Blog
Molar Mass of Benzene
Molar Mass of Benzene: Benzene, a six-carbon, six-hydrogen ring structure, is a quintessential compound in the world of organic chemistry. This article will explore the molar mass of benzene (C6H6) and its significance in various chemical applications.

Molar Mass of Benzene
The Significance of Benzene
Benzene is an aromatic hydrocarbon and one of the fundamental building blocks in organic chemistry. Its hexagonal ring structure and resonance contribute to its stability, making it a cornerstone of various chemical reactions and the synthesis of countless organic compounds. Benzene is also well-known for its unique odor and its presence in many natural sources like crude oil and gasoline.
Molecular Structure of Benzene
Before delving into the molar mass of benzene, it’s essential to understand its molecular structure. A benzene molecule consists of six carbon (C) atoms and six hydrogen (H) atoms, arranged in a symmetrical hexagonal ring. Each carbon atom forms a sigma bond with one hydrogen atom and two sigma bonds with neighboring carbon atoms. This arrangement results in a highly stable, planar, and symmetrical molecule.
Molar Mass of Benzene
The molar mass of a compound is defined as the mass of one mole of that compound, measured in grams per mole (g/mol). To calculate the molar mass of benzene (C6H6), we sum the atomic masses of its constituent atoms.
- Carbon (C) has an atomic mass of approximately 12.01 g/mol.
- Hydrogen (H) has an atomic mass of approximately 1.01 g/mol.
Now, let’s calculate the molar mass of benzene:
Molar Mass of Benzene (C6H6) = (6 × Atomic Mass of Carbon) + (6 × Atomic Mass of Hydrogen)
Molar Mass of Benzene (C6H6) = (6 × 12.01 g/mol) + (6 × 1.01 g/mol)
Molar Mass of Benzene (C6H6) ≈ 72.06 g/mol
So, the molar mass of benzene is approximately 72.06 grams per mole.
Significance of Molar Mass in Chemistry
Understanding the molar mass of benzene is crucial for several reasons. It is a fundamental parameter for stoichiometry, allowing chemists to determine the number of moles of benzene involved in a chemical reaction. This, in turn, helps in calculating reactant and product quantities, which is essential for formulation and balancing of chemical equations.
Additionally, the molar mass of benzene is integral in various applications, such as in gas chromatography and mass spectrometry, where it is used to identify and quantify compounds in complex mixtures.
Conclusion
The mol mass of benzene, approximately 72.06 g/mol, is a key piece of information for chemists and scientists working with this iconic aromatic compound. It facilitates precise measurements, stoichiometry calculations, and a deeper understanding of benzene’s role in the vast realm of organic chemistry. Whether in the laboratory or industrial settings, a grasp of benzene’s molar mass is indispensable for harnessing the versatility of this fundamental organic molecule.
Read More:
- Molar Mass of Calcium
- Electromagnetic Spectrum X Rays
- Electric Circuit Electrical Symbols
- Difference Between Watts And Volts
- Molar Mass Of Sulphur
Frequently Asked Questions (FAQs) mol Mass of Benzene
Q1: What is the mol mass of benzene?
A1: The mol mass of benzene (C6H6) is approximately 72.06 grams per mole (g/mol).
Q2: Why is the mol mass of benzene important in chemistry?
A2: The mol mass of benzene is crucial because it allows chemists to relate the mass of benzene molecules to the number of molecules present. It is essential for stoichiometry, which involves determining reactant and product quantities in chemical reactions, as well as for identifying and quantifying compounds in analytical techniques like gas chromatography and mass spectrometry.
Q3: What is the molecular structure of benzene?
A3: Benzene has a hexagonal ring structure consisting of six carbon (C) atoms and six hydrogen (H) atoms. Each carbon atom forms a sigma bond with one hydrogen atom and two sigma bonds with neighboring carbon atoms, resulting in a highly stable and symmetrical molecule.
Q4: How is the mol mass of benzene calculated?
A4: The mol mass of benzene is calculated by adding the atomic masses of its constituent atoms. Carbon (C) has an atomic mass of approximately 12.01 g/mol, and hydrogen (H) has an atomic mass of approximately 1.01 g/mol. To find the mol mass of benzene, you sum the masses of the six carbon atoms and six hydrogen atoms in its chemical formula.
Q5: What are some practical applications of benzene in chemistry and industry?
A5: Benzene is a versatile compound used in the synthesis of various organic chemicals. Its applications include the production of plastics, synthetic fibers, dyes, drugs, and pesticides. It is also a key component in the production of gasoline and other fuels. However, due to its toxicity and carcinogenicity, its use has become more restricted in recent years, and safer alternatives are sought in many applications.
Molar Mass of Calcium
Molar Mass of Calcium: Calcium is an element that plays a pivotal role in various aspects of our lives, from maintaining healthy bones and teeth to its widespread use in industrial applications.
In this article, we will explore the molar mass of calcium (Ca) and delve into its significance in the realm of chemistry.

Molar Mass of Calcium
The Importance of Calcium
Calcium is a chemical element with the atomic number 20 and the symbol Ca. It is an essential nutrient for living organisms, including humans. In our bodies, calcium is primarily found in our bones and teeth, where it provides structural support and strength. Beyond its biological importance, calcium also has significant industrial applications, making it a valuable element in many industries.
Atomic Structure of Calcium
To understand the molar mass of calcium, we need to explore its atomic structure. A calcium atom contains 20 electrons, arranged in four energy levels or electron shells. The electron configuration of calcium is 2-8-8-2, indicating that it has two electrons in the first shell, eight electrons in the second and third shells, and two electrons in the fourth shell. The outermost shell, with its two electrons, is known as the valence shell.
Calcium’s atomic number, 20, signifies that it has 20 protons in its nucleus, giving it a positive charge. To maintain electrical neutrality, calcium also has 20 electrons. In addition to protons and electrons, the nucleus of a calcium atom contains 20 neutrons, which are electrically neutral particles with a mass similar to that of protons.
Molar Mass of Calcium
The molar mass of an element is defined as the mass of one mole of atoms of that element, expressed in grams per mole (g/mol). It is a fundamental concept in chemistry, as it enables chemists to link the mass of a substance to the number of atoms or molecules it contains.
To calculate the molar mass of calcium, we consider the contribution of its isotopes. Calcium has several isotopes, with calcium-40 being the most abundant, followed by calcium-44, calcium-42, and a few others. The molar mass of calcium is determined by taking a weighted average of the masses of these isotopes, using their natural abundance as the weighting factor:
Molar Mass of Calcium (Ca) = (Fractional Abundance of Ca-40 × Mass of Ca-40) + (Fractional Abundance of Ca-44 × Mass of Ca-44) + …
In practice, the calculation considers all isotopes and their abundances, resulting in the molar mass of approximately 40.08 g/mol for calcium.
