Blog
Chemical Properties of Acid
Chemical Properties of Acid: Acids are a class of chemical compounds known for their distinct chemical properties. They play a vital role in various chemical reactions, industrial processes, and natural phenomena.
In this article, we will explore the chemical properties of acids, including their behavior in aqueous solutions, their reactivity, and their importance in chemistry and everyday life.

Chemical Properties of Acid
1. Acids and Their Properties
1.1. Definition of Acids
Acids are substances that can donate protons (H+) or accept pairs of electrons in chemical reactions. This proton-donating characteristic is at the heart of their chemical properties.
1.2. Acidic Taste and pH
Acids often have a sour taste, which is why substances like lemon juice and vinegar taste sour. The acidity of a substance is measured on the pH scale, where acids have pH values less than 7. The lower the pH, the stronger the acid.
2. Behavior of Acids in Aqueous Solutions
2.1. Formation of Hydronium Ions
When acids dissolve in water, they release protons (H+ ions), which immediately bond with water molecules to form hydronium ions (H3O+). This is represented in chemical equations as follows:
Acid (HA) + Water (H2O) → Hydronium Ion (H3O+) + Conjugate Base (A-)
2.2. Strong and Weak Acids
Acids can be classified as strong or weak based on their ability to dissociate in water. Strong acids dissociate almost completely in solution, while weak acids only partially dissociate. The degree of dissociation determines the concentration of hydronium ions in the solution, influencing the acidity.
3. Chemical Reactivity of Acids
3.1. Neutralization Reactions
One of the most well-known chemical properties of acids is their ability to react with bases to form water and salts. This reaction is called neutralization and is represented by the following general equation:
Acid (HA) + Base (BOH) → Water (H2O) + Salt (BA)
For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) results in the formation of water and sodium chloride (table salt):
HCl + NaOH → H2O + NaCl
3.2. Corrosive Properties
Concentrated acids can exhibit corrosive properties when they come into contact with various materials, including metals, due to their ability to donate protons and react with surfaces.
3.3. Reactivity with Carbonates
Acids react vigorously with carbonates to produce carbon dioxide gas, water, and a salt. For example, when hydrochloric acid (HCl) is added to calcium carbonate (CaCO3), the following reaction occurs:
2HCl + CaCO3 → CO2 + H2O + CaCl2
4. Importance of Acids in Everyday Life and Industry
4.1. Food and Beverage Industry
Acids are commonly used in the food and beverage industry to impart tartness and flavor to various products. Citric acid, for instance, is found in citrus fruits and used as a food additive.
4.2. Pharmaceuticals
Acids are essential in pharmaceuticals for drug formulation and chemical synthesis. They play a role in adjusting the pH of medications and are used in the production of various pharmaceutical compounds.
4.3. Chemical Manufacturing
Acids are crucial in the chemical manufacturing industry for processes like polymerization, catalysis, and the production of fertilizers, explosives, and dyes.
4.4. Environmental Impact
Acids can have a significant environmental impact, especially when they are released into water bodies. Acid rain, which results from the presence of sulfuric acid (H2SO4) and nitric acid (HNO3) in the atmosphere, can harm ecosystems, damage buildings, and affect aquatic life.
Conclusion
Acids exhibit a wide range of chemical properties that make them essential in various scientific, industrial, and everyday applications. From their ability to donate protons and react with bases to their role in adjusting pH and their impact on the environment, the chemical properties of acids play a crucial role in shaping the world of chemistry and our daily lives. Understanding these properties is fundamental for both scientists and the general public.
Read More
- Molar Mass of Benzene
- Molar Mass of Calcium
- Electromagnetic Spectrum X Rays
- Electric Circuit Electrical Symbols
- Difference Between Watts And Volts
Frequently Asked Questions (FAQs) Chemical Properties of Acid
Q1: What is the pH scale, and how does it relate to the chemical properties of acids?
A1: The pH scale is a measure of the acidity or alkalinity of a substance. It ranges from 0 to 14, here pH 7 is neutral, values below 7 indicate acidity (with lower numbers indicating stronger acidity), and values above 7 indicate alkalinity (with higher numbers indicating stronger alkalinity). Acids have pH values below 7, with lower pH values indicating stronger acidity.
Q2: Can you provide examples of common acids found in everyday life?
A2: Certainly! Common acids found in everyday life include:
- Hydrochloric acid (HCl) in gastric juice.
- Citric acid in citrus fruits like lemons and oranges.
- Acetic acid (vinegar) used in cooking.
- Sulfuric acid (H2SO4) used in car batteries.
- Carbonic acid (H2CO3) in carbonated beverages.
Q3: What is the difference between a strong acid and a weak acid in terms of their chemical properties?
A3: The main difference between strong acids and weak acids is their degree of dissociation in water. Strong acids almost completely dissociate into ions (e.g., H+ and A-) in aqueous solutions, resulting in high concentrations of hydronium ions (H3O+). In contrast, weak acids only partially dissociate, leading to lower concentrations of hydronium ions.
Q4: How do acids contribute to corrosion, and what are some examples of corrosive acids?
A4: Acids can contribute to corrosion by reacting with metals to produce metal salts and hydrogen gas. For example, hydrochloric acid (HCl) can corrode metals like iron, zinc, and aluminum. Sulfuric acid (H2SO4) is another extremely corrosive acid employed across a wide range of industrial applications.
Q5: What happens when an acid reacts with a carbonate substance?
A5: When an acid reacts with a carbonate substance, it produces carbon dioxide gas (CO2), water (H2O), and a salt. This reaction is characterized by effervescence or the release of bubbles of carbon dioxide gas. For example, the reaction between hydrochloric acid (HCl) and calcium carbonate (CaCO3) results in the formation of carbon dioxide, water, and calcium chloride (CaCl2).
Molecular Weight of Co2
Molecular Weight of Co2: Carbon dioxide (CO2) is a chemical compound that plays a significant role in various natural processes, climate change, and industrial applications.
Understanding its molecular weight is crucial in chemistry, environmental science, and engineering. In this article, we will explore the molecular weight of CO2, its composition, and its relevance in different fields.

mol Weight of Co2
mol Composition of CO2
Carbon dioxide (CO2) is composed of one carbon atom (C) and two oxygen atoms (O), bonded together through covalent bonds. Its chemical formula, CO2, reflects this composition, indicating one carbon atom and two oxygen atoms in a single molecule.
mol Weight of CO2
The mol weight of a substance is calculated by adding the atomic weights of all the atoms in its chemical formula. To determine the mol weight of CO2, we consider the atomic weights of carbon (C) and oxygen (O):
- The atomic weight of carbon (C) is approximately 12 atomic mass units (amu).
