Blog
Molecular Weight Of Air
Molecular Weight Of Air: Air, the invisible mixture of gases that surrounds our planet, plays a vital role in sustaining life. While it may seem weightless, it does indeed have a molecular weight. In this article, we will explore the concept of the mole weight of air, its composition, and its significance in various scientific and practical applications.

Molecular Weight Of Air
Composition of Air:
The predominant components of air are the two major gases: nitrogen (N2) and oxygen (O2). These two gases make up the majority of the Earth’s atmosphere, accounting for approximately 99% of the total volume. Other gases, such as carbon dioxide (CO2), argon (Ar), neon (Ne), helium (He), and traces of other elements and compounds, make up the remaining 1%.
Calculating the Molecular Weight of Air:
To calculate the mole weight of air, we must consider the molecular weights of its constituent gases and their relative abundances. Here are the key steps to determine the mole weight of air:
- Identify the Constituent Gases: As mentioned earlier, nitrogen (N2) and oxygen (O2) are the two most abundant gases in the atmosphere. Their molecular weights are approximately 28 g/mol (for N2) and 32 g/mol (for O2).
- Determine the Relative Abundances: The Earth’s atmosphere consists of approximately 78% nitrogen (N2) and 21% oxygen (O2). The remaining gases make up the remaining 1%.
- Calculate the Weighted Average: To find air’s molecular weight, calculate a weighted average using the molecular weights and relative gas abundances.
Using this formula:
mole Weight of Air (g/mol) = (Abundance of N2 × mole Weight of N2) + (Abundance of O2 × mole Weight of O2) + …
mole Weight of Air (g/mol) = (0.78 × 28 g/mol) + (0.21 × 32 g/mol) + …
The calculated mole weight of air is approximately 28.97 g/mol.
Significance of Molecular Weight of Air:
- Avogadro’s Law: The mole weight of air is crucial for understanding Avogadro’s law, which states that equal volumes of gases, at the same temperature and pressure, contain an equal number of molecules. This principle is essential in the study of gases.
- Gas Laws: Knowledge of the mole weight of air is essential in various gas laws, such as the ideal gas law (PV = nRT), where it helps in calculating the properties of gases under different conditions.
- Atmospheric Science: Understanding the mole weight of air is vital in atmospheric science, including weather forecasting and the study of air pollutants.
- Aeronautics and Aviation: In aviation, engineers and pilots use air’s molecular weight to evaluate aircraft performance, including lift and thrust.
Conclusion:
The mole weight of air, approximately 28.97 g/mol, provides a foundational understanding of the Earth’s atmosphere. It is a critical factor in various scientific fields, including chemistry, physics, atmospheric science, and engineering. Comprehending air’s composition and properties aids in grasping natural phenomena and advancing technologies dependent on gas behavior in our environment.
Read More
- Molecular Weight Of K2Cr2O7
- Molecular Weight Of Phosphorus
- Molecular Weight Of SO2
- Molecular Weight of Na2CO3
- Molecular Mass Of Sodium
Frequently Asked Questions (FAQs) Molecular Weight Of Air
Q1: What is the molecular weight of air?
A1: The mole weight of air is approximately 28.97 grams per mole (g/mol). This value is calculated based on the molecular weights and relative abundances of its constituent gases, primarily nitrogen (N2) and oxygen (O2).
Q2: Why is it important to know the molecular weight of air?
A2: Understanding the mole weight of air is important in various scientific fields and practical applications. It helps in gas law calculations, atmospheric science, aeronautics, and aviation, among others. It also serves as a fundamental concept in the study of gases.
Q3: How is the molecular weight of air calculated?
A3: The mole weight of air is calculated by considering the mole weights of its constituent gases (e.g., nitrogen and oxygen) and their relative abundances in the atmosphere. It involves a weighted average calculation based on these factors.
Q4: Does the molecular weight of air change with altitude or location?
A4: The mole weight of air remains relatively constant at different altitudes and locations on Earth. However, variations in the composition of air, such as changes in the concentration of water vapor or pollutants, can cause minor fluctuations in the molecular weight of air.
Q5: How is the molecular weight of air used in aviation and aeronautics?
A5: In aviation and aeronautics, the mole weight of air is used to determine aircraft performance, including lift, thrust, and engine efficiency. It plays a crucial role in calculations related to aircraft design and operation.
Molar Mass Of Ag
Molar Mass Of Ag: In the realm of chemistry, the concept of molar mass is fundamental. It is a crucial parameter that helps chemists determine the quantity of a substance in a given sample, balance chemical equations, and predict reaction outcomes.
In this article, we will delve into the molar mass of silver (Ag) and explore its significance in the world of chemistry.

Molar Mass Of Ag
Silver (Ag) – A Brief Overview:
Silver is a chemical element with the symbol Ag and atomic number 47. It is a lustrous, white metal known for its excellent electrical conductivity, ductility, and malleability. Silver boasts a millennia-old legacy, serving diverse roles such as currency, adornment, and photographic material throughout history.
Determining the Molar Mass of Silver:
The molar mass of an element is defined as the mass of one mole of that element, expressed in grams per mole (g/mol). To calculate the molar mass of silver (Ag), we need to consider its atomic mass, which can be found on the periodic table. The atomic mass of silver is approximately 107.87 atomic mass units (amu).
In chemical calculations, the atomic mass is converted to grams per mole (g/mol), resulting in silver’s (Ag) molar mass of approximately 107.87 g/mol.
Significance of Molar Mass in Chemistry:
- Stoichiometry: Molar mass is essential in stoichiometry, which deals with the quantitative relationships between reactants and products in chemical reactions. It helps in determining the amount of substances involved in a reaction.
- Empirical and Molecular Formulas: Molar mass is used to find the empirical and molecular formulas of compounds. By comparing the molar mass of a compound with its elemental composition, chemists can deduce the ratios of atoms and determine its formula.
- Concentration Calculations: Molar mass is crucial for calculating concentrations of solutions. For example, it finds application in determining the molarity (M) of a solution, which represents moles of solute per liter of solution.
- Gas Laws: In gas law calculations, including the ideal gas law (PV = nRT), molar mass is essential for connecting gas mass, moles, and volume.
- Chemical Reactions: Molar mass is pivotal for balancing chemical equations, maintaining the law of mass conservation.
Applications of Silver (Ag) in Chemistry:
Silver has various applications in chemistry, including:
- Photography: Silver compounds in traditional photography undergo chemical reactions to create images.