The Significance of Molar Mass
The molar mass of calcium is a critical value in chemistry, serving as a cornerstone for various chemical calculations. It is indispensable in determining the amount of calcium required for specific chemical reactions, stoichiometry calculations, and the formulation of chemical equations. In industries such as metallurgy, agriculture, and construction, knowing the molar mass of calcium is vital for precise measurements and formulations.
In conclusion, the molar mass of calcium, approximately 40.08 g/mol, underlines the importance of this element in both biological and industrial contexts. Its role in maintaining our health and contributing to technological advancements makes understanding its atomic properties and molar mass an essential part of our scientific knowledge.
Read More
- Electromagnetic Spectrum X Rays
- Electric Circuit Electrical Symbols
- Difference Between Watts And Volts
- Molar Mass Of Sulphur
- Electronics In Daily Life
Frequently Asked Questions (FAQs) Molar Mass of Calcium
Q1: What is the mol mass of calcium?
A1: The mol mass of calcium (Ca) is approximately 40.08 grams per mole (g/mol).
Q2: Why is the mol mass of calcium important in chemistry?
A2: The mol mass of calcium is crucial in chemistry because it allows chemists to relate the mass of calcium atoms or molecules to the number of atoms or molecules present. This value is fundamental for stoichiometry calculations, chemical equations, and precise measurements in various industries.
Q3: How is the molar mass of calcium calculated?
A3: The mol mass of calcium is calculated by taking a weighted average of the masses of its isotopes, considering their natural abundances as weighting factors. Calcium has several isotopes, with calcium-40 being the most abundant. The calculation accounts for all isotopes and their abundances.
Q4: What are the main applications of calcium in industry and daily life?
A4: Calcium has numerous applications, including:
- In daily life: It is essential for the formation and maintenance of healthy bones and teeth.
- In agriculture: Calcium is used as a soil conditioner and in fertilizers to enhance plant growth.
- In construction: Calcium is used in the production of cement and concrete.
- In metallurgy: Calcium is employed to extract various metals from their ores.
- In the food industry: Calcium is added as a dietary supplement and preservative.
Q5: Are there any health implications associated with calcium’s molar mass?
A5: Calcium’s molar mass itself doesn’t have direct health implications. However, maintaining an adequate intake of calcium through diet or supplements is important for overall health, particularly for bone health, muscle function, and nerve signaling.
Molar Mass of Chlorine
Molar Mass of Chlorine: The molar mass of an element is a fundamental concept in chemistry, crucial for various calculations in the field. In this article, we will delve into the molar mass of chlorine (Cl), one of the essential elements on the periodic table.

Molar Mass of Chlorine
Introduction to Chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. It is a halogen, belonging to Group 17 (formerly Group VIIA) of the periodic table. Chlorine is a diatomic molecule in its natural form, existing as Cl2 gas. It is known for its distinctive pungent odor and is commonly used in a variety of industrial and household applications, such as water disinfection, bleach production, and as a component in various chemicals.
Atomic Structure of Chlorine
To understand the molar mass of chlorine, we must first examine its atomic structure. A chlorine atom consists of 17 electrons, arranged in three energy levels or electron shells. The electron configuration of chlorine is 2-8-7, indicating that it has two electrons in the first shell, eight electrons in the second shell, and seven electrons in the third shell. The outermost shell, with its seven electrons, is referred to as the valence shell.
Chlorine’s atomic number, 17, tells us it has 17 protons in its nucleus, giving it a positive charge. It also has 17 electrons to balance this charge, making it electrically neutral. In addition to the protons and electrons, the nucleus of a chlorine atom contains 18 neutrons, which are electrically neutral particles with a mass similar to that of protons.
Molar Mass of Chlorine
The molar mass of an element is defined as the mass of one mole of atoms of that element, measured in grams per mole (g/mol). It is a crucial quantity for chemical calculations because it allows chemists to relate the mass of a substance to the number of atoms or molecules it contains. To calculate the molar mass of chlorine, we need to consider the contributions of its isotopes.
Chlorine has two stable isotopes: chlorine-35 (17 protons and 18 neutrons) and chlorine-37 (17 protons and 20 neutrons). These isotopes occur in nature in different proportions. Approximately 75.77% of naturally occurring chlorine is chlorine-35, while the remaining 24.23% is chlorine-37.
To calculate the molar mass of chlorine, we take a weighted average of the masses of these isotopes, using their natural abundance as the weighting factor:
Molar Mass of Chlorine (Cl) = (Fractional Abundance of Cl-35 × Mass of Cl-35) + (Fractional Abundance of Cl-37 × Mass of Cl-37)
Molar Mass of Chlorine (Cl) = (0.7577 × 35.453 g/mol) + (0.2423 × 37.967 g/mol)
Molar Mass of Chlorine (Cl) ≈ 26.94 g/mol
So, the molar mass of chlorine is approximately 26.94 grams per mole.
Importance of Molar Mass
The molar mass of chlorine is a fundamental value used in various chemical calculations. It is essential in determining the amount of chlorine needed for specific reactions, stoichiometry calculations, and the formulation of chemical equations. Additionally, molar mass plays a critical role in determining the concentration of chlorine in solutions and mixtures, such as in water treatment processes.
Understanding the molar mass of elements is a fundamental step in mastering chemistry, as it forms the basis for various chemical calculations and laboratory experiments. Chlorine, with its molar mass of approximately 26.94 g/mol, is no exception, and its properties and behavior in chemical reactions are deeply influenced by this important value.
Read More
- Electrical Energy And Power
- Dynamics Of Circular Motion
- Molecular Weight Of Acetic Acid
- Difference Between Torque And Power
- Difference Between Emission And Absorption Spectra
Frequently Asked Questions (FAQs) Molar Mass of Chlorine
Q1: What is the mol mass of chlorine?
A1: The mol mass of chlorine (Cl) is approximately 26.94 grams per mole (g/mol).
Q2: Why is the mol mass of chlorine important in chemistry?
A2: The mol mass of chlorine is crucial in chemistry because it allows chemists to relate the mass of chlorine atoms or molecules to the number of atoms or molecules present. It plays a fundamental role in stoichiometry, chemical calculations, and the formulation of chemical equations.
Q3: How is the mol mass of chlorine calculated?
