gas, its chemical reactivity, and its significance in various fields, including biology, industry, and environmental science. Understanding its molecular weight helps scientists and researchers make informed decisions about its use and applications.
-
How is nitrogen’s molecular weight calculated?
To calculate the molecular weights of nitrogen (N2), you sum the atomic weights of the constituent atoms, which are two nitrogen (N) atoms. The atomic weights of a single nitrogen atom is approximately 14.00674 amu. Therefore, the molecular weights of N2 is 2 times the atomic weights of nitrogen.
-
What are the main properties of nitrogen related to its molecular weight?
Nitrogen’s relatively low molecular weight compared to other atmospheric gases makes it lighter, allowing it to stay close to the Earth’s surface. Its stable diatomic form (N2) with a strong triple bond makes it unreactive under normal conditions, contributing to the stability of the Earth’s atmosphere.
-
In what industries is nitrogen commonly used?
Nitrogen has diverse industrial applications. It is used in the food industry for packaging and preservation, in electronics manufacturing to prevent oxidation, and in the production of ammonia for fertilizers, among others. Its inert nature and low molecular weight make it valuable in various processes.
Difference Between Asteroid And Meteoroid
Difference Between Asteroid And Meteoroid : Asteroids and meteoroids are celestial objects that inhabit our solar system, but they differ in several key aspects, including their size, location, and origin. Here’s a breakdown of the differences between asteroids and meteoroids:

Difference Between Asteroid And Meteoroid
1. Size:
- Asteroid: Asteroids are relatively larger celestial bodies. They can range in size from a few meters to hundreds of kilometers in diameter. Some of the largest asteroids are even classified as dwarf planets, such as Ceres.
- Meteoroid: Meteoroids are much smaller than asteroids. They can vary in size from a few millimeters to several meters in diameter. Meteoroids are typically significantly smaller than asteroids.
2. Location:
- Asteroid: Asteroids are primarily found in the asteroid belt, which is a region located between the orbits of Mars and Jupiter. However, asteroids can also be found elsewhere in the solar system, including near-Earth orbits.
- Meteoroid: Meteoroids can be found throughout the solar system. They are not confined to a specific region like the asteroid belt. Some meteoroids are part of asteroid families or are remnants of comets.
3. Composition:
- Asteroid: Asteroids are composed of various materials, including rock, metal, and sometimes even ice. Their composition can vary depending on their location and origin within the solar system.
- Meteoroid: Meteoroids can also have diverse compositions, but they are generally smaller and less massive than asteroids. Some meteoroids are composed of rock, while others may contain metal or other materials.
4. Origin:
- Asteroid: Asteroids are believed to be remnants from the early solar system’s formation, similar to planets. They are often considered leftover building blocks that never coalesced into full-fledged planets.
- Meteoroid: Meteoroids can have various origins. Some are fragments of asteroids or comets that have broken apart due to collisions or other forces. Others may be ejected material from the Moon or Mars.
5. Movement:
- Asteroid: Asteroids generally follow predictable orbits around the Sun. Their movements are influenced by gravitational forces, and they do not exhibit the rapid changes in brightness associated with meteoroids.
- Meteoroid: Meteoroids move through space at various speeds and trajectories. When they enter Earth’s atmosphere, they are often referred to as meteors or shooting stars. These meteoroids produce visible streaks of light due to their high-speed entry.
6. Visibility:
- Asteroid: Asteroids are not typically visible to the naked eye from Earth without the aid of telescopes. They remain distant and relatively stable in their orbits.
- Meteoroid: Meteoroids become visible when they enter Earth’s atmosphere and create bright streaks of light called meteors. If a meteoroid survives its journey through the atmosphere and reaches the Earth’s surface, it is referred to as a meteorite.
Read More
- Class 6 Maths Question Paper With Solutions NCERT PDF
- Sample Question Paper For Class 6 CBSE English Grammar
- NCERT Maths Book Class 6 Solutions PDF Free Download
Frequently Asked Question (FAQs) Difference Between Asteroid And Meteoroid
1. What is the main difference between asteroids and meteoroids?
The main difference between asteroids and meteoroids is their size. Asteroids are relatively larger celestial bodies, ranging from a few meters to hundreds of kilometers in diameter, while meteoroids are much smaller, typically measuring from a few millimeters to several meters.