- The atomic weight of oxygen (O) is approximately 16 amu.
Now, let’s calculate the mol weight of CO2:
mol Weight of CO2 = (Atomic Weight of C) + 2 × (Atomic Weight of O) mol Weight of CO2 = 12 amu + 2 × 16 amu Molecular Weight of CO2 = 12 amu + 32 amu Molecular Weight of CO2 = 44 amu
Therefore, the mol weight of carbon dioxide (CO2) is 44 atomic mass units (amu).
Significance of the mol Weight of CO2
The mol weight of CO2 holds significant importance across various scientific disciplines:
1. Chemistry:
In chemistry, the mol weight of CO2 is essential for stoichiometry, which involves the calculation of reactant and product quantities in chemical reactions. It helps determine the balanced chemical equations and the molar relationships between substances in a reaction.
2. Environmental Science:
Understanding the mol weight of CO2 is vital in environmental science and climate change research. CO2 is a greenhouse gas, and its molecular weight influences its behavior in the atmosphere, including its role in trapping heat and contributing to global warming.
3. Industry and Engineering:
In industrial processes, the molecular weight of CO2 is relevant for designing equipment, such as compressors and separators, used in the extraction, storage, and transport of carbon dioxide. It is also crucial in carbon capture and storage (CCS) technologies.
4. Health and Safety:
In safety assessments and industrial hygiene, the molecular weight of CO2 is considered when evaluating its effects on human health. High concentrations of CO2 can be hazardous, affecting respiration and leading to asphyxiation.
5. Education and Research:
The mol weight of CO2 is a fundamental concept taught in chemistry and science education. It forms the basis for understanding chemical reactions, gas properties, and environmental issues.
Conclusion
The mol weight of carbon dioxide (CO2), calculated as 44 atomic mass units (amu), is a critical parameter with broad implications in chemistry, environmental science, industry, and safety. It governs the behavior of CO2 in various contexts, from chemical reactions to its role as a greenhouse gas. As we continue to grapple with climate change and environmental challenges, a deep understanding of CO2’s molecular weight remains indispensable in addressing these global issues.
Read More
- Molecular Mass of Water
- Difference Between Cyclone And Hurricane
- Molecular Weight Of Na
- Molecular Mass Of O2
- Molecular Mass of H2
Frequently Asked Questions (FAQs) Molecular Weight of Co2
Q1: What is the mol weight of CO2 (carbon dioxide)?
A1: The mol weight of carbon dioxide (CO2) is 44 atomic mass units (amu). This value is calculated by adding the atomic weights of one carbon atom (approximately 12 amu) and two oxygen atoms (approximately 16 amu each) in a CO2 molecule.
Q2: Why is knowing the mol weight of CO2 important?
A2: Understanding the mol weight of CO2 is important in various fields. In chemistry, it’s essential for stoichiometry and reaction calculations. In environmental science, it’s crucial for studying climate change and greenhouse gas emissions. It also has significance in industrial processes, safety assessments, and education.
Q3: How is the molecular weight of CO2 used in stoichiometry?
A3: In stoichiometry, the molecular weight of CO2 is used to calculate the molar relationships between substances in a chemical reaction. It helps determine the balanced chemical equations and allows for the calculation of reactant and product quantities.
Q4: What is the role of CO2’s mol weight in climate change research?
A4: CO2’s mol weight plays a role in climate change research because it influences how CO2 behaves in the atmosphere. CO2 is a greenhouse gas, and its molecular weight affects its ability to trap heat, contributing to global warming and climate change.
Q5: How is the mol weight of CO2 relevant in industry and engineering?
A5: In industry and engineering, the mol weight of CO2 is relevant for designing equipment used in processes involving CO2, such as compressors and separators. It’s also crucial in carbon capture and storage (CCS) technologies, which aim to mitigate CO2 emissions from industrial sources.
Molecular Mass of Water
Difference Between Cyclone And Hurricane
The Molecular Weight Of Na
Molecular Mass Of O2
Molecular Mass of H2
Molecular Mass of Water
Molecular Mass of Water: Water is a fundamental and ubiquitous substance in our world, covering about 71% of the Earth’s surface and making up a significant portion of living organisms.
At a molecular level, water consists of two hydrogen atoms and one oxygen atom bonded together, represented as H2O. In this article, we will explore the molecular mass of water and its significance in chemistry and everyday life.
Molecular Mass of Water
Molecular Composition of Water
Water’s molecular formula, H2O, describes the specific arrangement of its atoms. In a water molecule, two hydrogen atoms (H) are covalently bonded to one oxygen atom (O). This simple structure belies the remarkable properties and importance of water in various natural and chemical processes.
Molecular Mass of Water
The molecular mass of a substance is the sum of the atomic masses of all the atoms in a molecule. To calculate the molecular mass of water (H2O), we add the atomic masses of its constituent elements:
- The atomic mass of hydrogen (H) is approximately 1 atomic mass unit (amu).
- The atomic mass of oxygen (O) is approximately 16 amu.
Therefore, the molecular mass of water is:
Molecular mass of H2O = (2 × Atomic mass of H) + Atomic mass of O Molecular mass of H2O = (2 × 1 amu) + 16 amu Molecular mass of H2O = 2 amu + 16 amu Molecular mass of H2O = 18 amu
The molecular mass of water is 18 atomic mass units (amu).
Significance of the Molecular Mass of Water
The molecular mass of water is a critical concept in chemistry and has several significant implications:
1. Stoichiometry:
In chemical reactions, the molecular mass of water is essential for stoichiometric calculations. It helps determine the quantities of reactants and products involved in a chemical reaction. As an illustration, in the reaction where hydrogen gas (H2) combines with oxygen gas (O2) to produce water (H2O), the mol. mass of water is employed to determine the stoichiometry of the reaction.
2. Concentrations and Solutions:
The molecular mass of water is employed for the computation of substance concentrations within solutions. The concept of molarity (moles of solute per liter of solution) relies on knowing the mol. mass of solutes, including water itself when it acts as a solvent.
3. Physical Properties:
Water’s mol mass contributes to its unique physical properties, such as its high boiling point, melting point, and heat capacity. These properties are essential for life and various industrial processes.
4. Environmental Science:
Understanding the mol mass of water is crucial in environmental science and ecology. It plays a role in the study of water pollution, the water cycle, and the behavior of substances dissolved in water bodies.
5. Health and Nutrition:
The mol mass of water is also relevant in health and nutrition. It is used to calculate the molecular mass of nutrients and substances in food and beverages, aiding in dietary planning and analysis.