A3: The mol mass of chlorine is calculated by taking a weighted average of the masses of its isotopes, chlorine-35 and chlorine-37, using their natural abundances as weighting factors. Approximately 75.77% of naturally occurring chlorine is chlorine-35, and about 24.23% is chlorine-37. The calculation is as follows:
mol Mass of Chlorine (Cl) = (Fractional Abundance of Cl-35 × Mass of Cl-35) + (Fractional Abundance of Cl-37 × Mass of Cl-37)
Q4: What is the significance of chlorine’s atomic number and electron configuration?
A4: Chlorine’s atomic number, 17, indicates the number of protons in its nucleus, which defines its chemical identity. Its electron configuration, 2-8-7, reveals the arrangement of its electrons in energy levels or electron shells, helping us understand its chemical behavior and reactivity.
Q5: Are there any practical applications of chlorine’s mol mass?
A5: Yes, there are practical applications of chlorine’s molar mass. It is used in various industries and applications, including water treatment, where the concentration of chlorine in solutions must be precisely controlled for effective disinfection. Additionally, the mol mass of chlorine is used in chemical manufacturing processes and in calculating the quantities of chlorine needed for specific reactions.
Molar Mass of Helium
Molar Mass of Helium: Helium, symbolized as He on the periodic table, is the second most abundant element in the universe, following hydrogen. It is renowned for its lightness, its ability to make balloons float, and its critical role in various scientific and industrial applications.
One essential aspect of helium is its molar mass, a fundamental concept in chemistry. In this article, we explore the molar mass of helium, its significance, and how it is calculated.
Molar Mass of Helium
Table of Contents:
Introduction to Helium
- Helium’s Discovery and Occurrence
- Unique Properties of Helium
What is Molar Mass?
- Definition and Significance
- The Concept of Mole in Chemistry
Calculating the Molar Mass of Helium
- Atomic Mass Units (amu)
- Molar Mass Calculation
The Significance of Helium’s Low Molar Mass
- Lightness and Buoyancy
- Helium in Balloons and Airships
Helium in Scientific and Industrial Applications
- Cryogenics and Superconductivity
- Welding and Leak Detection
- Medical Applications
Conclusion
- The Light but Essential Element
Introduction to Helium
Helium’s Discovery and Occurrence: Helium was first discovered in 1868 by French astronomer Pierre Janssen during a solar eclipse. It was subsequently identified on Earth in natural gas fields. Today, helium is primarily extracted from natural gas reservoirs, particularly in the United States.
Unique Properties of Helium: Helium possesses several unique properties, including being colorless, odorless, and tasteless. It is inert, meaning it does not readily react with other elements. However, its most famous property is its lightness, which makes it invaluable in various applications.
2. What is Molar Mass?
Definition and Significance: Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It is a crucial concept in chemistry because it allows chemists to relate the mass of a substance to the number of atoms or molecules it contains.
The Concept of Mole in Chemistry: A mole is a unit used in chemistry to count entities like atoms, molecules, or ions. One mole of any substance contains approximately 6.022 x 10^23 entities, a value known as Avogadro’s number.
3. Calculating the Molar Mass of Helium
Atomic Mass Units (amu): To calculate the molar mass of helium, we start with its atomic mass, which is expressed in atomic mass units (amu). Helium has an atomic mass of approximately 4.0026 amu.
Molar Mass Calculation: The molar mass of helium is numerically equivalent to its atomic mass in amu. Therefore, the molar mass of helium is approximately 4.0026 g/mol.
4. The Significance of Helium’s Low Molar Mass
Lightness and Buoyancy: Helium’s exceptionally low molar mass is the reason it is so buoyant. It is significantly lighter than air, making helium-filled balloons and airships float effortlessly.
Helium in Balloons and Airships: Helium is commonly used to fill balloons for celebrations, scientific research, and advertising. It also played a vital role in the era of airships, such as the famous Hindenburg.
5. Helium in Scientific and Industrial Applications
Cryogenics and Superconductivity: Helium is a crucial cryogenic refrigerant used to reach extremely low temperatures, near absolute zero. It enables the operation of superconducting magnets in devices like MRI machines and particle accelerators.
Welding and Leak Detection: Helium is used in welding processes to shield materials from atmospheric contamination. Its small atomic size makes it ideal for leak detection in pipelines and containers.
Medical Applications: In the medical field, helium-oxygen mixtures are used to treat patients with respiratory conditions. Its low density reduces the effort required for breathing.
6. Conclusion
The molar mass of helium, approximately 4.0026 g/mol, reflects its lightness and unique properties. Helium’s low molar mass is not only responsible for its buoyancy but also makes it indispensable in a wide range of scientific, industrial, and medical applications. From filling celebratory balloons to enabling cutting-edge research, helium’s significance in our world is as light as the element itself, yet its impact is profound.
Read More
- Molecular Mass of CH3COOH
- Molecular Weight of kmno4
- Molecular Weight of CaCO3
- Molecular Mass Of CaCO3
- Molecular Mass Of Sulphur
Frequently Asked Questions (FAQs) Molar Mass of Helium
1. What is the molar mass of helium?
The molar mass of helium (He) is approximately 4.0026 grams per mole (g/mol).
2. Why is the molar mass of helium important?
The mol mass is a fundamental property of an element and is essential in chemistry for various calculations, including stoichiometry, determining the mass of a specific number of helium atoms or molecules, and understanding its behavior in chemical reactions.
3. How is the mol mass of helium calculated?
The mol mass of helium is calculated based on its atomic mass, which is approximately 4.0026 atomic mass units (amu). This atomic mass, when expressed in grams per mole (g/mol), gives the molar mass of helium.
4. Why is helium used to fill balloons?
Helium is used to fill balloons because it is much lighter than air, which causes the balloons to float. This property of helium makes it ideal for creating floating balloons for celebrations and events.
5. Are there any safety considerations when using helium-filled balloons?
Helium is generally safe when used for filling balloons. However, it should not be inhaled directly from a helium tank, as inhaling helium can lead to oxygen deprivation and fainting. It’s important to use helium for its intended purpose, which is filling balloons.
Electromagnetic Spectrum X Rays
Electromagnetic Spectrum X Rays: The electromagnetic spectrum is a vast continuum of electromagnetic waves, each with its own unique properties and applications. X-rays occupy a crucial position within this spectrum, known for their ability to penetrate matter and provide valuable insights into the inner workings of our world.
In this article, we’ll dive into the world of X-rays, exploring their characteristics, generation, applications, and safety considerations.