2. Where are asteroids primarily located in the solar system?
Asteroids are primarily located in the asteroid belt, which is a region between the orbits of Mars and Jupiter. However, asteroids can also be found elsewhere in the solar system, including near-Earth orbits.
3. Do meteoroids have a specific location in the solar system like the asteroid belt?
No, meteoroids do not have a specific region in the solar system like the asteroid belt. They can be found throughout the solar system and are not confined to a particular area.
4. What are asteroids made of?
Asteroids are composed of various materials, including rock, metal, and sometimes ice. Their composition can vary depending on their location and origin within the solar system.
5. What materials are meteoroids typically composed of?
Meteoroids can have diverse compositions, but they are generally smaller and less massive than asteroids. Some meteoroids are composed of rock, while others may contain metal or other materials.
Types Of Chemical Bonding
Types Of Chemical Bonding: Chemical bonding is a fundamental concept in chemistry that helps us understand how atoms combine to form molecules and compounds.
It governs the physical and chemical properties of matter and is the basis for the diversity of substances we encounter in our daily lives. In this article, we will delve into the different types of chemical bonding and their significance in the world of chemistry.

Types Of Chemical Bonding
1. Ionic Bonding
Ionic bonding occurs when one or more electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges. This type of bonding typically occurs between metals and nonmetals. The metal loses electrons to become a positively charged cation, while the nonmetal gains those electrons to become a negatively charged anion. The strong electrostatic attraction between these oppositely charged ions holds them together in ionic compounds.
Significance: Ionic compounds, such as table salt (sodium chloride, NaCl) and calcium carbonate (CaCO3), are vital in various applications, including in the food industry, medicine, and the production of ceramics and glass.
2. Covalent Bonding
Covalent bonding results from the sharing of electrons between atoms, typically between nonmetals. In a covalent bond, atoms share one or more pairs of electrons, resulting in the formation of molecules. This type of bonding is characterized by the strong attraction between the shared electrons and the positively charged nuclei of the atoms involved.
Significance: Covalent compounds, like water (H2O) and methane (CH4), are widespread in nature and have diverse uses, from serving as essential compounds for life to forming the basis of many organic chemicals.
3. Metallic Bonding
Metallic bonding occurs in metals, where atoms within a solid lattice structure share their valence electrons freely. This sharing of electrons creates a “sea of electrons” that moves throughout the structure, giving metals their unique properties such as electrical conductivity and malleability.
Significance: Metallic bonds are crucial in the construction of electrical wires, the production of structural materials, and the formation of alloys, which have applications in aerospace, construction, and manufacturing.
4. Hydrogen Bonding
Hydrogen bonding is a special type of dipole-dipole interaction that occurs when a hydrogen atom is covalently bonded to a highly electronegative atom (usually oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom in a nearby molecule. This bond is responsible for the unique properties of water, such as its high boiling point and surface tension.
Significance: Hydrogen bonding is essential in biology, contributing to the structure of DNA, the folding of proteins, and the properties of water that support life.
5. Van der Waals Forces
Van der Waals forces, also known as London dispersion forces and dipole-dipole interactions, are relatively weak attractions that exist between all molecules, regardless of their polarity. These forces are caused by temporary fluctuations in electron distribution within molecules, creating temporary positive and negative charges that attract nearby molecules.
Significance: Van der Waals forces play a significant role in the behavior of gases, the condensation of liquids, and the adsorption of molecules onto surfaces, influencing phenomena like adhesion and cohesion.
6. Coordinate Covalent Bonding
In coordinate covalent bonding, also known as dative bonding, one atom donates a pair of electrons to be shared in a covalent bond with another atom. This type of bonding is commonly found in complex ions and coordination compounds.
Significance: Coordinate covalent bonds are essential in the formation of metal complexes, which are widely used in catalysis, as pigments, and in the study of coordination chemistry.
Understanding the various types of chemical bonding is fundamental to comprehending the behavior of matter, the properties of substances, and the countless applications of chemistry in our daily lives.