6. Laboratory Techniques:
In laboratories, chemists use the mol mass of water to calibrate instruments, prepare solutions of known concentrations, and analyze samples using techniques like mass spectrometry and chromatography.
Conclusion
The mol mass of water, represented as H2O, is a fundamental concept in chemistry and plays a vital role in various scientific, industrial, and everyday applications. It not only defines the composition of water but also influences its physical and chemical properties. Understanding the mol mass of water is essential for stoichiometry, concentration calculations, environmental studies, and numerous other fields, underlining its significance in our understanding of the natural world and the chemical processes that govern it.
Read More
- Difference Between Cyclone And Hurricane
- Molecular Weight Of Na
- Molecular Mass Of O2
- Molecular Mass of H2
- Molecular Mass Of NH3
Frequently Asked Questions (FAQs) Mol Mass of Water
Q1: What is the mol mass of water (H2O)?
The mol mass of water (H2O) is 18 atomic mass units (amu). The determination of this value involves summing the atomic masses of two hydrogen atoms (each roughly 1 amu) and one oxygen atom (approximately 16 amu) within a water molecule.
Q2: Why is the mol mass of water important in chemistry?
The mol mass of water holds significant importance in chemistry as it plays a pivotal role in stoichiometry, where it helps ascertain the amounts of substances involved in chemical reactions, both as reactants and products. It is also essential for calculating concentrations in solutions and plays a role in understanding the physical and chemical properties of water.
Q3: How is the molecular mass of water relevant in everyday life?
The mol. mass of water is relevant in everyday life in various ways. It contributes to the unique physical properties of water, such as its high boiling and melting points, which are essential for cooking and various industrial processes. Additionally, it is important in health and nutrition for calculating nutrient content in foods and beverages.
Q4: Is the molecular mass of water used in environmental science?
Certainly, the molecular mass of water finds application in the field of environmental science. It is relevant in studies of water pollution, the water cycle, and the behavior of substances dissolved in water bodies. Understanding water’s molecular mass is crucial for assessing its impact on the environment.
Q5: How is the molecular mass of water determined experimentally?
The molecular mass of water can be determined experimentally using various analytical techniques, including mass spectrometry and chromatography. These methods involve measuring the mass-to-charge ratios of H2O molecules and their fragments, allowing for precise determination of molecular mass.
Blind Visually Impaired Braille
Blind Visually Impaired Braille: The ability to read and write is a fundamental skill that opens doors to knowledge, education, and communication. For individuals who are blind or visually impaired, accessing written information can be a challenge, but Braille offers a solution.
Braille is a tactile writing system that enables people with visual disabilities to read and write through touch. In this comprehensive guide, we will explore Braille in depth, covering its history, significance, learning process, and modern applications.

Blind Visually Impaired Braille
1. The History of Braille
The story of Braille begins in 19th-century France with a remarkable individual named Louis Braille. Louis was born in 1809, and at the age of three, he accidentally injured his eye with a stitching awl, leading to an infection that left him completely blind by the age of five. Despite his blindness, Louis was determined to learn and sought an education.
1.1 Louis Braille’s Contribution
Louis Braille attended the Royal Institute for Blind Youth in Paris, where he encountered a tactile writing system called “night writing.” This system, created by Charles Barbier, was designed for military communication but proved impractical for blind individuals due to its complexity. Louis Braille recognized the potential of Barbier’s system but believed it needed simplification.
At the age of 15, Louis Braille developed the Braille system that we know today. He reduced the number of dots in each character from twelve to six, creating a compact and efficient system. Braille’s innovation was groundbreaking, as it allowed blind individuals to read and write quickly and independently.
2. The Significance of Braille
Braille has had a profound impact on the lives of blind and visually impaired individuals worldwide. Its significance can be understood through various dimensions:
2.1. Education
Braille is the primary medium through which blind individuals access education. It enables them to read textbooks, take notes, and complete assignments independently. Access to Braille materials ensures that students with visual disabilities can participate in mainstream educational settings.
2.2. Literacy and Employment
Literacy is a cornerstone of employment and independence. Braille literacy opens doors to a wide range of career opportunities, from computer programming to journalism. It also empowers individuals to manage daily tasks such as reading labels, cooking recipes, and using public transportation.
2.3. Inclusivity
Braille promotes inclusivity by providing access to printed materials. It allows blind individuals to participate fully in social, cultural, and recreational activities, such as reading novels, newspapers, or menus at restaurants.
2.4. Independence and Empowerment
One of the most profound aspects of Braille is the sense of independence and empowerment it offers. It allows individuals to communicate without assistance, make informed choices, and advocate for themselves.
3. Learning Braille
Learning Braille is a valuable skill for individuals who are blind or visually impaired, and it is typically introduced at a young age. Here is an overview of the Braille learning process:
3.1. Braille Alphabet
The Braille system consists of characters or cells, each comprising six dots arranged in two columns of three dots each. Each dot in the cell is assigned a number, and different combinations of dots represent letters, numbers, punctuation, and special symbols. For example, the letter “A” is represented by dot 1, while the letter “B” is represented by dots 1 and 2, and so on.
3.2. Learning Materials
Braille learners use special tools and materials. A slate and stylus or a Braille embosser is commonly used to create Braille characters. Braille books, worksheets, and tactile graphics are essential resources for practice and learning.
3.3. Braille Instruction
Learning Braille often begins with instruction from a trained teacher or specialist in blindness and visual impairment. Instruction may take place in specialized schools or through itinerant teachers who visit students in mainstream educational settings.
3.4. Practice and Fluency
Becoming proficient in Braille requires consistent practice. Students learn to recognize and produce Braille characters accurately and efficiently. Building fluency is a key goal of Braille instruction.
3.5. Technology and Braille
Modern technology has expanded the ways in which individuals can access Braille. Braille displays, which convert digital text into tactile Braille characters, are widely used in conjunction with computers and smartphones. This technology enhances accessibility in various aspects of life, from reading emails to browsing the web.
4. The Evolution of Braille
Braille has evolved over the years to adapt to changing technologies and the needs of blind individuals. Some notable developments include:
4.1. Unified English Braille (UEB)
In an effort to standardize Braille codes and improve accessibility, many English-speaking countries adopted Unified English Braille (UEB). UEB streamlines and simplifies some Braille contractions and symbols, making Braille more consistent across regions.
4.2. Electronic Braille
Advancements in technology have given rise to electronic Braille displays, which allow users to access digital content in Braille format. These displays use a series of small pins or dots to create Braille characters that change as the user reads.