- Electronics: Silver’s conductivity makes it valuable in electronic components, including conductive pastes and circuit boards.
- Medicine: Silver compounds are antimicrobial, finding use in medical applications such as wound dressings and surgical instruments.
Conclusion:
Silver’s (Ag) molar mass is a foundational concept in chemistry, indispensable for numerous chemical calculations and practical applications. Comprehending the molar mass of elements, such as silver, is vital for precise chemical analysis and advancing products and technologies.
Read More
- Molar Mass Of K
- Molar Mass Of Magnesium
- Law Of Conservation Of Energy
- Difference Between Equinox And Solstice
- Molecular Weight Of K2Cr2O7
Frequently Asked Questions (FAQs) On Molar Mass Of Ag
Q1: What is the molar mass of silver (Ag)?
A1: The molar mass of silver (Ag) is approximately 107.87 grams per mole (g/mol).
Q2: Why is the molar mass of silver important in chemistry?
A2: Silver’s molar mass is pivotal in chemistry for determining silver quantities in reactions, solution concentrations, and deriving silver compound formulas.
Q3: How is the molar mass of silver calculated?
A3: Calculate silver’s molar mass by summing the atomic masses of all atoms in a mole of silver (Ag). The atomic mass of silver, approximately 107.87 atomic mass units (amu), is readily available on the periodic table.
Q4: What is the significance of molar mass in stoichiometry?
A4: Molar mass is essential in stoichiometry, as it helps determine the quantity of substances involved in chemical reactions. It allows chemists to balance chemical equations and predict reaction outcomes accurately.
Q5: Are there any practical applications of silver (Ag) in chemistry beyond its molar mass?
A5: Yes, silver has various applications in chemistry, including its use in traditional photography, electronics due to its excellent conductivity, and medicine for its antimicrobial properties in wound dressings and medical instruments.
Molar Mass Of Potassium (K)
Molar Mass Of K: In the world of chemistry, one of the fundamental concepts is the molar mass of an element. It is essential in diverse chemical computations, encompassing substance quantification in samples and forecasting reaction results.
This article delves into potassium’s (K) molar mass, examining its importance within the realm of chemistry and its various applications.
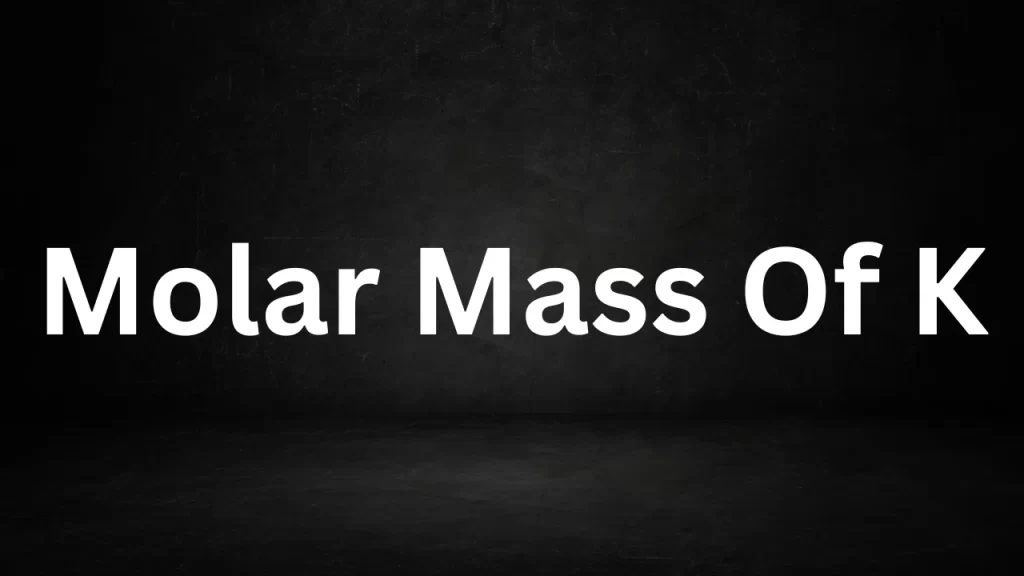
Molar Mass Of K
Potassium (K) – A Brief Overview:
Potassium, symbolized as K with atomic number 19, is classified within the alkali metal group on the periodic table. This element holds significant importance as an essential nutrient vital for the well-being of all living organisms, including humans. Potassium ions (K+) play a pivotal role in various physiological processes, such as nerve signal transmission, muscle contractions, and cellular functionality, making them indispensable in the realm of biology.
Determining the Molar Mass of Potassium:
The molar mass of an element is the mass of one mole of that element, in grams per mole (g/mol). To calculate potassium’s molar mass, we use its atomic mass, found on the periodic table, approximately 39.10 atomic mass units (amu).
However, in chemical calculations, we usually consider the molar mass of potassium as 39.10 g/mol. This is because the atomic mass unit (amu) is a very small unit of measurement, and it is more convenient to work with grams per mole in laboratory settings and chemical equations.
The molar mass of potassium (K) is, therefore, 39.10 g/mol.
Significance of Molar Mass in Chemistry:
- Stoichiometry: Molar mass is crucial in stoichiometry, the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It helps in determining the amount of reactants needed or products formed during a chemical reaction.
- Empirical and Molecular Formulas: Molar mass aids in deducing the empirical and molecular formulas of compounds by comparing it with elemental composition. This reveals atom ratios and compound formulas.
- Concentration Calculations: In solutions and mixtures, molar mass is essential for calculating concentrations. For example, it is employed in calculating the molarity (M) of a solution, defined as the moles of solute per liter of solution.
- Gas Laws: Molar mass is crucial in gas law calculations, such as the ideal gas law (PV = nRT). It helps convert between mass, moles, and volume when dealing with gases.
- Chemical Reactions: Molar mass is instrumental in achieving balance in chemical equations, ensuring compliance with the law of conservation of mass.
Applications of Potassium (K) in Chemistry:
Aside from its importance in understanding molar mass, potassium itself has various applications in chemistry. Some notable examples include:
- Potassium Hydroxide (KOH): Used in the manufacture of soap and as a strong base in chemical processes.
- Potassium Nitrate (KNO3): Used in fireworks, fertilizers, and as a food preservative.
- Potassium Permanganate (KMnO4): A powerful oxidizing agent used in various chemical reactions and water treatment.