Read More
- Chemistry In Everyday Life
- Energy Stored In A Capacitor
- Compound Lenses Thin Lenses In Contact
- 12th Class English Book Question Answer Pdf Download
Frequently Asked Question (FAQs) Types Of Chemical Bonding
1. What is chemical bonding?
Chemical bonding is the process by which atoms combine to form molecules or compounds. It involves the sharing or transfer of electrons between atoms to achieve a stable electronic configuration.
2. What are the main types of chemical bonding?
The main types of chemical bonding are ionic bonding, covalent bonding, metallic bonding, hydrogen bonding, Van der Waals forces, and coordinate covalent bonding.
3. What is ionic bonding, and how does it work?
Ionic bonding occurs when one atom transfers electrons to another, resulting in the formation of ions with opposite charges. The electrostatic attraction between these oppositely charged ions holds them together in an ionic compound.
4. Can you provide examples of substances with ionic bonding?
Examples of substances with ionic bonding include table salt (sodium chloride, NaCl), calcium carbonate (CaCO3), and magnesium oxide (MgO).
5. What is covalent bonding, and how does it differ from ionic bonding?
Covalent bonding involves the sharing of electrons between atoms, typically between nonmetals. In contrast to ionic bonding, where electrons are transferred, covalent bonds result from the mutual attraction between shared electrons and the positively charged nuclei of the atoms involved.
Chemistry In Everyday Life
Chemistry in everyday life: Chemistry is not confined to laboratory experiments and chemical equations; it is an integral part of our everyday lives.

Chemistry In Everyday Life
Medicine and Healthcare: Chemistry plays a pivotal role in the development of pharmaceuticals and medical treatments. From pain relievers to antibiotics, chemistry enables the creation of life-saving drugs that combat diseases and alleviate suffering.
Food and Nutrition: Chemistry helps us understand the composition of the food we consume. It involves processes like cooking, baking, and fermentation, which transform raw ingredients into flavorful and nutritious meals.
Cleaning and Hygiene: Household cleaning products, detergents, and disinfectants are formulated using chemical principles. They aid in maintaining cleanliness and preventing the spread of infections.
Cosmetics and Personal Care: Chemistry is behind the creation of cosmetics, shampoos, soaps, and skincare products. These items enhance our appearance and personal hygiene.
Environmental Protection: Understanding chemical processes in the environment is essential for addressing pollution, climate change, and conservation. Chemistry contributes to cleaner energy sources and sustainable technologies.
Water Purification: Chemical processes purify water, making it safe for drinking and everyday use. This is a critical aspect of public health.
Textiles and Apparel: Chemistry plays a role in fabric dyeing, textile manufacturing, and the creation of wrinkle-resistant and stain-repellent clothing.
Transportation: Chemistry is crucial in the development of fuels, lubricants, and materials used in transportation, making vehicles more efficient and eco-friendly.
Plastics and Packaging: Chemistry is responsible for producing versatile and lightweight materials like plastics, which are used in countless everyday items and packaging.
Electronics: Advances in materials science and semiconductor chemistry drive the development of electronic devices, making them smaller, faster, and more energy-efficient.
Agriculture and Food Production: Chemical fertilizers, pesticides, and herbicides boost crop yields and safeguard crops from pests and diseases. Soil chemistry and nutrient uptake are also areas where chemistry is vital.
Cooking and Baking: Everyday culinary activities involve chemical reactions like caramelization, Maillard reactions, and leavening, contributing to the delicious flavors and textures of our meals.
Energy Generation: Chemistry is central to energy production through processes such as combustion, nuclear reactions, and renewable energy technologies.
Aesthetics and Fragrances: Chemistry plays a significant role in creating fragrances, scents, and colors used in perfumes, cosmetics, and art.
Healthcare Diagnostics: Chemistry-based diagnostic tests, including blood tests and urinalysis, help healthcare professionals diagnose and monitor various medical conditions.
In summary, chemistry surrounds us and enriches our daily existence in countless ways. Its contributions to medicine, food, hygiene, the environment, and technology are evident throughout our lives, making it a vital and fascinating field of study and application.
Read More
- Energy Stored In A Capacitor
- Compound Lenses Thin Lenses In Contact
- 12th Class English Book Question Answer Pdf Download
Frequently Asked Question (FAQs) Chemistry In Everyday Life
1. What is the significance of chemistry in our daily lives?
Chemistry is essential in our daily lives because it helps us understand and improve various aspects of life, including health, nutrition, hygiene, energy production, and environmental conservation.