4.3. Braille Translation Software
Braille translation software helps convert digital text into Braille, making it easier to produce Braille materials. This software can handle various languages and Braille codes, enhancing accessibility for users worldwide.
4.4. Audio-Braille Books
In addition to traditional Braille books, audio-Braille books combine Braille with audio narration, providing a multisensory reading experience. This format is particularly beneficial for individuals with dual sensory impairments.
5. Modern Applications of Braille
Braille continues to play a significant role in the lives of blind and visually impaired individuals in the modern world. Its applications extend beyond reading and writing:
5.1. Education
Braille remains essential in the education of blind students. It allows them to access textbooks, educational materials, and assignments independently, ensuring equal educational opportunities.
5.2. Accessible Technology
Braille displays and Braille-compatible software have made technology more accessible. These tools enable users to read digital content, access screen readers, and interact with computers and smartphones.
5.3. Employment
Braille literacy enhances employment prospects. Blind individuals who are proficient in Braille can pursue a wide range of careers, including computer programming, teaching, and administrative roles.
5.4. Independent Living
Braille supports independent living by enabling individuals to manage daily tasks such as cooking, labeling personal items, and reading instructions on appliances and medication labels.
5.5. Advocacy and Empowerment
Braille empowers individuals to advocate for themselves, participate in community activities, and engage in social and cultural events. It fosters self-confidence and self-advocacy.
6. Challenges and Future of Braille
While Braille has made significant advancements, challenges remain. These include:
6.1. Limited Resources
Access to Braille materials can be limited, particularly in resource-constrained regions. The production of Braille books and materials requires specialized equipment and trained personnel.
6.2. Digital Accessibility
As digital content becomes more prevalent, ensuring the accessibility of online resources and technology for Braille users remains an ongoing challenge.
6.3. Braille Literacy Rates
Braille literacy rates vary worldwide, and efforts are needed to promote Braille education and proficiency among blind and visually impaired individuals.
6.4. Advocacy and Awareness
Advocacy efforts are crucial to raise awareness about the importance of Braille and ensure that it remains a viable and accessible tool for the blind and visually impaired.
Conclusion
Braille has been a beacon of hope and independence for individuals who are blind or visually impaired. It has evolved from its humble beginnings into a versatile and essential tool that empowers people to access education, employment, information, and the world around them. The legacy of Louis Braille and the enduring relevance of Braille in the digital age demonstrate the resilience and adaptability of this tactile writing system. By promoting Braille literacy and accessibility, we can continue to break down barriers and create a more inclusive society for all. Braille is not merely a system of raised dots; it is a symbol of empowerment, independence, and the boundless potential of the human spirit.
Read More
- Examples Of Rotational Motion
- Addition And Subtraction Of Fractions
- Difference Between Asteroid And Meteoroid
- Class 6 Maths Question Paper With Solutions NCERT PDF
- Sample Question Paper For Class 6 CBSE English Grammar
Frequently Asked Questions (FAQs) Blind Visually Impaired Braille
Q1: What is Braille ?
A1: Braille is a tactile writing system used by individuals who are blind or visually impaired. It consists of raised dots arranged in cells, with each cell representing a letter, number, punctuation mark, or special symbol.
Q2: Who invented Braille?
A2: Braille was invented by Louis Braille, a blind Frenchman, in the 19th century. Louis Braille developed the Braille system to create a more efficient and accessible method of reading and writing for individuals with visual disabilities.
Q3: How do blind individuals learn Braille?
A3: Blind individuals typically learn Braille through specialized instruction from teachers or specialists in blindness and visual impairment. They use tools like Braille slates and styluses, Braille embossers, and Braille books to practice and become proficient in reading and writing Braille.
Q4: What is Unified English Braille (UEB)?
A4: Unified English Braille (UEB) is a standardized Braille code used in many English-speaking countries. It aims to streamline and simplify Braille contractions and symbols, making Braille more consistent across regions and improving accessibility.
Q5: How is technology changing the way Braille is used?
A5: Technology has significantly impacted Braille by introducing electronic Braille displays and Braille translation software. Electronic Braille displays allow users to access digital content in Braille, while Braille translation software converts digital text into Braille format.
Difference Between Cyclone And Hurricane
Difference Between Cyclone And Hurricane: Cyclones and hurricanes are both powerful weather phenomena known for their destructive potential, but they are given different names based on their location and characteristics.
These storms are essentially the same natural disasters, but they are called by different names in different parts of the world. In this article, we will explore the key differences between cyclones and hurricanes.

Difference Between Cyclone And Hurricane
Cyclone:
1. Geographic Location:
- Cyclones are tropical storms that occur in the Indian Ocean and the South Pacific Ocean.
- In the South Pacific, they are known as cyclones.
- In the Indian Ocean, they are referred to as cyclones as well.
2. Nomenclature:
- Cyclones are typically named by the meteorological agencies of the countries affected by them.
- Names are assigned alphabetically, often based on a predetermined list of names for the region.
3. Wind Direction:
- In the Southern Hemisphere, cyclones rotate counterclockwise (anticlockwise).
- In the Northern Hemisphere, they rotate clockwise.
4. Notable Cyclones:
- Some notable cyclones include Cyclone Tracy, which devastated Darwin, Australia in 1974, and Cyclone Phailin, which hit the eastern coast of India in 2013.
Hurricane:
1. Geographic Location:
- Hurricanes occur in the Atlantic Ocean and the northeastern Pacific Ocean.
- In the Atlantic, they are known as hurricanes.
- In the northeastern Pacific, they are referred to as hurricanes as well.
2. Nomenclature:
- Hurricanes are named according to a predetermined list of names established by the World Meteorological Organization (WMO).
- Names are assigned alphabetically and alternate between male and female names.
3. Wind Direction:
- In the Northern Hemisphere, hurricanes rotate counterclockwise.
- In the Southern Hemisphere, hurricanes rotate clockwise.
4. Notable Hurricanes:
- Some famous hurricanes include Hurricane Katrina, which struck the Gulf Coast of the United States in 2005, and Hurricane Maria, which devastated Puerto Rico in 2017.
Similarities:
- While cyclones and hurricanes have distinct names and are found in different ocean basins, they share several common characteristics:
- Formation: Both cyclones and hurricanes originate as tropical storms over warm ocean waters, where they gain energy from the heat and moisture in the atmosphere.
- Destructive Potential: Both can intensify into extremely powerful and destructive storms, with high winds, heavy rainfall, and the potential for storm surges that can lead to coastal flooding.
- Tracking and Prediction: Meteorological agencies closely monitor the development and path of both cyclones and hurricanes, providing advance warning to potentially affected regions.