Conclusion:
Potassium’s (K) molar mass is a foundational concept in chemistry, with a pivotal role in multiple aspects of the field. Understanding the molar mass of elements is essential for accurate chemical calculations, stoichiometry, and the study of chemical reactions. Potassium’s molar mass, 39.10 g/mol, is significant not just in chemistry but also vital as a nutrient for all living organisms.
Read More
- Molar Mass Of Magnesium
- Law Of Conservation Of Energy
- Difference Between Equinox And Solstice
- Molecular Weight Of K2Cr2O7
- Molecular Weight Of Phosphorus
Frequently Asked Questions (FAQs) On Molar Mass Of K
Q1: What is molar mass?
A1: Molar mass, measured in grams per mole (g/mol), represents the mass of a single mole of a substance. This foundational concept in chemistry plays a pivotal role in various calculations, encompassing stoichiometry and the determination of substance quantities within samples.
Q2: What is the molar mass of potassium (K)?
A2: The molar mass of potassium (K) is approximately 39.10 grams per mole (g/mol). This value is commonly used in chemical calculations and is found on the periodic table.
Q3: Why is molar mass important in chemistry?
A3: Molar mass is important in chemistry because it allows chemists to relate the mass of a substance to the number of moles of that substance. It is essential for stoichiometry, determining chemical formulas, calculating concentrations, and balancing chemical equations.
Q4: How is the molar mass of an element determined?
A4: The molar mass of an element is calculated by adding up the atomic masses of all the atoms in one mole of that element. This information is typically found on the periodic table and is expressed in atomic mass units (amu). To apply it in chemical computations, the atomic mass is converted to grams per mole (g/mol).
Q5: Is the molar mass of potassium used only in chemistry?
A5: Although potassium’s molar mass is predominantly utilized in chemistry, potassium has diverse applications beyond the field. For instance, potassium compounds are integral to fireworks, fertilizers, and soap production.
Molar Mass Of Magnesium
Molar Mass Of Magnesium: Magnesium, a versatile and essential element, holds a significant place in the periodic table.
Its role in various natural processes, industrial applications, and biological systems makes it a subject of great interest in the world of chemistry. In this article, we’ll explore the concept of the molar mass of magnesium, understand its significance, and uncover the atomic weight of this mighty element.
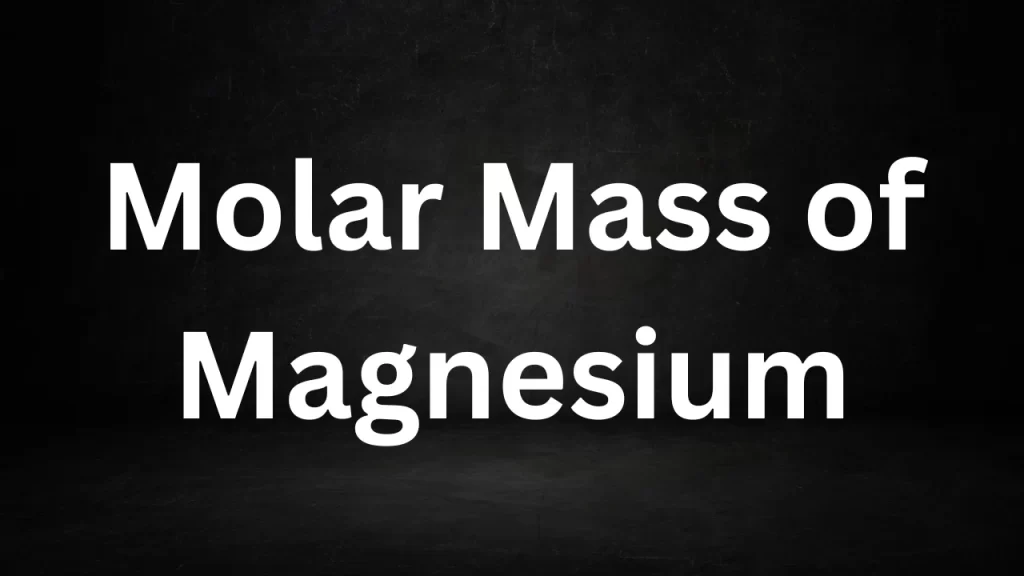
Molar Mass Of Magnesium
Understanding Molar Mass
Before we delve into the specifics of magnesium, let’s establish what molar mass signifies. Molar mass, often referred to as molecular weight or atomic weight, is a fundamental concept in chemistry. It represents the mass of a substance in grams per mole (g/mol) and is expressed in unified atomic mass units (u) or atomic mass units (amu).
The molar mass is calculated by summing the atomic masses of all the atoms in a chemical compound, considering their respective proportions. It serves as a bridge between the microscopic world of atoms and molecules and the macroscopic world of measurable quantities in chemistry.
The Atomic Structure of Magnesium
Magnesium, with the atomic symbol Mg and atomic number 12, is a light, silvery-white, and highly abundant element in the Earth’s crust. It falls within Group 2 (Group IIA) on the periodic table, a category recognized as the alkaline earth metals. Magnesium’s atomic configuration exhibits the following features:
Atomic Number: 12 (indicating the number of protons in the nucleus)
- Atomic Mass: Approximately 24.31 atomic mass units (u)
- Electron Configuration: [Ne] 3s² (indicating its electron arrangement)
- Number of Electrons: 12 (equal to the number of protons in a neutral atom)
Calculating the Molar Mass of Magnesium
The mol mass of magnesium is determined by summing the atomic mass of a single magnesium atom. Since magnesium exists as individual atoms in its elemental form (Mg), the mol mass is equal to its atomic mass. The atomic mass of magnesium is approximately 24.31 u.
Therefore, the mol mass of magnesium (Mg) is approximately 24.31 g/mol. This numerical value finds application in diverse chemical calculations, including the determination of magnesium quantities in a given sample and assessing the composition of chemical compounds containing magnesium atoms.
Significance of the Molar Mass of Magnesium
The mol mass of magnesium is of great importance in several domains:
1. Chemical Reactions: In stoichiometry, the molar mass plays a vital role in determining the quantities of reactants and products involved in chemical reactions. It enables chemists to balance chemical equations and calculate the mass of substances consumed or produced.
2. Laboratory Analysis: When conducting experiments involving magnesium, scientists and researchers need to know its molar mass to make precise measurements and ensure accurate results.