2. How does chemistry impact our health?
Chemistry plays a crucial role in healthcare by enabling the development of pharmaceuticals, diagnostics, and medical treatments that improve our well-being and combat diseases.
3. What are some common examples of chemical reactions in cooking and baking?
Common chemical reactions in cooking and baking include caramelization, the Maillard reaction (responsible for browning), and the leavening of dough in baking.
4. How does chemistry contribute to the food we eat?
Chemistry helps us understand food composition, preservation techniques, and flavor development. It’s involved in processes like fermentation, pasteurization, and food additives.
5. What are some environmentally significant applications of chemistry?
Chemistry helps address environmental challenges by contributing to clean energy technologies, waste management, and pollution control. It also aids in understanding climate change.
Molecular Weight Of NaOH
Molecular Weight Of NAOH: The Molecular Weight of NaOH (Sodium Hydroxide) Understanding its Significance in Chemistry Chemistry, the study of matter and its properties, often involves the measurement and analysis of substances at the molecular level.

Molecular Weight Of NaOH
Defining Molecular Weight:
Molecular weight is a measure of the mass of a molecule, and it is expressed in atomic mass units (amu) or grams per mole (g/mol). It provides valuable information about the composition of a substance by summing up the atomic masses of all the atoms present in a molecule.
The Composition of NaOH:
Sodium hydroxide (NaOH) is a chemical compound composed of three elements: sodium (Na), oxygen (O), and hydrogen (H). Its chemical formula, NaOH, tells us that each molecule of sodium hydroxide contains one atom of sodium (Na), one atom of oxygen (O), and one atom of hydrogen (H).
Calculating the Molecular Weight of NaOH:
To find the molecular weight of NaOH, we need to add up the atomic weights of its constituent elements:
- Sodium (Na) has an atomic weight of approximately 22.99 g/mol.
- Oxygen (O) has an atomic weight of roughly 16.00 g/mol.
- Hydrogen (H) has an atomic weight of approximately 1.01 g/mol.
Now, let’s calculate the molecular weight of NaOH:
Molecular Weight (NaOH) = Atomic Weight (Na) + Atomic Weight (O) + Atomic Weight (H)
Molecular Weight (NaOH) = 22.99 g/mol + 16.00 g/mol + 1.01 g/mol
Molecular Weight (NaOH) ≈ 40.00 g/mol
The Significance of Molecular Weight:
Understanding the molecular weight of a compound like NaOH is crucial in various aspects of chemistry:
- Stoichiometry: Molecular weight is used to calculate the number of moles of a substance in a given sample. This is essential for determining the proportions of reactants and products in chemical reactions.
- Molar Mass: Molecular weight is also referred to as molar mass, and it represents the mass of one mole of a substance. This value is used extensively in chemical calculations.
- Formulas and Empirical Formulas: Molecular weight helps in determining the chemical formula of a compound and its empirical formula, which represents the simplest whole number ratio of its constituent atoms.
- Concentration Calculations: In solutions, the molecular weight of a solute is used to calculate its concentration in terms of molarity (moles per liter).
- Chemical Analysis: Molecular weight aids in identifying and quantifying substances through techniques like mass spectrometry and chromatography.
Practical Applications of Sodium Hydroxide:
Sodium hydroxide (NaOH) is a versatile chemical with numerous applications in various industries. It is commonly used in:
- Chemical Manufacturing: NaOH is a key ingredient in the production of various chemicals, including soaps, detergents, and pharmaceuticals.
- Food Processing: It is used in food preparation and production, particularly in the manufacture of products like pretzels and chocolate.
- Water Treatment: NaOH is employed to adjust the pH of water and wastewater in water treatment plants.
- Cleaning Products: It is a vital component of household cleaning products, as it effectively dissolves grease and oils.
- Petroleum Industry: NaOH plays a role in refining petroleum products and removing impurities.
- Paper and Pulp Industry: It is used to break down lignin in wood fibers during the papermaking process.
Understanding the molecular weight of NaOH and its properties allows for precise and controlled use in these applications.