Electromagnetic Spectrum X Rays
Table of Contents:
Introduction to the Electromagnetic Spectrum
- The Diversity of Electromagnetic Waves
- Where X-Rays Reside in the Spectrum
What Are X-Rays
- The Discovery of X-Rays
- Characteristics of X-Rays
Generation of X-Rays
- Production Methods
- X-Ray Tubes and Sources
Applications of X-Rays
- Medical Imaging
- Industrial Inspection and Non-Destructive Testing
- Security Screening
- Astrophysics and Astronomy
Safety and Risks Associated with X-Rays
- Radiation Exposure and Protection
- Medical X-Ray Safety
- Industrial and Research X-Ray Safety
Advancements in X-Ray Technology
- Digital X-Ray Imaging
- X-Ray Crystallography
Conclusion
- The Ongoing Impact of X-Rays
1. Introduction to the Electromagnetic Spectrum
The Diversity of Electromagnetic Waves: The electromagnetic spectrum encompasses a wide range of waves, from radio waves with long wavelengths to gamma rays with incredibly short wavelengths. Each type of wave has its unique characteristics and applications.
Where X-Rays Reside in the Spectrum: X-rays fall within the spectrum between ultraviolet (UV) radiation and gamma rays. They have shorter wavelengths than visible light and carry higher energy, making them suitable for a range of applications.
2. What Are X-Rays?
The Discovery of X-Rays: X-rays were discovered by Wilhelm Conrad Roentgen in 1895 while he was experimenting with cathode rays. His discovery earned him the first Nobel Prize in Physics in 1901.
Characteristics of X-Rays: X-rays are high-energy electromagnetic waves characterized by their ability to penetrate matter. They can ionize atoms, causing electrons to be ejected, and are invisible to the human eye. X-rays have applications in imaging and various scientific fields.
3. Generation of X-Rays
Production Methods: X-rays are produced through processes that involve the acceleration of charged particles, typically electrons, and their interaction with matter. This interaction results in the emission of X-rays.
X-Ray Tubes and Sources: X-ray tubes are commonly used devices for generating X-rays. They consist of a cathode and an anode within a vacuum tube. When high-speed electrons from the cathode strike the anode, X-rays are produced. Synchrotrons and X-ray generators are other sources of X-rays.
4. Applications of X-Rays
Medical Imaging: X-ray imaging is widely used in medicine for diagnostic purposes. It allows healthcare professionals to visualize the internal structures of the body, detect fractures, identify diseases, and guide surgical procedures.
Industrial Inspection and Non-Destructive Testing: X-rays are employed in industrial settings to inspect welds, detect defects in materials, and ensure the quality of manufactured products. This non-destructive testing technique is crucial for safety and quality control.
Security Screening: X-ray scanners are used for security purposes, such as at airports and border crossings. They can detect concealed weapons, explosives, and other contraband items.
Astrophysics and Astronomy: X-rays provide valuable insights into the universe. X-ray telescopes in space capture X-ray emissions from distant celestial objects, revealing the high-energy processes occurring in stars, galaxies, and black holes.
5. Safety and Risks Associated with X-Rays
Radiation Exposure and Protection: X-rays can pose health risks when exposure levels are excessive. Radiation protection measures, such as lead shielding and limiting exposure time, are essential to mitigate these risks.
Medical X-Ray Safety: In medical settings, practitioners follow strict safety protocols to minimize patient and staff exposure to X-rays. Lead aprons, collimators, and dose monitoring are commonly used safeguards.
Industrial and Research X-Ray Safety: Industrial and research facilities adhere to safety guidelines to protect workers from radiation exposure. Protective barriers, dosimetry, and training are crucial for safety.
6. Advancements in X-Ray Technology
Digital X-Ray Imaging: Digital X-ray imaging has replaced traditional film-based radiography in many applications. It offers faster image acquisition, lower radiation doses, and the ability to enhance and share images digitally.
X-Ray Crystallography: X-ray crystallography is a powerful technique used to determine the atomic and molecular structure of crystals. It has profound applications in chemistry, biology, and materials science.
Conclusion
X-rays have revolutionized various fields, from healthcare to industry and astrophysics. Their ability to penetrate matter and reveal hidden details continues to drive advancements in technology and scientific understanding. As technology evolves, X-rays will remain a valuable tool for exploring the world around us and the mysteries of the universe.
Read More
- Electric Circuit Electrical Symbols
- Difference Between Watts And Volts
- Molar Mass Of Sulphur
- Electronics In Daily Life
- Molecular Weight Of Hcl
Frequently Asked Questions (FAQs) Electromagnetic Spectrum X Rays
1. What is the electromagnetic spectrum?
The electromagnetic spectrum is the entire range of electromagnetic waves, including radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays. It spans from low-energy, long-wavelength waves to high-energy, short-wavelength waves.
2. Where do X-rays fit in the electromagnetic spectrum?
X-rays fall between ultraviolet (UV) radiation and gamma rays in the electromagnetic spectrum. They have shorter wavelengths and higher energy compared to visible light.
3. How were X-rays discovered?
X-rays were discovered by Wilhelm Conrad Roentgen in 1895 while he was conducting experiments with cathode rays. His discovery was accidental and led to the first X-ray image of the human body.
4. What are the characteristics of X-rays?
X-rays are high-energy electromagnetic waves.
- They can penetrate matter, including human tissues and materials.
- X-rays are invisible to the human eye.
- They can ionize atoms, causing the ejection of electrons.
5. How are X-rays generated?
X-rays are typically generated by accelerating electrons and directing them at a target material, usually made of metal like tungsten. When high-speed electrons strike the target, X-rays are produced as a result of interactions between electrons and atoms in the target.
Electrical Energy And Power
Electrical Energy And Power: Electricity is a ubiquitous force that powers our modern world. It lights up our homes, charges our devices, and drives our industries.
Understanding the concepts of electrical energy and power is fundamental to harnessing and managing this vital resource efficiently. In this article, we delve into the principles of electrical energy and power, exploring their definitions, formulas, units, and practical applications.

Electrical Energy And Power
Table of Contents:
1. Introduction to Electrical Energy and Power
- The Role of Electricity in Modern Life
- The Difference Between Electrical Energy and Power
2. What is Electrical Energy?
- Definition and Basics
- Units of Electrical Energy
- Calculating Electrical Energy Consumption
3. Understanding Electrical Power
- Definition and Basics
- Units of Electrical Power
- Calculating Electrical Power
4. The Relationship Between Energy and Power
- The Power-Energy Time Relationship
- Energy Efficiency and Conservation
5. Practical Applications of Electrical Energy and Power
- Residential Electricity Consumption
- Industrial Applications
- Renewable Energy Sources
5. Measuring and Billing for Electrical Energy
- Electricity Meters
- Understanding Your Electricity Bill
6. Challenges and Future Trends
- Power Generation and Sustainability
- Energy Storage Technologies
Conclusion
- The Endless Potential of Electrical Energy and Power
1. Introduction to Electrical Energy and Power
The Role of Electricity in Modern Life: Electricity is a versatile form of energy that plays a pivotal role in our daily lives. It powers our homes, enabling lighting, heating, and cooling, as well as operating our appliances and electronics. Beyond residential use, electricity drives industries, fuels transportation, and is a cornerstone of technological advancements.