In conclusion, the main difference between cyclones and hurricanes lies in their geographic locations and the names used to refer to them in different parts of the world. Despite these variations, they share common characteristics and pose similar threats to coastal areas and communities, emphasizing the importance of preparedness and effective disaster response.
Read More
- Molecular Weight Of Na
- Molecular Mass Of O2
- Molecular Mass of H2
- Molecular Mass Of NH3
- Molecular Mass Of Ch4
Frequently Asked Questions (FAQs) Difference Between Cyclone And Hurricane
Q1: What is the primary difference between a cyclone and a hurricane?
A1: The primary difference between a cyclone and a hurricane is their geographic location and the terminology used to describe them. Cyclones are tropical storms that occur in the Indian Ocean and the South Pacific, while hurricanes are tropical storms that occur in the Atlantic Ocean and the northeastern Pacific. They are essentially the same meteorological phenomenon but are given different names based on their location.
Q2: How do the names of cyclones and hurricanes differ?
A2: Cyclones are typically named by the meteorological agencies of the countries affected by them, and names are often assigned alphabetically from predetermined lists for their respective regions. In contrast, hurricanes are named according to a predetermined list established by the World Meteorological Organization (WMO). The names for hurricanes are chosen internationally and alternate between male and female names.
Q3: Do cyclones and hurricanes rotate in the same direction?
A3: No, the direction of rotation of cyclones and hurricanes depends on their hemisphere. In the Northern Hemisphere, both cyclones and hurricanes rotate counterclockwise. In the Southern Hemisphere, they rotate clockwise. This is due to the Coriolis effect, caused by the Earth’s rotation, which influences the direction of motion in large-scale weather systems.
Q4: Are cyclones and hurricanes equally destructive?
A4: Both cyclones and hurricanes have the potential to be extremely destructive, with high winds, heavy rainfall, and the potential for storm surges that can lead to coastal flooding. The level of destruction depends on factors such as the storm’s intensity, size, and the vulnerability of the affected areas.
Q5: Can a cyclone turn into a hurricane or vice versa?
A5: Cyclones and hurricanes are essentially the same meteorological phenomenon, but they are given different names based on their location. While they can have similar characteristics, they do not spontaneously change from one to the other. They maintain their names and characteristics based on the ocean basin in which they form.
Effect Of Magnet On Current Carying Wire
Effect Of Magnet On Current Carying Wire: The interaction between magnets and electric currents is a fascinating aspect of electromagnetism, a fundamental branch of physics. This interaction has led to the development of various technological applications, from electric motors to generators.
In this article, we will explore the intriguing effect of magnets on current-carrying wires, shedding light on the principles behind it and its real-world applications.

Effect Of Magnet On Current Carying Wire
The Basics of Electromagnetism
Electromagnetism is a branch of physics that deals with the relationship between electric currents and magnetic fields. It was James Clerk Maxwell who, in the 19th century, formulated the fundamental equations that describe how electric charges and currents generate magnetic fields and how changing magnetic fields induce electric currents.
The Right-Hand Rule
One of the key principles in understanding the interaction between magnets and current-carrying wires is the right-hand rule. This rule states that if you point your thumb in the direction of the current flow (conventional current, from positive to negative), and you curl your fingers, the direction in which your fingers curl represents the direction of the magnetic field lines around the wire. The strength of the magnetic field depends on the current intensity and the distance from the wire.
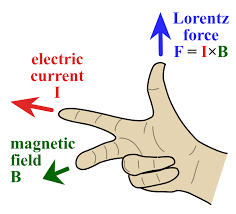
The Effect of Magnets on Current-Carrying Wires
When a magnetic field interacts with a current-carrying wire, several important phenomena occur:
- Magnetic Force: The magnetic field exerts a force on the wire. This force is perpendicular to both the current direction and the magnetic field direction and is described by the magnetic force formula: F = I * B * L * sin(θ), where F is the force, I is the current, B is the magnetic field strength, L is the length of the wire in the field, and θ is the angle between the current and magnetic field directions.
- Lorentz Force: The magnetic force on the wire is part of the larger Lorentz force, which includes the electric force on charged particles within the wire. This combined force can cause the wire to move or experience a torque.
Applications of the Effect
- Understanding the effect of magnets on current-carrying wires has a multitude of practical applications:
- Electric Motors: Electric motors utilize the interaction between magnetic fields and current-carrying wires to convert electrical energy into mechanical motion. This principle is at the core of various appliances and machines.
- Generators: In generators, mechanical motion is used to move wires through magnetic fields, inducing electric currents. This process is how electrical energy is generated in power plants.
- Magnetic Sensors: Magnetic sensors and compasses rely on the interaction between magnetic fields and current in coils to detect and measure magnetic fields, providing critical information in navigation and various industries.
- Transformers: Transformers use the principles of electromagnetic induction to change the voltage levels in electrical circuits, making power distribution and voltage conversion efficient.
Conclusion
The effect of magnets on current-carrying wires is a fundamental aspect of electromagnetism with profound implications in modern technology. It enables the creation of electric motors, generators, sensors, and transformers, all of which are integral to our daily lives. By harnessing the principles of electromagnetism, scientists and engineers have revolutionized the way we generate, distribute, and use electrical energy, making the world of technology and innovation possible. Understanding these principles is crucial for anyone interested in the fields of physics and electrical engineering.
Read More
- Simple Harmonic Motion Formulas
- Dipole Uniform Magnetic Field
- Light Filtering Polaroid Films Polarization
- Animals Biology Science Lion Zoology
- Difference Between Voltage And Current
Frequently Asked Questions (FAQs) Effect Of Magnet On Current Carying Wire
Q1: What is the basic principle behind the interaction between magnets and current-carrying wires?
A1: The basic principle is that a magnetic field exerts a force on a current-carrying wire. This force is perpendicular to both the current direction and the magnetic field direction and is described by the magnetic force formula.
Q2: What is the right-hand rule in electromagnetism?
A2: The right-hand rule is a convention used to determine the direction of magnetic field lines generated by a current-carrying wire. If you point your right thumb in the direction of the current, the curling of your fingers represents the direction of the magnetic field lines around the wire.
Q3: How does the strength of the magnetic field affect the force on a current-carrying wire?
A3: The strength of the magnetic field (denoted as B) directly affects the force (F) experienced by the wire. The greater the magnetic field strength, the stronger the force on the wire, assuming other factors like current and length remain constant.
Q4: What is the Lorentz force, and how does it relate to the effect of magnets on current-carrying wires?
A4: The Lorentz force is the combined force acting on a charged particle due to both an electric field and a magnetic field. In the context of current-carrying wires, it includes the magnetic force on the wire as well as the electric force on the charged particles (electrons) within the wire.