In Conclusion:
The moleculars weight of NaOH, approximately 40.00 g/mol, provides a fundamental insight into the composition of this chemical compound.
Read More
- Difference Between Force And Pressure
- Difference Between Diode And Rectifier
- Difference Between AM And FM
- Dielectric Material And Dipole Moment
- Difference Between Centre Of Gravity And Centroid
Frequently Asked Question (FAQs) Molecular Weight Of NaOH
What is the molecular weight of NaOH?
The moleculars weight of sodium hydroxide (NAOH) is approximately 40.00 grams per mole (g/mol). This value is calculated by adding the atomic weights of the constituent elements: sodium (Na), oxygen (O), and hydrogen (H).
Why is it important to know the molecular weight of NaOH?
Knowing the moleculars weight of NaOH is essential in chemistry and science in general because it helps in various calculations, including molar mass, stoichiometry, and determining the amount of a substance in a given sample.
How can I calculate the molecular weight of other compounds?
To calculate the molecular weight of any compound, add up the atomic weight of all the atoms in the compound according to their chemical formula. You can find atomic weights on the periodic table. Multiply the atomic weight of each element by the number of atoms of that element in the compound, and then sum these values.
Is the molecular weight of NaOH the same as its molar mass?
Yes, in chemistry, the terms “molecular weight” and “molar mass” are often used interchangeably. They both refer to the mass of one mole of a substance in grams. So, the moleculars weight of NaOH is also its molar mass.
Why is NaOH commonly referred to as “caustic soda”?
Sodium hydroxide (NaOH) is commonly known as “caustic soda” because it is a highly caustic and corrosive substance. It has the ability to burn or corrode various materials and is used in a wide range of industrial applications, including in the production of soaps, detergents, and cleaning agents.
Distance Time Velocity Time Graph
Distance Time Velocity Time Graph: A “Distance-Time Velocity-Time Graph,” often referred to as a “velocity-time graph” or “speed-time graph,” is a graphical representation that illustrates how an object’s velocity or speed changes over time. Here’s how to understand and interpret such a graph:

Distance Time Velocity Time Graph
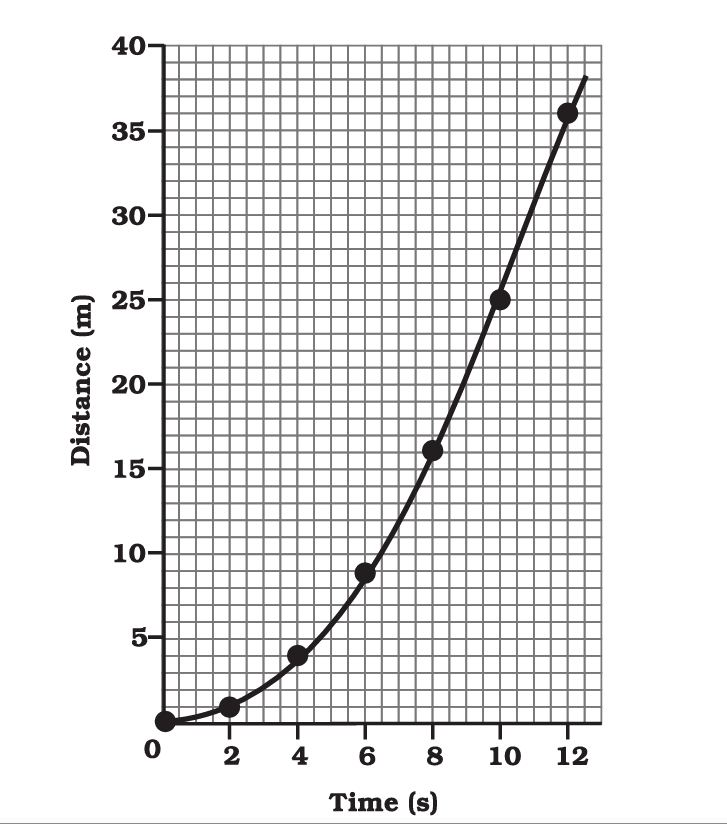
Distance-Time Graph:
- On the horizontal axis (x-axis), you’ll find time, typically measured in seconds (s).