The Difference Between Electrical Energy and Power: Electrical energy and power are related but distinct concepts. Electrical energy refers to the total amount of energy consumed or produced over time, while electrical power represents the rate at which energy is used or generated.
Units of Electrical Energy:
- Joule (J): The joule is the standard unit of energy in the International System of Units (SI).
- Kilowatt-hour (kWh): The kilowatt-hour is commonly used for billing and represents the energy consumption of 1 kilowatt of power used for 1 hour.
Calculating Electrical Energy Consumption: To calculate electrical energy consumption, you can use the formula: Energy (kWh) = Power (kW) × Time (hours)
3. Understanding Electrical Power
Definition and Basics: Electrical power is the rate at which electrical energy is used or generated. It measures how quickly work is done by electric currents and is typically expressed in watts (W) or kilowatts (kW).
Units of Electrical Power:
- Watt (W): The watt is the SI unit of power, representing one joule of energy per second.
- Kilowatt (kW): One kilowatt is equal to 1,000 watts.
Calculating Electrical Power: The formula for calculating electrical power is: Power (kW) = Energy (kWh) / Time (hours)
4. The Relationship Between Energy and Power
The Power-Energy Time Relationship: The relationship between energy, power, and time is crucial. Power (P) is the rate at which energy (E) is used or generated, and time (t) is the duration over which this power is applied. This relationship is expressed by the formula: E = P × t
Energy Efficiency and Conservation: Efficiency is a critical consideration when using electrical energy. Efficient devices and systems consume less energy for the same amount of work, reducing costs and environmental impact. Conservation measures, such as using energy-efficient appliances and practices, contribute to sustainability.
5. Practical Applications of Electrical Energy and Power
Residential Electricity Consumption: Electricity consumption in homes varies depending on factors like location, climate, and lifestyle. Appliances, lighting, heating, and cooling systems are significant contributors to residential energy use.
Industrial Applications: Industries rely heavily on electrical power for manufacturing processes, machinery, and equipment. Optimizing power usage is crucial for productivity and cost-effectiveness.
Renewable Energy Sources: Renewable energy sources, such as solar and wind power, are harnessed to generate electricity sustainably. These sources play a vital role in reducing greenhouse gas emissions and transitioning to cleaner energy production.
6. Measuring and Billing for Electrical Energy
Electricity Meters: Electricity meters, also known as watt-hour meters, measure the amount of electrical energy consumed in kilowatt-hours (kWh). They are installed by utility companies to monitor usage and determine billing.
Understanding Your Electricity Bill: Electricity bills typically detail your energy consumption, rate structure, and charges. Understanding your bill can help you manage and reduce your electricity costs.
7. Challenges and Future Trends
Power Generation and Sustainability: One of the major challenges in the energy sector is transitioning to sustainable power generation. This involves reducing reliance on fossil fuels, increasing renewable energy capacity, and improving energy storage technologies.
Energy Storage Technologies: Developing efficient energy storage solutions, such as advanced batteries and supercapacitors, is crucial for storing excess energy from renewable sources and ensuring a stable power supply.
8. Conclusion
Electricity is a cornerstone of modern civilization, powering our homes, industries, and technological advancements. Understanding electrical energy and power is essential for using this resource efficiently and sustainably. As we strive to reduce our environmental impact and develop cleaner energy sources, the concepts explored in this article will continue to be at the forefront of our efforts to illuminate and power the world.
Read More
- Dynamics Of Circular Motion
- Molecular Weight Of Acetic Acid
- Difference Between Torque And Power
- Difference Between Emission And Absorption Spectra
- Ionic Product Of Water
Frequently Asked Questions (FAQs) Electric Energy And Power
1. What is the difference between electric energy and electrical power?
Electrical Energy: Electrical energy is the total amount of energy consumed or generated over a period. It is typically measured in units such as joules (J) or kilowatt-hours (kWh).
Electric Power: Electric power is the rate at which electric energy is used or generated per unit of time. It is typically measured in watts (W) or kilowatts (kW).
2. How is electrical energy calculated?
To calculate electrical energy consumption, use the formula: Energy (kWh) = Power (kW) × Time (hours). This formula multiplies the power used (in kilowatts) by the time it is used (in hours) to determine energy consumption in kilowatt-hours (kWh).
3. What are some common examples of electrical power ratings for appliances?
Common household appliances and their power ratings include:
- Lightbulbs: 60W to 100W
- Refrigerators: 100W to 800W
- Air Conditioners: 1,000W to 5,000W (1kW to 5kW)
- Microwave Ovens: 600W to 1,200W
4. Why is it important to understand electric power and energy?
Understanding electric power and energy consumption helps individuals and industries make informed decisions about energy usage, efficiency, and cost savings. It also contributes to environmental sustainability by promoting efficient energy use.
5. What is the significance of energy efficiency in electrical devices?
Energy-efficient devices consume less electric power while providing the same or better performance. This reduces electricity bills, lowers environmental impact, and extends the lifespan of electrical equipment.
Electric Circuit Electrical Symbols
Electric Circuit Electrical Symbols: Creating and understanding electrical circuits is a fundamental skill in the field of electrical engineering and electronics.
These circuits are composed of various components that work together to perform specific tasks, from powering your household appliances to controlling complex industrial systems. To design and analyze circuits effectively, engineers and technicians use a standardized set of electrical symbols to represent components and connections.
Electric Circuit Electrical Symbols
Table of Contents:
Introduction to Electrical Symbols
- The Importance of Standardized Symbols
- Historical Development of Electrical Symbols
Common Electrical Symbols
- Passive Components
- Active Components
- Power Sources and Generators
- Connectors and Wires
- Switches and Relays
- Measuring Instruments
- Transformers and Inductors
- Capacitors and Batteries
- Diodes and Transistors
Understanding Electrical Circuit Diagrams
- Schematic Diagrams
- Wiring Diagrams
- Block Diagrams
How to Read Electrical Symbols
- Identifying Components and Their Functions
- Analyzing Circuit Connections
Applications and Industries
- Residential Electrical Wiring
- Automotive Electronics
- Industrial Control Systems
- Telecommunications
- Aerospace and Avionics
Designing Electrical Circuits
- Circuit Design Software
- Prototyping and Testing
Safety Considerations
- Electrical Hazards
- Safety Symbols
Conclusion
- The Ubiquity of Electrical Circuits
- The Role of Electrical Symbols in Engineering
1. Introduction to Electrical Symbols
Electrical symbols are graphical representations of electrical and electronic components used in circuit diagrams. These symbols serve as a universal language for engineers, electricians, and technicians to communicate circuit designs and concepts efficiently. Standardized symbols ensure that anyone familiar with electrical diagrams can understand and work with them regardless of their geographic location or language.