3. Industrial Applications: Industries that utilize magnesium and its compounds, such as the automotive and aerospace sectors, rely on the molar mass for quality control and product development.
4. Environmental Studies: Environmental scientists use the molar mass of magnesium to analyze soil composition, study groundwater chemistry, and assess the impact of magnesium-containing substances on ecosystems.
5. Dietary Considerations: Magnesium is an essential mineral in the human diet, and its molar mass is relevant for nutritional assessments and dietary recommendations.
Conclusion: Magnesium’s Atomic Weight Unveiled
In conclusion, the molar mass of magnesium, approximately 24.31 g/mol, is a fundamental parameter in chemistry. It represents the atomic weight of this versatile element, empowering scientists, researchers, and industries to explore its diverse applications. Beyond the lab, magnesium’s importance extends to advancing science, technology, and enhancing our comprehension of the natural world.
Read More
- Law Of Conservation Of Energy
- Difference Between Equinox And Solstice
- Molecular Weight Of K2Cr2O7
- Molecular Weight Of Phosphorus
- Molecular Weight Of SO2
Frequently Asked Questions (FAQs) Molar Mass Of Magnesium
1. What is the molar mass of magnesium?
The mol mass of magnesium (Mg) is approximately 24.31 grams per mole (g/mol).
2. How is the mol mass of magnesium calculated?
The mol mass of magnesium is calculated by summing the atomic mass of a single magnesium atom. In the case of magnesium, the atomic mass is approximately 24.31 atomic mass units (u), which is equivalent to 24.31 g/mol.
3. Why is the mol mass of magnesium important in chemistry?
Magnesium’s molar mass is vital for chemical calculations like stoichiometry, aiding in balancing equations and precisely measuring reactants and products.
4. Is the mol mass of magnesium used in industries and manufacturing processes?
Yes, industries that utilize magnesium or magnesium-containing compounds rely on the mol mass for quality control, product development, and ensuring the proper composition of materials.
5. How does the mol mass of magnesium impact environmental studies?
Environmental scientists use the mol mass of magnesium to analyze soil composition, assess groundwater chemistry, and study the environmental impact of magnesium-containing substances.
Chemistry In Everyday Life
Chemistry In Everyday Life: Chemistry, often referred to as the central science, plays a crucial role in shaping the world around us. It is the science that explores the properties, composition, and transformations of matter.
While many people associate chemistry with laboratories and complex equations, the truth is that chemistry is an integral part of our daily lives, influencing everything from the food we eat to the medicines we take and the materials that surround us.
In this article, we will delve into the fascinating world of chemistry in everyday life, exploring its applications, impact, and significance.

Chemistry In Everyday Life
The Chemistry of Food: From Farm to Fork
Let’s begin our journey by exploring the role of chemistry in the food we consume every day. Chemistry is intimately connected to food production, preservation, and flavor enhancement.
1. Food Production: The journey of food from farm to table involves various chemical processes. Farmers rely on chemistry to optimize crop yields, utilizing fertilizers to provide essential nutrients to plants. Pesticides and herbicides are chemical compounds designed to protect crops from pests and weeds.
2. Food Preservation: Chemistry is the backbone of food preservation methods. Refrigeration, freezing, and canning are all based on chemical principles that slow down or inhibit the growth of microorganisms and enzymes that can spoil food. Additionally, additives like preservatives and antioxidants are used to extend the shelf life of many food products.
3. Flavor Chemistry: The taste and aroma of food are strongly influenced by chemistry. The interaction between various molecules in food and our taste receptors on the tongue creates the sensations of sweetness, sourness, bitterness, saltiness, and umami. Aromas are the result of volatile organic compounds, and the chemistry of spices and seasonings adds depth and complexity to culinary experiences.
Medicine and Healthcare: Healing through Chemistry
Chemistry plays a central role in the field of medicine and healthcare. From the development of life-saving drugs to diagnostic tests and medical imaging, chemistry impacts every aspect of healthcare.
1. Drug Discovery: Pharmaceutical chemistry focuses on the design, synthesis, and testing of drugs. Chemists work to create compounds that target specific diseases by understanding the chemical processes within the human body. This field has led to the development of antibiotics, painkillers, and treatments for chronic illnesses.
2. Diagnostic Chemistry: Clinical chemistry and medical testing rely on chemistry to detect and measure biomarkers, such as blood glucose levels or cholesterol levels, that provide critical information about a patient’s health. Diagnostic tests, such as blood tests and urine analysis, are based on chemical reactions and molecular interactions.
3. Imaging Technologies: Advanced imaging technologies like MRI (Magnetic Resonance Imaging) and CT (Computed Tomography) scans rely on the principles of nuclear magnetic resonance and X-ray technology, which are rooted in chemistry. These technologies enable non-invasive visualization of the human body’s internal structures.
Household Chemistry: Cleanliness and Comfort
In our daily lives, we encounter numerous household products that owe their effectiveness to chemistry. Cleaning agents, personal care products, and materials in our homes all have chemistry at their core.
1. Cleaning Agents: Household cleaners and detergents contain chemical compounds designed to break down and remove dirt, stains, and unwanted microorganisms. Surfactants, enzymes, and disinfectants are examples of chemicals used in cleaning products.
2. Personal Care Products: From shampoo and soap to toothpaste and deodorants, personal care products are formulated using chemistry to ensure product safety, stability, and efficacy. Chemistry is also responsible for the pleasant scents and textures of these products.
3. Materials Science: Chemistry contributes to the development of materials used in construction, electronics, and everyday items. Polymers, composites, and nanomaterials are all products of chemical research and engineering.
Environmental Chemistry: Sustaining Our Planet
As we grapple with environmental challenges, chemistry plays a vital role in addressing pollution, waste management, and the development of sustainable technologies.
1. Pollution Control: Environmental chemistry focuses on understanding and mitigating the impact of pollutants on ecosystems. Chemical processes are used to remove contaminants from air and water, improving environmental quality.
2. Waste Management: Chemistry informs waste reduction and recycling efforts. Chemical reactions and processes can transform waste into valuable resources or safely dispose of hazardous materials.
3. Sustainable Technologies: Green chemistry seeks to develop environmentally friendly products and processes. It emphasizes reducing the use of hazardous substances and minimizing waste in manufacturing and other industries.
Chemistry at Work: Industry and Innovation
Chemistry is a driving force behind industrial processes and innovations. It fuels technological advancements and economic growth.