- On the vertical axis (y-axis), you’ll find distance or displacement, typically measured in meters (m) or kilometers (km).
- A straight line on a distance-time graph represents constant velocity or speed. The slope of the line indicates the velocity.
- A horizontal line indicates that the object is at rest (not moving).
- An upward-sloping line indicates positive velocity (moving away from the starting point).
- A downward-sloping line indicates negative velocity (moving toward the starting point).
- Curved lines represent changing velocities or acceleration.
Velocity-Time Graph:
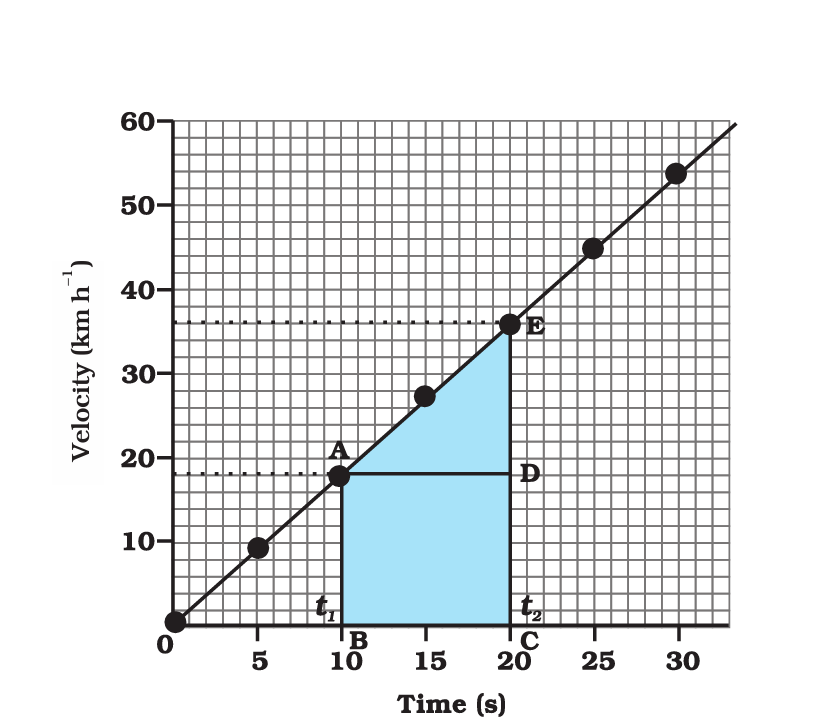
- On the horizontal axis (x-axis), you’ll find time, typically measured in seconds (s).
- On the vertical axis (y-axis), you’ll find velocity or speed, typically measured in meters per second (m/s) or kilometers per hour (km/h).
- A straight line on a velocity-time graph represents constant acceleration or deceleration.
- The slope of the line indicates acceleration (positive or negative) when the line is not horizontal.
- A horizontal line represents constant velocity (no acceleration).
- Areas under the curve represent displacement or distance traveled. The area between the graph and the time axis indicates the total distance covered.
- Points where the velocity-time graph crosses the time axis represent moments when the object is at rest (velocity = 0).
Interpreting the Graphs:
- On a distance-time graph, the steeper the slope, the greater the speed or velocity.
- On a velocity-time graph, the slope indicates acceleration. Positive slopes indicate acceleration in the positive direction, while negative slopes indicate acceleration in the negative direction.
- A horizontal line on a velocity-time graph implies constant speed.
- The area under a velocity-time graph curve represents the change in distance or displacement.
In summary, a distance-time graph shows how an object’s position changes with time, while a velocity-time graph reveals how an object’s velocity or speed changes with time.
Read More
- Average Speed And Average Velocity Class 9
- Differences Between Acceleration And Velocity
- Matter In Our Surroundings Class 9 Summary
- Class 9th Chapter 2 Science Question Answer of NCERT
- NCERT Physics Class 9 Chapter 1 Solutions PDF Download
Frequently Asked Question (FAQs) on Distance Time Velocity Time Graphs:
What is a distance-time graph, and how is it used?
A distance-time graph illustrates how an object’s position or distance changes over time. It’s used to visualize an object’s motion, calculate its speed, and understand whether it is moving, at rest, or changing its speed.
How do you interpret a straight line on a distance-time graph?