Q5: What are some practical applications of the effect of magnets on current-carrying wires?
A5: Some practical applications include electric motors, generators, magnetic sensors, compasses, and transformers. These devices rely on the interaction between magnetic fields and current-carrying wires to perform various functions, such as generating electricity, providing directional information, and converting electrical energy into mechanical motion.
Simple Harmonic Motion Formulas
Simple Harmonic Motion Formulas: Simple Harmonic Motion (SHM) is a fundamental concept in physics that describes the repetitive back-and-forth motion of an object around an equilibrium position.
Many physical phenomena, from a swinging pendulum to vibrating guitar strings, can be modeled as simple harmonic motion.To analyze and describe this motion, several key formulas come into play. In this article, we will explore these formulas, their significance, and how they help us understand and predict simple harmonic motion.

Simple Harmonic Motion Formulas
1. Displacement (x): The displacement (x) of an object undergoing SHM at any given time is the distance and direction from its equilibrium position.
Formula: x(t) = A * cos(ωt + φ)
- x(t) represents the displacement at time t.
- A is the amplitude, the maximum displacement from the equilibrium position.
- ω (omega) is the angular frequency, which depends on the system’s characteristics.
- φ (phi) is the phase angle, indicating the initial position of the oscillating object.
2. Velocity (v): The velocity of an object in SHM describes how fast it is moving at any point in its oscillation.
Formula: v(t) = -A * ω * sin(ωt + φ)
- v(t) represents the velocity at time t.
- A is the amplitude.
- ω is the angular frequency.
- φ is the phase angle.
3. Acceleration (a): The acceleration of an object undergoing SHM represents its rate of change of velocity and is directed toward the equilibrium position.
Formula: a(t) = -A * ω^2 * cos(ωt + φ)
- a(t) represents the acceleration at time t.
- A is the amplitude.
- ω is the angular frequency.
- φ is the phase angle.
4. Angular Frequency (ω): The angular frequency (ω) is a measure of how quickly an object oscillates in SHM.
Formula: ω = 2πf
- ω is the angular frequency.
- f is the frequency, which represents the number of oscillations per unit time (usually in hertz, Hz).
5. Period (T): The period (T) is the time it takes for one complete oscillation, and it is the inverse of frequency.
Formula: T = 1/f
- T is the period.
- f is the frequency.
6. Frequency (f): The frequency (f) measures the number of oscillations per unit time.
Formula: f = 1/T
- f is the frequency.
- T is the period.
These formulas provide a comprehensive framework for analyzing and understanding simple harmonic motion. They allow us to calculate key parameters such as displacement, velocity, and acceleration at any given time, helping scientists and engineers model and predict the behavior of systems exhibiting SHM.
Applications of Simple Harmonic Motion Formulas:
- Mechanical Systems: Formulas for Simple Harmonic Motion (SHM) find application in the examination of the movement of pendulums, vibrating springs, and oscillating objects within mechanical systems.
- Electromagnetic Waves: In physics, the equations governing electromagnetic waves, such as light, can be described using SHM principles.
- Sound Waves: Vibrating objects, like guitar strings or tuning forks, produce sound waves that can be analyzed using SHM formulas.
- Quantum Mechanics: SHM concepts and equations are used in quantum mechanics to describe the behavior of particles within potential wells.
In conclusion, simple harmonic motion formulas provide a powerful tool for understanding and quantifying the behavior of oscillating systems. Whether in physics, engineering, or other scientific disciplines, these formulas are essential for predicting and explaining a wide range of phenomena involving repetitive motion around an equilibrium position.
Read More
- Dipole Uniform Magnetic Field
- Light Filtering Polaroid Films Polarization
- Animals Biology Science Lion Zoology
- Difference Between Voltage And Current
- Difference Between Two Stroke And Four Stroke
Frequently Asked Questions (FAQs) Simple Harmonic Motion Formulas
Q1: What is Simple Harmonic Motion (SHM)?
A1: Simple Harmonic Motion (SHM) is a type of mechanical wave-like motion in which an object oscillates back and forth around an equilibrium position under the influence of a restoring force. It is characterized by a constant frequency and amplitude.
Q2: What is the equilibrium position in SHM?
A2: The equilibrium position is the central point in an oscillating system where the object would naturally come to rest if not subjected to any external forces. It is the point around which the object oscillates.
Q3: What is the amplitude of an oscillating object in SHM?
A3: The amplitude (A) in SHM is the maximum displacement of the oscillating object from its equilibrium position. It represents the distance between the equilibrium position and the extreme points reached during the oscillation.
Q4: How do you calculate the angular frequency (ω) in SHM?
A4: The angular frequency (ω) for Simple Harmonic Motion (SHM) can be determined through the equation ω = 2πf, where f represents the oscillation frequency, typically measured in hertz (Hz).
Q5: What is the relationship between the period (T) and frequency (f) in SHM?
A5: The period (T) of an oscillation is the time it takes to complete one full cycle, while frequency (f) is the number of cycles per second. The relationship is T = 1/f, or equivalently, f = 1/T.
Molecular Mass Of Calcium
Mol Mass Of Calcium: Calcium, with its symbol Ca and atomic number 20, is a fundamental element in the periodic table. It is renowned for its crucial role in biological processes, as well as its various applications in industry, agriculture, and everyday life.
Understanding the mol mass of calcium is essential for comprehending its behavior and significance in the world of chemistry. In this article, we delve into the concept of molecular mass, its calculation for calcium, and the implications of this fundamental property.

Mol Mass Of Calcium
The Nature of Calcium
Calcium is a metallic element found in group 2 (formerly group IIA) of the periodic table, along with other alkaline earth metals. It is a silvery-white, soft metal that reacts vigorously with water and oxygen, forming compounds like calcium oxide (CaO) and calcium hydroxide (Ca(OH)2). In its natural state, calcium is often found in the form of calcium carbonate (CaCO3), which makes up materials like limestone, chalk, and shells.
Importance of Molecular Mass
Mol mass, also known as molar mass or molecular weight, is the mass of a molecule or compound expressed in atomic mass units (amu) or grams per mole (g/mol). It is a fundamental concept in chemistry and has several key implications:
- Stoichiometry: Molecular mass is crucial for calculating the molar ratio of reactants and products in chemical reactions, aiding in the determination of reaction yields and limiting reagents.
- Synthesis and Formulation: Chemists use molecular mass to precisely measure and combine reactants when synthesizing compounds or formulating chemical solutions.
- Analytical Chemistry: Molecular mass is essential in the identification and quantification of compounds using techniques like mass spectrometry.