1. Industrial Chemistry: The chemical industry produces a wide range of products, including chemicals, plastics, pharmaceuticals, and fuels. Chemistry is integral to manufacturing processes, quality control, and product development.
2. Energy Production: Chemistry is at the heart of energy production, from the combustion of fossil fuels to the development of renewable energy technologies such as solar cells and batteries.
3. Research and Innovation: Scientific research, including chemistry, drives innovation across industries. Breakthroughs in chemistry have led to the development of new materials, technologies, and scientific understanding.
Education and Scientific Advancement
Chemistry education is essential for fostering scientific literacy and training the next generation of scientists and innovators. It provides a foundational understanding of the scientific method, critical thinking, and problem-solving skills.
1. Science Education: Chemistry is a fundamental subject in science education curricula worldwide. It introduces students to the scientific method, laboratory techniques, and the principles that govern matter and energy.
2. Scientific Research: Chemistry research contributes to our understanding of the natural world and leads to discoveries that benefit society. Scientific advancements in chemistry have the potential to address global challenges, from climate change to disease prevention.
Conclusion: Embracing the Chemistry of Everyday Life
In conclusion, chemistry is not confined to laboratories or academic textbooks; it is woven into the fabric of our daily lives. From the food we eat to the medicines we take, from the products we use to the air we breathe, chemistry is the invisible force that shapes our world. Embracing the chemistry of everyday life allows us to make informed choices, promote sustainability, and appreciate the beauty of the natural world at the molecular level. It is a reminder that science is not an abstract concept but a tangible and transformative force that surrounds us every day.
Read More
- Difference Between Diffraction And Interference
- Alcohols Phenols And Ethers Class 12
- Alcohols Phenols And Ethers
- Effect Of Magnet On Current Carying Wire
- Simple Harmonic Motion Formulas
Frequently Asked Questions (FAQs) On Chemistry In Everyday Life
1. What is the significance of chemistry in our daily lives?
Chemistry is crucial in understanding and improving various aspects of daily life, including food production, healthcare, cleaning, personal care products, environmental sustainability, and technological advancements.
2. How does chemistry impact the food we consume?
Chemistry influences food production, preservation, flavor enhancement, and nutritional analysis. It plays a role in creating safe, delicious, and nutritious food products.
3. What is the role of chemistry in healthcare and medicine?
Chemistry is vital for drug discovery, diagnostic tests, medical imaging, and the development of pharmaceuticals and medical treatments that save lives and improve health.
4. What household products rely on chemistry for their effectiveness?
Cleaning agents, personal care products (such as shampoos and soaps), and materials used in construction and electronics all incorporate chemistry to function effectively.
5. How does chemistry contribute to environmental sustainability?
Chemistry is essential for addressing pollution, waste management, and the development of sustainable technologies, including renewable energy sources and green chemistry practices.
Molecular Weight Of Sodium Carbonate
Molecular Weight Of Sodium Carbonate: Sodium carbonate, commonly known as soda ash or washing soda, is a chemical compound with the molecular formula Na2CO3.
Understanding its molecular weight is fundamental in various fields, including chemistry, industry, and environmental science.
In this article, we delve into the concept of molecular weight and explore the significance of knowing the molecular weight of sodium carbonate.

Molecular Weight Of Sodium Carbonate
Definition of Molecular Weight
Molecular weight, also known as molar mass or molecular mass, is a measure of the mass of a molecule. It is defined as the sum of the atomic weights (masses) of all the atoms in a molecule. The unit of molecular weight is the atomic mass unit (amu) or unified atomic mass unit (u). For convenience, molecular weights are often expressed in grams per mole (g/mol).
Calculating the Molecular Weight of Sodium Carbonate
To calculate the molecular weight of sodium carbonate (Na2CO3), we need to sum the atomic weights of all the constituent atoms:
- Sodium (Na) has an atomic weight of approximately 22.99 amu.
- Carbon (C) has an atomic weight of roughly 12.01 amu.
- Oxygen (O) has an atomic weight of about 16.00 amu.
Now, let’s calculate the molecular weight:
Molecular Weight of Na2CO3 = (2 * Atomic Weight of Na) + Atomic Weight of C + (3 * Atomic Weight of O)
Molecular Weight of Na2CO3 = (2 * 22.99 amu) + 12.01 amu + (3 * 16.00 amu)
Mole Weight of Na2CO3 ≈ 45.98 amu + 12.01 amu + 48.00 amu
Mole Weight of Na2CO3 ≈ 105.99 amu (or g/mol)
So, the molecular weight of sodium carbonate is approximately 105.99 g/mol.
Significance of Mole Weight of Sodium Carbonate
Understanding the mole weight of sodium carbonate holds several practical implications:
- Chemical Formulation: The mole weight provides the exact mass of one mole of sodium carbonate molecules. This is essential for precise chemical formulations and reactions in laboratories and industries.
- Stoichiometry: In chemical reactions, the molecular weight plays a crucial role in stoichiometry, helping scientists and engineers determine the quantities of reactants and products.
- Dosage Calculation: In various applications, such as water treatment and detergents, the molecular weight is vital for calculating the appropriate dosage of sodium carbonate needed to achieve specific results.
- Environmental Impact: In environmental science, understanding the molecular weight aids in assessing the impact of sodium carbonate on ecosystems and water quality when it is used or released.
- Quality Control: Industries that produce sodium carbonate products rely on accurate molecular weight data to maintain quality control standards.
In conclusion, the mole weight of sodium carbonate, approximately 105.99 g/mol, is a crucial parameter in various scientific, industrial, and environmental contexts. It enables precise calculations, formulations, and assessments, ultimately contributing to the efficient and responsible use of this important chemical compound.
Read More
- Difference Between Isothermal And Adiabatic Process
- Simple Harmonic Motion Examples
- Molecular Mass Of Calcium
- Lumen Meaning In Biology
- Molar Mass of Chlorine
Frequently asked questions (FAQs) On Mole Weight Of Sodium Carbonate
1. What is the mole formula of sodium carbonate?
The mole formula of sodium carbonate is Na2CO3, indicating that each molecule contains two sodium (Na) atoms, one carbon (C) atom, and three oxygen (O) atoms.
2. What is the mole weight of sodium carbonate?
The mole weight of sodium carbonate (Na2CO3) is approximately 105.99 grams per mole (g/mol).
3. Why is it important to know the mole weight of sodium carbonate?
Knowing the mole weight is essential for accurate chemical calculations, including determining reactant and product quantities in chemical reactions, dosage calculations in various applications, and quality control in industries.