A straight line on a distance-time graph represents constant speed. The slope of the line indicates the speed, with a steeper slope representing a greater speed.
What does a horizontal line on a distance-time graph signify?
A horizontal line indicates that the object is at rest or not changing its position with time.
What is a velocity-time graph, and what does it show?
A velocity-time graph depicts how an object’s velocity (speed and direction) changes over time. It provides insights into an object’s acceleration, constant velocity, and moments of rest.
How is acceleration represented on a velocity-time graph?
Acceleration is represented by the slope of a line on a velocity-time graph. A positive slope indicates acceleration in the positive direction, while a negative slope represents acceleration in the negative direction.
Difference Between Kinetics And Kinematics
Difference Between Kinetics And Kinematics: Kinetics and kinematics are both subfields of physics that encompass the study of an object’s motion. While these terms may appear similar, they do exhibit distinct differences.

Difference Between Kinetics And Kinematics
Nature of Study:
Kinematics: Kinematics is the branch of physics that deals with the study of motion of objects without considering the forces causing that motion. It focuses on describing the position, velocity, and acceleration of objects as they move through space and time.
Kinetics: Kinetics, on the other hand, is the branch of physics that delves into the causes of motion. It is concerned with the analysis of the forces, torques, and energy transfers that lead to changes in an object’s motion.
Fundamental Concepts:
Kinematics: Kinematics primarily deals with concepts like displacement, velocity, acceleration, and time. It provides a framework for describing how objects move and change position relative to time.
Kinetics: Kinetics is centered on concepts like force, mass, inertia, work, energy, and momentum. It investigates how these factors interact to produce or change motion.
Representation:
Kinematics: Kinematics is often represented mathematically through equations and graphs that describe an object’s motion in terms of position, velocity, and acceleration as functions of time.
Kinetics: Kinetics involves the use of Newton’s laws of motion, equations of motion, and various mathematical principles to analyze and predict the forces acting on objects and their resulting motion.
Key Questions:
Kinematics: Kinematics answers questions like “What is the object’s position at a given time?” or “How fast is the object moving?” It focuses on the “what” and “how” of motion.
Kinetics: Kinetics addresses questions like “Why is the object accelerating?” or “What force is causing the object to move?” It investigates the underlying causes and the “why” of motion.
Example:
Kinematics: When describing the motion of a car on a highway, kinematics would provide information about the car’s speed, its change in position over time, and how its velocity is changing.
Kinetics: Kinetics, in the context of the same car, would analyze the forces acting on it, such as engine force, friction, and air resistance, to explain why the car is accelerating, decelerating, or maintaining a constant speed.
In summary, kinematics is concerned with describing the motion of objects and their geometric characteristics (position, velocity, acceleration) without considering the underlying forces.
Kinetics, on the other hand, focuses on understanding the forces and interactions that cause objects to move or change their state of motion. Both branches are essential in the study of mechanics and the analysis of physical systems.
Read More
- Changing States Of Matter Class 9
- Average Speed And Average Velocity Class 9
- Differences Between Acceleration And Velocity
- Matter In Our Surroundings Class 9 Summary
- Class 9th Chapter 2 Science Question Answer of NCERT
Frequently Asked Question (FAQs)
What is the primary focus of kinematics?
Kinematics primarily focuses on describing the motion of objects, including parameters such as position, velocity, acceleration, and time, without considering the forces causing that motion.
How does kinetics differ from kinematics in terms of focus?
Kinetics is concerned with understanding the causes of motion, particularly the forces, torques, and energy transfers that lead to changes in an object’s motion.
Can you provide an example illustrating the difference between kinetics and kinematics?
Certainly. Consider a car moving along a road. Kinematics would describe its speed, position changes, and acceleration patterns. Kinetics, on the other hand, would analyze the forces responsible for the car’s motion, such as engine force, friction, and air resistance.
What are some fundamental concepts in kinematics?
In kinematics, fundamental concepts include displacement, velocity, acceleration, and time. These concepts help describe how objects move without delving into the causes of motion.
What key concepts are involved in kinetics?
Kinetics involves concepts like force, mass, inertia, work, energy, and momentum. It investigates how these factors interact to produce or change motion and explores the underlying causes of motion.