The Importance of Standardized Symbols: Standardized symbols simplify the design, analysis, and troubleshooting of electrical circuits. They eliminate ambiguity and reduce the chances of errors in circuit diagrams. This consistency is crucial in industries where safety and reliability are paramount.
Historical Development of Electrical Symbols: The history of electrical symbols dates back to the early days of electrical engineering. Engineers and scientists like Charles Wheatstone, Michael Faraday, and Samuel Morse developed rudimentary symbols to represent electrical components in their diagrams. Over time, these symbols evolved and were standardized by organizations like the International Electrotechnical Commission (IEC) and the Institute of Electrical and Electronics Engineers (IEEE).
2. Common Electrical Symbols
Electrical symbols encompass a wide range of components, including passive and active components, power sources, connectors, switches, measuring instruments, and more. Here’s an overview of some common electrical symbols:
Passive Components:
- Resistor (R): Represents a passive component that resists the flow of electrical current.
- Capacitor (C): Symbolizes a device used to store electrical energy.
- Inductor (L): Represents a component that stores energy in a magnetic field.
Active Components:
- Voltage Source: Depicts a source of electrical voltage, such as a battery.
- Current Source: Represents a source that provides a constant current.
- Transistor (NPN and PNP): Symbols for NPN and PNP bipolar junction transistors.
- Operational Amplifier (Op-Amp): Symbolizes a versatile analog component used in amplification and signal processing.
Power Sources and Generators:
- Battery: Represents a battery or a collection of batteries.
- Generator: Symbolizes a power generator, such as a turbine or alternator.
Connectors and Wires:
- Connector: Signifies a connection point or terminal.
- Wire: Represents a conductor used to connect components.
- Ground: Symbol for electrical ground, typically connected to the Earth.
Switches and Relays:
- Switch (SPST, SPDT, DPST, DPDT): Various symbols for switches with different configurations.
- Relay: Represents an electromagnetic switch controlled by a separate circuit.
Measuring Instruments:
- Voltmeter: Symbol for a device used to measure voltage.
- Ammeter: Symbol for a device used to measure current.
- Ohmmeter: Represents a device used to measure resistance.
Transformers and Inductors:
- Transformer: Symbolizes a device used to change the voltage level in an AC circuit.
- Mutual Inductor: Represents a transformer with multiple windings.
Capacitors and Batteries:
- Polarized Capacitor: Symbolizes a capacitor with polarity.
- Non-Polarized Capacitor: Represents a capacitor without polarity.
- Cell (Battery): Symbol for an individual cell or battery.
Diodes and Transistors:
- Diode (Rectifier, Zener, Light-Emitting Diode): Various symbols for diodes with different functions.
- Field-Effect Transistor (FET): Symbolizes a type of transistor.
- These symbols form the building blocks of electrical and electronic circuit diagrams, allowing engineers and technicians to represent complex systems succinctly.
3. Understanding Electrical Circuit Diagrams
- Electrical circuit diagrams, also known as schematics, are graphical representations of electrical circuits using standardized symbols. These diagrams convey the relationships between components and the flow of electrical current within a circuit. There are different types of circuit diagrams, including schematic diagrams, wiring diagrams, and block diagrams.
Schematic Diagrams:
- Schematic diagrams provide a detailed representation of individual components and their connections within a circuit.
- They are commonly used for complex electronic circuits, control systems, and integrated circuits.
- Schematic diagrams are essential for circuit design, analysis, and troubleshooting.
Wiring Diagrams:
- Wiring diagrams focus on the physical layout of components and their interconnections within a system.
- They are often used in the automotive industry, household electrical wiring, and building control systems.
- Wiring diagrams help technicians understand how to physically wire a circuit.
Block Diagrams:
- Block diagrams provide a high-level overview of a system or circuit.
- They use blocks to represent major components or subsystems and arrows to indicate the flow of signals or information between them.
- Block diagrams are useful for understanding the functional relationships within a complex system.
4. How to Read Electrical Symbols
- Reading electrical symbols and understanding their meanings is crucial for interpreting circuit diagrams. Here are some key principles for reading electrical symbols effectively:
- Identifying Components and Their Functions:
- Familiarize yourself with common electrical symbols and their corresponding components.
- Understand the functions of each component, including passive and active elements, sources, and connectors.
Analyzing Circuit Connections:
- Follow the lines and connections in the diagram to trace the path of electrical current.
- Note the direction of arrowheads, which indicate the flow of signals or currents.
- Pay attention to the labels and annotations to understand component values, such as resistance or voltage.
Referencing a Key:
- Some circuit diagrams include a key or legend that provides explanations for symbols used in the diagram.
- Refer to the key when encountering unfamiliar symbols or components.
Consider Context:
- Analyze the overall context of the circuit and the specific function it is designed to perform.
- Take into account the purpose of the circuit, whether it’s for amplification, control, signal processing, or power distribution.
5. Applications and Industries
- Electrical circuit diagrams and symbols find applications in various industries and sectors, each with its specific requirements and standards:
Residential Electrical Wiring:
- In residential settings, electrical symbols are used in house wiring diagrams to plan and install electrical systems safely.
- These diagrams ensure that electrical connections meet building codes and safety standards.
Automotive Electronics:
- Electrical symbols are prevalent in automotive wiring diagrams, enabling technicians to diagnose and repair electrical issues in vehicles.
- Modern automobiles rely on complex electrical systems for engine control, infotainment, lighting, and safety features.
Industrial Control Systems:
- Industrial circuits often employ complex control systems with sensors, actuators, and programmable logic controllers (PLCs).
- Electrical symbols help engineers design and maintain industrial control systems used in manufacturing and process industries.
Telecommunications:
- The telecommunications industry uses circuit diagrams for designing and troubleshooting communication systems, including network infrastructure and mobile devices.
Aerospace and Avionics:
- Avionics systems on aircraft, spacecraft, and drones heavily rely on electrical symbols and diagrams to ensure reliable communication, navigation, and control.
6. Designing Electrical Circuits
- Designing electrical circuits involves creating schematics, selecting components, and ensuring the circuit meets specific requirements. Engineers use specialized software tools for circuit design and simulation. Some common steps in designing electrical circuits include:
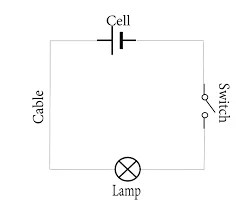
Circuit Design Software:
- Engineers use software tools like AutoCAD Electrical, Eagle, OrCAD, and KiCad to create schematic diagrams, simulate circuit behavior, and generate Bill of Materials (BOM).