4. How is the mole weight of sodium carbonate calculated?
The mole weight of sodium carbonate is calculated by summing the atomic weights of all the constituent atoms in its chemical formula: 2 * Atomic Weight of Sodium (Na) + Atomic Weight of Carbon (C) + 3 * Atomic Weight of Oxygen (O).
5. What are some common uses of sodium carbonate in everyday life?
Sodium carbonate has a wide range of applications, including as a cleaning agent (in laundry detergents and household cleaners), in water treatment, as a food additive, and in various industrial processes.
Law Of Conservation Of Energy
Law Of Conservation Of Energy: Energy is a fundamental concept that governs the behavior of the universe. It powers our homes, drives our cars, and fuels the processes of life itself.
The Law of Conservation of Energy is one of the most fundamental principles in physics, revealing the remarkable fact that energy cannot be created or destroyed but can only change from one form to another.
In this article, we will explore the essence of this law, its historical development, and its profound implications across various scientific disciplines.

Law Of Conservation Of Energy
Understanding the Law of Conservation of Energy
The Law of Conservation of Energy, also known as the First Law of Thermodynamics, states that the total energy of an isolated system remains constant over time. In simpler terms, energy cannot be created or destroyed within a closed system; it can only change its form or be transferred from one part of the system to another. This law underlies numerous physical phenomena and is the cornerstone of many scientific principles.
Historical Development
The concept of conservation of energy has a rich history of development:
- Early Ideas: The idea that something akin to energy conservation existed dates back to ancient philosophers, such as Aristotle, who postulated that the total amount of motion (kinetic energy) in the universe remains constant.
- Work by Julius Mayer and James Joule: In the mid-19th century, scientists like Julius Mayer and James Joule independently formulated the idea that energy is conserved. Joule’s famous experiments with mechanical work and heat led to the formulation of the mechanical equivalent of heat.
- Hermann Helmholtz: Hermann Helmholtz, a German physicist, further clarified the law by introducing the concept of conservation of energy in a broader sense, encompassing various forms of energy, including potential, kinetic, thermal, and chemical energy.
- The First Law of Thermodynamics: This principle was codified as the First Law of Thermodynamics, which articulates that the alteration in the internal energy of a closed system equals the heat added to the system minus the work performed by the system.
Implications and Applications
The Law of Conservation of Energy has far-reaching implications across various scientific fields:
- Physics: In classical physics, this law is crucial for understanding the behavior of mechanical systems, the motion of objects, and the transfer of energy in various forms.
- Thermodynamics: The law forms the foundation of thermodynamics, a branch of physics dealing with heat, work, and energy transfer. It underlies the principles governing engines, refrigerators, and the behavior of gases.
- Chemistry: In chemical reactions, energy conservation plays a vital role. Comprehending the conservation and transfer of energy is essential for predicting the outcomes of reactions and investigating the behavior of molecules.
- Engineering: Engineers rely on the conservation of energy when designing efficient systems and machinery. The law is essential in fields like electrical engineering, where energy transfer and conversion are fundamental.
- Environmental Science: In environmental science, the law is pivotal for assessing energy flows in ecosystems and understanding the impact of human activities on energy conservation and sustainability.
Challenges and Expanding Horizons
While the Law of Conservation of Energy has been a cornerstone of classical physics, it faced challenges and expansions with the advent of modern physics. The development of quantum mechanics and the theory of relativity introduced new perspectives on energy conservation, particularly at microscopic scales and in situations involving extremely high speeds or strong gravitational fields.
Applications Across Diverse Fields
The Law of Conservation of Energy has found applications in various scientific, technological, and everyday contexts:
- Renewable Energy: The development of renewable energy sources, such as solar panels and wind turbines, relies on energy conservation principles. These technologies harness energy from natural processes and convert it into electricity while adhering to the law.
- Electric Circuits: In electrical engineering, energy conservation is fundamental for analyzing and designing circuits. It ensures that electrical energy input equals the output, accounting for losses due to resistance and inefficiencies.
- Climate Science: Understanding the Earth’s energy balance is essential in climate science. The law helps scientists assess the energy absorbed from the Sun, radiated back into space, and retained in the atmosphere, influencing climate patterns.
- Medicine: Medical devices like pacemakers and defibrillators operate based on the principles of energy conservation, ensuring precise delivery of electrical energy to the heart to maintain proper function.
- Space Exploration: Space missions and satellite operations adhere to the law when planning energy budgets, ensuring that spacecraft have enough energy to perform their tasks efficiently.
Challenges and Expanding Horizons
While the Law of Conservation of Energy remains a fundamental pillar of physics, it faced challenges and expansions in the 20th century:
- Quantum Mechanics: At the quantum level, the conservation of energy takes on unique characteristics. Energy levels in quantum systems are quantized, leading to phenomena like electron energy states in atoms and energy conservation within subatomic particles.
- Theory of Relativity: Albert Einstein’s theory of relativity introduced the famous equation E=mc², demonstrating that energy (E) and mass (m) are interchangeable. This theory expanded our understanding of energy conservation in the context of space-time.
- Dark Energy and Dark Matter: In cosmology, the nature of dark energy and dark matter challenges our understanding of energy conservation on cosmic scales. These mysterious components of the universe may not adhere to conventional energy conservation laws.
Conclusion
The Law of Conservation of Energy, a cornerstone of physics, guides our understanding of energy’s role in the universe. It has applications ranging from everyday engineering and environmental science to cutting-edge research in quantum mechanics and cosmology. Despite challenges and expansions, this law endures as a fundamental principle, offering valuable insights into the workings of the physical world and opening doors to new discoveries and technological advancements. As science continues to evolve, the conservation of energy remains a timeless and indispensable concept.
Read More
- Difference Between Equinox And Solstice
- Molecular Weight Of K2Cr2O7
- Molecular Weight Of Phosphorus
- Molecular Weight Of SO2
- Molecular Weight of Na2CO3
Frequently Asked Questions (FAQs) On Law Of Conservation Of Energy
1. What is the Law of Conservation of Energy?
The Law of Energy Conservation in physics asserts the unchanging total energy of an isolated system throughout time. Energy cannot be created or destroyed but can only change from one form to another.
2. Who formulated the Law of Conservation of Energy?
Although the concept of conserving energy has ancient origins, the modern formulation of this principle is credited to 19th-century scientists such as Julius Mayer, James Joule, and Hermann Helmholtz.