- Physical Properties: Molecular mass influences a compound’s physical properties, including melting and boiling points, density, and solubility.
Calculating the Molecular Mass of Calcium
To calculate the molecular mass of calcium (Ca), you consider the atomic mass of a single calcium atom. The atomic mass of calcium is approximately 40.08 atomic mass units (amu).
Now, let’s calculate the molecular mass of calcium (Ca):
Molecular Mass of Calcium (Ca) = Atomic Mass of Calcium Molecular Mass of Calcium (Ca) = 40.08 amu
The molecular mass of calcium is approximately 40.08 amu.
Implications of Calcium’s Molecular Mass
The molecular mass of calcium, approximately 40.08 amu, has several implications in the field of chemistry and various applications:
- Quantitative Analysis: In analytical chemistry, calcium’s molecular mass is essential for accurately determining its concentration in samples, such as in water quality testing.
- Chemical Reactions: Calcium’s molecular mass plays a key role in stoichiometric calculations for chemical reactions involving calcium compounds, ensuring precise measurements and predictions.
- Biological Role: In biology, calcium ions (Ca2+) are vital for numerous physiological processes, including muscle contraction, nerve signaling, and blood clotting.
- Industrial Applications: Calcium and its compounds are used in diverse industrial applications, including the production of steel, cement, and fertilizers, as well as in the food and pharmaceutical industries.
Conclusion
The molecular mass of calcium, approximately 40.08 amu, is a fundamental property that plays a pivotal role in chemistry and various scientific disciplines. It is indispensable for stoichiometric calculations, accurate measurements, and an in-depth understanding of calcium’s chemical and physical properties. As a versatile element with significant applications in industry, agriculture, and biology, calcium’s molecular mass continues to be a critical parameter for researchers and professionals working with this essential element.
Read More
- Molecular Weight Of Sucrose
- Molecular Mass Of Carbon
- Molar Mass Of Phosphorus
- Molar Mass of Chlorine
- Electrical Energy And Power
Frequently Asked Questions (FAQs) Molecular Mass Of Calcium
Q1: What is molecular mass or molecular weight?
A1: Molecular mass, also known as molar mass or molecular weight, is the mass of a molecule or compound expressed in atomic mass units (amu) or grams per mole (g/mol). It is a fundamental property used in chemistry to quantify the mass of chemical substances.
Q2: What is the chemical symbol for calcium?
A2: The chemical symbol for calcium is Ca.
Q3: Is calcium a metal or non-metal?
A3: Calcium is a metal. It belongs to the group of metals known as alkaline earth metals.
Q4: Why is calcium important in biology?
A4: Calcium plays a crucial role in biology as a key intracellular signaling ion. It is involved in various physiological processes, including muscle contraction, nerve transmission, blood clotting, and cell communication.
Q5: How is the molecular mass of calcium calculated?
A5: To calculate the molecular mass of calcium (Ca), you consider the atomic mass of a single calcium atom, which is approximately 40.08 atomic mass units (amu).
Molecular Weight of Chlorine
Molecular Weight of Chlorine: Chlorine is a highly reactive and essential element in the world of chemistry. It plays a crucial role in various chemical processes, from water purification to the production of household chemicals and pharmaceuticals.
One of the fundamental characteristics of any chemical substance is its molecular weight, a property that holds significant importance in understanding and working with chlorine. In this article, we will explore the concept of molecular weight and its relevance in the context of chlorine.

Molecular Weight of Chlorine
The Nature of Chlorine
Before delving into the molecular weight of chlorine, it is important to understand the nature of this element. Chlorine, denoted by the chemical symbol Cl, is a halogen found in group 17 (formerly group VIIA) of the periodic table. It exists primarily as a diatomic molecule, Cl2, composed of two chlorine atoms bonded together. This diatomic form is the most common and stable form of chlorine.
Importance of Molecular Weight
Molecular weight, also known as molar mass, is the mass of a molecule or compound expressed in atomic mass units (amu) or grams per mole (g/mol). It is a fundamental property in chemistry and plays a pivotal role in various aspects of chemical science and industry, including:
- Stoichiometry: Molecular weight is essential for determining the stoichiometry of chemical reactions, helping chemists balance equations and calculate reaction yields.
- Synthesis: Chemists use molecular weight to measure and combine reactants accurately when synthesizing compounds or formulating solutions.
- Analytical Chemistry: Molecular weight is crucial in identifying and quantifying compounds through techniques like mass spectrometry.
- Physical Properties: Mol weight influences a substance’s physical properties, such as melting point, boiling point, density, and solubility.
Calculating the Mol Weight of Chlorine
To calculate the mol weight of chlorine (Cl2), you need to consider the atomic mass of a single chlorine atom. The atomic mass of chlorine is approximately 35.45 atomic mass units (amu).
Now, let’s calculate the mol weight of chlorine (Cl2):
Mol Weight of Chlorine (Cl2) = (2 × Atomic Mass of Chlorine) Molecular Weight of Chlorine (Cl2) = (2 × 35.45 amu) Molecular Weight of Chlorine (Cl2) = 70.90 amu
The mol weight of chlorine (Cl2) is approximately 70.90 amu.
Implications of Chlorine’s Molecular Weight
The molecular weight of chlorine, approximately 70.90 amu, holds several implications in the field of chemistry and industry:
- Quantitative Analysis: In analytical chemistry, chlorine’s molecular weight is crucial for accurately determining its concentration in samples, such as in water quality testing.
- Chemical Reactions: Chlorine’s mol weight is essential for stoichiometric calculations in chemical reactions involving chlorine gas, ensuring precise measurements and predictions.
- Safety: Understanding the mol weight of chlorine is important in handling and storage to prevent accidents since chlorine gas is toxic and can be hazardous when mishandled.
- Industrial Applications: Chlorine’s mol weight is a key parameter in industries using chlorine for various processes, including the production of chemicals, plastics, and pharmaceuticals.
Conclusion
- The molecular weight of chlorine, approximately 70.90 amu for the diatomic molecule Cl2, is a fundamental property that plays a pivotal role in chemistry and industry. It is crucial for stoichiometric calculations, precise measurements, and the understanding of chlorine’s physical and chemical properties. As an element of great significance in various applications, chlorine’s molecular weight continues to be a critical parameter for chemists and professionals working with this versatile and essential element.
Read More
- Molecular Weight Of Benzene
- Lumen Meaning In Biology
- Molecular Weight Of Sucrose
- Molecular Mass Of Carbon
- Molar Mass Of Phosphorus
Frequently Asked Questions (FAQs) Molecular Weight of Chlorine
Q1: What is molecular weight, and why is it important in chemistry?