Prototyping and Testing:
- After designing a circuit on paper or in software, engineers often build prototypes to test and verify its functionality.
- Prototyping involves selecting physical components, creating a physical circuit, and conducting tests.
Iterative Design:
- Engineers may go through multiple iterations of circuit design and testing to optimize performance, reduce costs, or address specific requirements.
7. Safety Considerations
- Working with electrical circuits presents inherent hazards, including electrical shock, fire, and equipment damage. To ensure safety, electrical diagrams and components may include safety symbols and labels. These symbols warn about potential dangers and provide guidance on safe practices.
Electrical Hazards:
- Electrical hazard symbols indicate the presence of electrical energy and warn individuals to take precautions.
- Symbols for high voltage, electrical shock, and grounding are common safety symbols.
Safety Symbols:
Safety symbols on electrical components or equipment may include information on safe operation, such as voltage limits and safety instructions.
Safety Precautions:
- When working with electrical circuits, always follow safety protocols, wear appropriate personal protective equipment (PPE), and disconnect power sources before handling electrical components.
8. Conclusion
- Electrical symbols play a crucial role in electrical engineering, electronics, and many other industries where electrical circuits are designed, analyzed, and maintained. These symbols provide a standardized and universally understood means of representing components and connections within circuits, simplifying communication among professionals worldwide.
- From the residential wiring in our homes to the intricate systems powering advanced technologies, electrical symbols and circuit diagrams are at the heart of modern electrical and electronic systems. As technology continues to advance, the need for clear and standardized electrical symbols remains vital in ensuring the safety, reliability, and efficiency of electrical circuits and systems.
Read More
- Difference Between Watts And Volts
- Molar Mass Of Sulphur
- Electronics In Daily Life
- Molecular Weight Of Hcl
- Asteroid And Comet Difference
Frequently Asked Questions (FAQs) Electric Circuit Electrical Symbols
1. What are electric circuit electrical symbols, and why are they important?
Electric circuit electrical symbols are graphical representations of electrical and electronic components used in circuit diagrams. They are essential for communicating circuit designs and concepts effectively. Standardized symbols ensure clarity, consistency, and precision in circuit diagrams.
2. How do electric circuit electrical symbols simplify circuit design and analysis?
Electric circuit electrical symbols simplify circuit design and analysis by providing a universal language for representing components. Engineers and technicians can quickly understand and work with circuit diagrams, making it easier to design, troubleshoot, and maintain electrical systems.
3. Where can I find a comprehensive list of electric circuit electrical symbols and their meanings?
You can find comprehensive lists of electric circuit electrical symbols, along with their meanings, in electrical engineering textbooks, online resources, and dedicated symbol libraries in circuit design software. These resources typically include symbols for passive components, active components, power sources, connectors, switches, and more.
4. Are there international standards for electric circuit electrical symbols?
Yes, there are international standards for electric circuit electrical symbols. Organizations like the International Electrotechnical Commission (IEC) and the Institute of Electrical and Electronics Engineers (IEEE) have established standardized symbols to ensure consistency in circuit diagrams worldwide. These standards facilitate cross-border communication and understanding.
5. How do I interpret circuit diagrams that use electric circuit electrical symbols?
Interpreting circuit diagrams involves understanding the meanings of electrical symbols and tracing the path of electrical current through the diagram. Key principles include identifying components, recognizing connections, following the flow of signals or currents, and considering the context and purpose of the circuit.
Dynamics Of Circular Motion
Dynamics Of Circular Motion: Circular motion, characterized by the continuous movement of an object along the circumference of a circle, is a ubiquitous phenomenon in our daily lives.
From the Earth revolving around the Sun to the spinning wheels of a car, circular motion is governed by a set of fundamental principles that involve forces, acceleration, and velocity. In this article, we delve into the dynamics of circular motion, shedding light on the key concepts that drive objects in circular paths.
Dynamics Of Circular Motion
The Basics of Circular Motion
Before delving into the dynamics, it’s important to understand the basic elements of circular motion:
- Centripetal Force: Circular motion requires a force directed toward the center of the circle, known as the centripetal force. This force keeps the object moving in a curved path and prevents it from moving in a straight line (according to Newton’s First Law).
- Tangential Velocity: Objects in circular motion have a tangential velocity, which is the velocity along the tangent to the circle at any given point. This velocity is always changing in magnitude because the direction of motion is constantly changing.
- Angular Velocity: Angular velocity measures the rate of change of angular displacement with respect to time. It is usually denoted by the Greek letter ω (omega) and is measured in radians per second (rad/s).
The Centripetal Force: A Fundamental Component
- The centripetal force is central to understanding the dynamics of circular motion. Its magnitude depends on the mass of the object in motion (m), the radius of the circular path (r), and the object’s tangential velocity (v). Mathematically, it can be expressed as:
- F_c = (mv^2) / r
Where:
- F_c represents the centripetal force.
- m is the mass of the object.
- v is the tangential velocity of the object.
- r is the radius of the circular path.
- Newton’s Second Law and Circular Motion
- Applying Newton’s second law to circular motion, we find that the net force acting on an object moving in a circle is equal to the mass of the object times its centripetal acceleration (a_c):
- ΣF = ma_c
- Since acceleration is the change in velocity over time, we can express the centripetal acceleration as:
- a_c = (Δv) / Δt
- In circular motion, the direction of velocity is constantly changing, which means there is an acceleration even if the speed remains constant. This acceleration is directed towards the center of the circle, in line with the centripetal force.
Banked Curves and Non-Uniform Circular Motion
- In some cases, circular motion involves banked curves, as seen in race tracks and highways. Banked curves are designed to allow vehicles to move safely around corners at high speeds. The angle of banking and the speed of the vehicle are crucial factors in these scenarios.
- Additionally, not all circular motion is uniform, where the object travels at a constant speed. Non-uniform circular motion involves changing speed, which requires a varying centripetal force.
Real-World Applications
- Understanding the dynamics of circular motion has far-reaching applications:
- Orbital Mechanics: The motion of planets, satellites, and celestial bodies in orbit is governed by circular motion principles.
- Automotive Engineering: Understanding circular motion is essential for designing safe and efficient roadways and racetracks.
- Amusement Rides: Roller coasters, carousels, and other amusement park rides rely on circular motion dynamics to create thrilling experiences.
- Sports: Concepts of circular motion are integral in sports such as racing, figure skating, and gymnastics.