3. What are the different forms of energy?
Energy can exist in various forms, including kinetic energy (associated with motion), potential energy (associated with position or configuration), thermal energy (associated with heat), chemical energy (associated with chemical reactions), and electromagnetic energy (associated with light and other electromagnetic waves), among others.
4. Can energy be lost or created in everyday processes?
In everyday occurrences, energy remains conserved, undergoing conversions from one manifestation to another. In a car engine, fuel’s chemical energy converts into kinetic (motion) and thermal (heat) energy.
5. How is the Law of Conservation of Energy applied in technology and engineering?
The law is crucial in designing and analyzing systems, such as engines, power plants, and electrical circuits. It ensures that energy input equals the energy output, accounting for losses due to inefficiencies.
Difference Between Transducer And Sensor
Difference Between Transducer And Sensor: Transducers and sensors are both devices used in various fields to measure and convert physical quantities into electrical signals, but they have distinct functions and characteristics. Here’s a comparison of the key differences between transducers and sensors:

Difference Between Transducer And Sensor
1. Function:
- Transducer: A transducer is a device that converts one form of energy or physical quantity into another. It can convert various types of energy, including mechanical, electrical, thermal, or optical, into electrical signals or vice versa.
- Sensor: A sensor is a specific type of transducer that is designed to detect and respond to a particular physical stimulus, such as light, temperature, pressure, or motion. Sensors convert this stimulus into electrical signals for further processing or measurement.
2. Purpose:
- Transducer: Transducers are versatile devices used in a wide range of applications, from measuring physical quantities to converting signals for transmission or processing. They serve as intermediaries between different systems or devices.
- Sensor: Sensors are designed for specific purposes, such as detecting and measuring specific physical properties like temperature, humidity, or light. They provide data for monitoring and control systems.
3. Output:
- Transducer: Transducers can have various types of output, including voltage, current, resistance, or frequency. The output signal may not always be directly related to the measured physical quantity.
- Sensor: Sensors typically produce a well-defined electrical output signal that directly corresponds to the physical quantity they are designed to measure. For example, a temperature sensor generates an electrical signal proportional to the temperature.
4. Examples:
- Transducer: Examples of transducers include microphones (convert sound waves into electrical signals), speakers (convert electrical signals into sound waves), and strain gauges (convert mechanical strain into changes in electrical resistance).
- Sensor: Examples of sensors include temperature sensors (thermocouples or thermistors), light sensors (photodetectors), motion sensors (infrared motion detectors), and pressure sensors (piezoelectric sensors).
5. Sensing Element:
- Transducer: Transducers may or may not have a specific sensing element. Some transducers, like microphones and speakers, consist of multiple components and processes.
- Sensor: Sensors always include a dedicated sensing element that directly interacts with the physical stimulus to generate an electrical signal. For example, a temperature sensor uses a thermocouple as its sensing element.
6. Range of Application:
- Transducer: Transducers can have broad applications in various fields, including telecommunications, automation, audio technology, and instrumentation, where their primary function is to convert one form of energy into another.
- Sensor: Sensors are specialized for specific applications and are commonly used in areas such as environmental monitoring, industrial control systems, healthcare, and consumer electronics, where precise measurements of physical quantities are essential.


In summary, while both transducers and sensors play crucial roles in converting physical quantities into electrical signals, transducers are versatile devices used for various conversions, while sensors are specialized devices designed for specific measurements and applications.
Read More
- Difference Between Alternator And Generator
- Physical & chemical properties of water
- Difference Between Fuse And Circuit Breaker
- Difference Between Electromagnet And Permanent Magnet
- Blind Visually Impaired Optical Low Vision Aids
Frequently Asked Questions (FAQs) Difference Between Transducer And Sensor
1. What is a transducer?
A transducer is a device that converts one form of energy or physical quantity into another. It can convert various types of energy, including mechanical, electrical, thermal, or optical, into electrical signals or vice versa.
2. What is a sensor?
A sensor is a specific type of transducer designed to detect and respond to a particular physical stimulus, such as light, temperature, pressure, or motion. Sensors convert this stimulus into electrical signals for measurement or further processing.
3. How do transducers and sensors differ in their functions?
Transducers have a broader function, serving as intermediaries between different energy forms, while sensors are specialized for detecting and measuring specific physical properties.
4. What types of output signals do transducers and sensors produce?
Transducers can have various types of output signals, including voltage, current, resistance, or frequency, which may not always directly relate to the measured physical quantity. Sensors produce well-defined electrical output signals directly proportional to the measured property.
5. Can you provide examples of transducers and sensors?
Examples of transducers include microphones (converting sound waves into electrical signals), speakers (converting electrical signals into sound waves), and strain gauges (converting mechanical strain into changes in electrical resistance). Examples of sensors include temperature sensors (measuring temperature), light sensors (detecting light levels), and motion sensors (detecting motion or presence).
Difference Between Equinox And Solstice
Difference Between Equinox And Solstice: Equinox and solstice are astronomical events that mark significant points in the Earth’s orbit around the Sun, bringing about changes in seasons and daylight hours. Here’s a comparison of the key differences between equinox and solstice:
Difference Between Equinox And Solstice
1. Definition:
- Equinox: An equinox occurs when the Sun is directly above the Earth’s equator, resulting in nearly equal lengths of day and night. There are two equinoxes each year: the vernal equinox (spring equinox) and the autumnal equinox (fall equinox).
- Solstice: A solstice is an astronomical event when the Sun reaches its highest or lowest point in the sky at noon, marking the longest day (summer solstice) or shortest day (winter solstice) of the year.
2. Occurrence:
- Equinox: Equinoxes occur twice a year, around March 20-21 (vernal equinox) and September 22-23 (autumnal equinox) in the Northern Hemisphere. In the Southern Hemisphere, the dates are reversed.
- Solstice: Solstices also occur twice a year, around June 20-21 (summer solstice) and December 21-22 (winter solstice) in the Northern Hemisphere. In the Southern Hemisphere, the dates are reversed.
3. Daylight Hours:
- Equinox: During equinoxes, day and night are approximately of equal length in most parts of the world. This balance of daylight and darkness is often referred to as “equilux.”
- Solstice: Solstices mark the extremes of daylight hours. The summer solstice has the longest day and shortest night, while the winter solstice has the shortest day and longest night.