A1: Mol weight, also known as molar mass, is the mass of a molecule or compound expressed in atomic mass units (amu) or grams per mole (g/mol). It is important in chemistry for various reasons. It including stoichiometry, determining reactant and product quantities in chemical reactions, and understanding physical properties of substances.
Q2: What is the chemical symbol for chlorine, and what is its common form?
A2: The chemical symbol for chlorine is Cl. Its common form is the diatomic molecule Cl2, where two chlorine atoms are bonded together.
Q3: How is the mol weight of chlorine (Cl2) calculated?
A3: To calculate the mol weight of chlorine (Cl2), you sum the atomic mass of a single chlorine atom, which is approximately 35.45 atomic mass units (amu). Then multiply by 2 (since there are two chlorine atoms in Cl2).
Q4: What is the mol weight of chlorine (Cl2)?
A4: The mol weight of chlorine (Cl2) is approximately 70.90 amu (atomic mass units).
Q5: Why is the mol weight of chlorine important in water treatment?
A5: Chlorine is commonly used in water treatment to disinfect and purify drinking water. Its mol weight is important because it helps in accurately measuring the amount of chlorine needed to effectively treat water. It also ensure water safety for consumption.
Molecular Weight Of Benzene
Molecular Weight Of Benzene: Benzene, a ubiquitous aromatic hydrocarbon, has long fascinated chemists and researchers alike due to its unique ring structure and remarkable stability.
One fundamental aspect of any chemical compound is its molecular weight, which plays a crucial role in various chemical and physical processes. In this article, we delve into the molecular weight of benzene, exploring its significance, calculation, and implications in the world of chemistry.

Molecular Weight Of Benzene
Benzene’s Chemical Structure
Before delving into its molecular weight, it is essential to understand the structure of benzene. Benzene consists of a hexagonal ring of six carbon atoms, with alternating single and double bonds between them. Each carbon atom is bonded to a single hydrogen atom, resulting in the chemical formula C6H6. The carbon-carbon (C-C) bonds in the benzene ring are often depicted as a resonance hybrid, as the electrons are delocalized, leading to the molecule’s exceptional stability.
Importance of Molecular Weight
Molecular weight, also known as molar mass or molecular mass, is the mass of a molecule or compound expressed in atomic mass units (amu) or grams per mole (g/mol). It is a fundamental property in chemistry and is crucial for various purposes, including:
- Stoichiometry: Molecular weight is vital for calculating the molar ratio of reactants and products in chemical reactions, aiding in the determination of reaction yields and limiting reagents.
- Synthesis and Formulation: Chemists use molecular weight to precisely measure and combine reactants when synthesizing compounds or formulating chemical solutions.
- Analytical Chemistry: Molecular weight helps in the identification and quantification of compounds using techniques like mass spectrometry.
- Physical Properties: Molecular weight influences a compound’s physical properties such as melting and boiling points, density, and solubility.
Calculating the Molecular Weight of Benzene
To calculate the molecular weight of benzene (C6H6), you need to consider the individual atomic masses of carbon (C) and hydrogen (H) and sum them up according to the molecular formula.
The atomic masses (in amu) are as follows:
- Carbon (C): 12.01 amu
- Hydrogen (H): 1.01 amu
Now, let’s calculate the molecular weight of benzene:
Molecular Weight of Benzene (C6H6) = (6 × Atomic Mass of Carbon) + (6 × Atomic Mass of Hydrogen) Molecular Weight of Benzene (C6H6) = (6 × 12.01 amu) + (6 × 1.01 amu) Molecular Weight of Benzene (C6H6) = 72.06 amu
The molecular weight of benzene is approximately 72.06 amu.
Implications of Benzene’s Molecular Weight
The molecular weight of benzene, 72.06 amu, has several implications in the field of chemistry:
- Mass Calculation: It aids in determining the amount of benzene needed for chemical reactions or formulations, helping chemists work with precise quantities.
- Stoichiometry: When benzene participates in chemical reactions, its molecular weight is essential for stoichiometric calculations, ensuring that reactions proceed as expected.
- Physical Properties: The molecular weight influences benzene’s physical properties, making it a useful solvent and a critical component in various industrial processes.
- Toxicology and Environmental Impact: Understanding benzene’s molecular weight is crucial in assessing its toxicity and environmental behavior, as it plays a role in the compound’s dispersal and degradation.
Conclusion
The molecular weight of benzene, calculated as approximately 72.06 amu, is a fundamental property that holds significant importance in the world of chemistry. It is essential for stoichiometric calculations, synthesis, and the understanding of benzene’s physical and chemical properties. As a widely used compound in various industries, benzene’s molecular weight continues to be a critical parameter for researchers and chemists working with this remarkable aromatic hydrocarbon.
Read More
- Lumen Meaning In Biology
- Molecular Weight Of Sucrose
- Molecular Mass Of Carbon
- Molar Mass Of Phosphorus
- Molar Mass of Chlorine
Frequently Asked Questions (FAQs) Molecular Weight Of Benzene
Q1: What is molecular weight?
A1: Mol weight, also known as molar mass or molecular mass. It is the mass of a molecule or compound expressed in atomic mass units (amu) or grams per mole (g/mol). It represents the sum of the atomic masses of all the atoms in a molecule.
Q2: What is benzene, and why is it significant in chemistry?
A2: Benzene is an aromatic hydrocarbon with a hexagonal ring structure consisting of six carbon atoms and six hydrogen atoms. CDhemical formula of benzene is C6H6. It is significant in chemistry due to its unique structure and exceptional stability, making it a fundamental building block in various organic compounds.
Q3: How is the mol weight of benzene calculated?
A3: To calculate the mol weight of benzene, you sum the atomic masses of its constituent atoms. The atomic masses (in amu) are approximately 12.01 amu for carbon (C) and 1.01 amu for hydrogen (H). So, for benzene (C6H6), you multiply the atomic mass of carbon by 6. The atomic mass of hydrogen by 6 and then add these values together.
Q4: What is the mol weight of benzene?
A4: The mol weight of benzene (C6H6) is approximately 72.06 amu (atomic mass units).
Q5: Why is knowing the mol weight of benzene important?
A5: Knowing the mol weight of benzene is important for several reasons:
- Stoichiometry: It helps in calculating reactant and product quantities in chemical reactions.
- Synthesis: Chemists use it to measure and combine reactants accurately.
- Physical Properties: Molecular weight influences properties like melting and boiling points, solubility, and density.
- Analytical Chemistry: It aids in the identification and quantification of benzene in various analytical techniques.