4. Sun’s Position:
- Equinox: In an equinox, the Sun is situated precisely above the Earth’s equator, resulting in the Sun rising directly in the east and setting directly in the west, regardless of the observer’s geographical location on Earth.
- Solstice: During a solstice, the Sun is at its highest (summer solstice) or lowest (winter solstice) point in the sky at noon, resulting in the longest or shortest noontime shadows, respectively.
5. Seasonal Significance:
- Equinox: Equinoxes signal the beginning of spring (vernal equinox) or fall (autumnal equinox) and are associated with the transition between the seasons.
- Solstice: Solstices mark the official start of summer (summer solstice) or winter (winter solstice) and represent the peak of the respective seasons.
6. Cultural and Celebratory Traditions:
- Equinox: Equinoxes have been celebrated in various cultures and religions as times of balance and renewal. For example, the vernal equinox is associated with Easter in Christianity.
- Solstice: Solstices have often been associated with major cultural and religious celebrations. For instance, the summer solstice is celebrated in festivals like Midsummer’s Eve in some cultures.
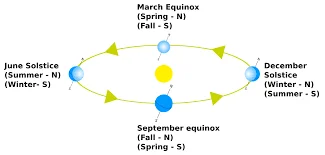
In summary, equinoxes and solstices are astronomical events that influence the length of day and night, the Sun’s position in the sky, and the changing of seasons. Equinoxes represent balance, while solstices represent extremes in daylight and are culturally significant in various parts of the world.
Read More
- Molecular Weight Of K2Cr2O7
- Molecular Weight Of Phosphorus
- Molecular Weight Of SO2
- Molecular Weight of Na2CO3
- Molecular Mass Of Sodium
Frequently Asked Questions (FAQs) On Difference Between Equinox And Solstice
1. What is the main difference between an equinox and a solstice?
The key difference lies in their astronomical significance. Equinoxes mark the times when day and night are nearly of equal length, signaling the transition between seasons, while solstices mark the moments when the Sun reaches its highest or lowest point in the sky, resulting in the longest or shortest day of the year.
2. How many equinoxes and solstices occur each year?
There are two equinoxes each year: the vernal (spring) equinox and the autumnal (fall) equinox. Likewise, there are two solstices: the summer solstice and the winter solstice.
3. When do equinoxes occur?
Equinoxes typically occur around March 20-21 (vernal equinox) and September 22-23 (autumnal equinox) in the Northern Hemisphere. The dates are reversed in the Southern Hemisphere.
4. When do solstices occur?
Solstices generally occur around June 20-21 (summer solstice) and December 21-22 (winter solstice) in the Northern Hemisphere, with reversed dates in the Southern Hemisphere.
5. How do equinoxes affect daylight hours?
Equinoxes bring nearly equal lengths of day and night, resulting in a balance of daylight and darkness.
Difference Between Diffraction And Interference
Difference Between Diffraction And Interference: Diffraction and interference are both wave phenomena that occur when waves interact with obstacles or slits, but they involve distinct mechanisms and produce different effects. Here’s a comparison of the key differences between diffraction and interference:

Difference Between Diffraction And Interference
1. Definition:
- Diffraction: Diffraction is the bending or spreading of waves as they encounter an obstacle or aperture (like a slit). It occurs when waves encounter an obstacle or aperture that is comparable in size to their wavelength.
- Interference: Interference is the interaction of two or more coherent waves (waves with the same frequency and phase) that results in the combination of their amplitudes. It occurs when two or more waves overlap in space and time.
2. Cause:
- Diffraction: Diffraction is caused by the bending of waves around obstacles or through apertures, leading to the wavefronts spreading out after passing through.
- Interference: Interference is caused by the superposition of waves from different sources or the splitting of a wave into multiple waves that then overlap and interfere constructively or destructively.
3. Wave Interaction:
- Diffraction: Diffraction occurs with a single wavefront encountering an obstacle or aperture. It involves the bending or spreading of that single wavefront.
- Interference: Interference involves the combination of multiple waves, often from different sources, that overlap in space and time, creating regions of constructive and destructive interference.
4. Resultant Pattern:
- Diffraction: Diffraction produces a pattern of waves that spread out in various directions after passing through an obstacle or aperture. The resulting pattern may exhibit a central maximum and secondary maxima and minima.
- Interference: Interference produces an interference pattern characterized by alternating regions of high (constructive) and low (destructive) intensity. It can create patterns with bright and dark fringes.
5. Wavelength Dependency:
- Diffraction: Diffraction is strongly dependent on the wavelength of the wave. It is more noticeable when the wavelength is comparable to the size of the obstacle or aperture.
- Interference: Interference is not directly dependent on the wavelength of the waves but on their phase relationship. Waves with different wavelengths can interfere constructively or destructively if their phases align or differ.
6. Examples:
- Diffraction: Examples of diffraction include the spreading of light waves when passing through a narrow slit, the bending of sound waves around obstacles, and the diffraction of water waves when passing through a harbor entrance.
- Interference: Examples of interference include the colorful patterns in soap bubbles and oil films (thin film interference), the creation of diffraction grating spectra, and the formation of interference patterns in double-slit experiments.
In summary, while both diffraction and interference involve wave phenomena, diffraction is primarily concerned with the bending or spreading of waves as they encounter obstacles or apertures, while interference involves the combination of multiple waves to create regions of constructive and destructive interference, leading to interference patterns.
Read More
- Alcohols Phenols And Ethers Class 12
- Alcohols Phenols And Ethers
- Effect Of Magnet On Current Carying Wire
- Simple Harmonic Motion Formulas
- Dipole Uniform Magnetic Field
Frequently Asked Questions (FAQs) Difference Between Diffraction And Interference
1. What is diffraction?
Diffraction is the bending or spreading of waves as they encounter an obstacle or aperture, leading to changes in the direction of the waves.
2. What is interference?
Interference is the interaction of two or more coherent waves (waves with the same frequency and phase) that results in the combination of their amplitudes, leading to regions of constructive and destructive interference.
3. What causes diffraction?
Diffraction is caused by the interaction of a single wavefront with an obstacle or aperture, leading to the bending or spreading of the wave.
4. What causes interference?
Interference is caused by the superposition of multiple waves, often from different sources, that overlap in space and time, resulting in regions of constructive and destructive interference.
5. Can diffraction occur with a single wave?
Yes, diffraction can occur with a single wave front when it encounters an obstacle or aperture comparable in size to its wavelength.