In conclusion, the dynamics of circular motion are a fundamental part of physics and have numerous practical applications. Whether it’s the Earth’s rotation around the Sun or the dynamics of a spinning top, the principles of circular motion help us comprehend the world around us and engineer solutions for a wide range of challenges.
Read More
- Molecular Weight Of Acetic Acid
- Difference Between Torque And Power
- Difference Between Emission And Absorption Spectra
- Ionic Product Of Water
- Hunds Rule Of Maximum Multiplicity
Frequently Asked Questions (FAQs) Dynamics Of Circular Motion
1. What is circular motion, and how does it differ from linear motion?
Circular Motion: Circular motion is the continuous movement of an object along the circumference of a circle or a curved path. In circular motion, the object’s direction changes at every point along the path.
Linear Motion: Linear motion is the movement of an object along a straight line, where its speed and direction remain constant.
2. What is centripetal force, and why is it necessary for circular motion?
Centripetal force is a force that acts on an object moving in a circular path, directing it toward the center of the circle. It is essential for circular motion because it keeps the object from moving in a straight line and ensures it follows the curved path.
3. How is centripetal force related to circular motion dynamics?
Centripetal force is the force responsible for maintaining an object’s circular motion. It is crucial for keeping the object on its curved path, as it continuously changes the object’s direction toward the center of the circle.
4. What determines the magnitude of the centripetal force in circular motion?
The magnitude of the centripetal force depends on several factors, including the mass of the object (m), the tangential velocity of the object (v), and the radius of the circular path (r). The formula for centripetal force is F_c = (mv^2) / r.
5. How does the velocity of an object change in circular motion?
In circular motion, the velocity of an object is not constant because its direction is continually changing. While the object may travel at a constant speed, its velocity vector keeps rotating with the motion, leading to acceleration.
Dipole Uniform Magnetic Field
Dipole Uniform Magnetic Field: The interaction between dipoles and magnetic fields is a fundamental concept in physics, particularly in the study of electromagnetism.
In this article, we will delve into the fascinating world of dipoles within a uniform magnetic field, exploring the principles, behaviors, and real-world applications of this phenomenon.

Dipole Uniform Magnetic Field
Understanding Dipoles
Before delving into the interaction between dipoles and magnetic fields, let’s clarify what a dipole is. In physics, a dipole refers to a pair of electric charges or magnetic poles with equal and opposite properties separated by some distance. Dipoles can be electric or magnetic.
Uniform Magnetic Fields
A uniform magnetic field, as the name suggests, is a magnetic field that has consistent strength and direction throughout a specified region of space. In such a field, magnetic lines of force run parallel and evenly spaced, creating a uniform environment.
Dipole in a Uniform Magnetic Field: The Torque Effect
When a magnetic dipole is placed within a uniform magnetic field, an interesting phenomenon occurs. The dipole experiences a rotational force known as torque. This torque is caused by the alignment of the dipole with the magnetic field, seeking the configuration of minimum potential energy.
Key points about this interaction:
- Alignment: The magnetic dipole will tend to align itself with the direction of the uniform magnetic fields. This alignment is analogous to a compass needle aligning with the Earth’s magnetic field.
- Torque: The torque applied to the dipole is proportional to the strength of the magnetic field, the dipole moment (a measure of the dipole’s strength), and the sine of the angle between the dipole moment and the magnetic field direction. The torque causes the dipole to rotate.
- Potential Energy: As the dipole aligns with the magnetic field, its potential energy decreases. When fully aligned, the potential energy is at a minimum.
Mathematical Representation
The torque (τ) experienced by a magnetic dipole in a uniform magnetic field is given by the formula:
τ = μBsinθ
Where:
- τ represents the torque.
- μ represents the dipole moment, a measure of the dipole’s strength.
- B represents the magnetic field strength.
- θ represents the angle between the dipole moment direction and the magnetic field direction.
Applications and Real-World Relevance
The interaction between dipoles and uniform magnetic fields finds applications in various real-world scenarios:
- Magnetic Compasses: Compass needles are essentially small magnetic dipoles that align with Earth’s magnetic field, aiding in navigation.
- MRI (Magnetic Resonance Imaging): In medical imaging, MRI machines use the principles of dipole interaction with magnetic fields to produce detailed images of the human body’s internal structures.
- Particle Accelerators: Magnetic dipoles are used in particle accelerators to steer and control the paths of charged particles.
- Electromagnetic Motors: The operation of electric motors involves the interaction of magnetic dipoles with magnetic fields, resulting in rotational motion.
In summary, the interaction between dipoles and uniform magnetic fields, characterized by the torque effect, is a fundamental concept in electromagnetism. This phenomenon underlies various technological applications, from compasses to medical imaging and particle accelerators, showcasing the profound impact of electromagnetic principles on our daily lives and scientific advancements.
Read More
- Light Filtering Polaroid Films Polarization
- Animals Biology Science Lion Zoology
- Difference Between Voltage And Current
- Difference Between Two Stroke And Four Stroke
- Difference Between Ac And Dc
Frequently Asked Questions (FAQs) Dipole Uniform Magnetic Field
1. What is a magnetic dipole in the context of a uniform magnetic field?
In physics, a magnetic dipole is a pair of magnetic poles with equal and opposite properties separated by some distance. When placed in a uniform magnetic field, it experiences a torque (rotational force) due to the alignment-seeking nature of magnetic dipoles.
2. How does a magnetic dipole align itself in a uniform magnetic field?
A magnetic dipole will tend to align itself with the direction of the uniform magnetic field. It aligns so that its magnetic moment (analogous to the dipole moment) points in the same direction as the magnetic field lines.
3. What causes the torque on a magnetic dipole in a uniform magnetic field?
The torque experienced by a magnetic dipole in a uniform magnetic field is due to the magnetic moment trying to align itself with the field direction. This torque is proportional to the strength of the magnetic field, the magnetic moment of the dipole, and the sine of the angle between the magnetic moment and the field direction.
4. What is the significance of potential energy in this interaction?
As the magnetic dipole aligns itself with the magnetic field, its potential energy decreases. When fully aligned, the potential energy reaches a minimum. This is a fundamental concept in understanding the stability of the dipole in the field.
5. How is the torque on a magnetic dipole mathematically represented?
The torque (τ) on a magnetic dipole in a uniform magnetic field is given by the formula τ = μBsinθ, where:
- τ represents the torque.
- μ represents the magnetic moment (dipole moment).
- B represents the magnetic field strength.
- θ represents the angle between the magnetic moment direction and the magnetic field direction.
Molecular Weight Of Acetic Acid
Molecular Weight Of Acetic Acid: The molecular weight of acetic acid (CH3COOH) can be calculated by adding up the atomic masses of all the atoms in one molecule of acetic acid:
Molecular Weight Of Acetic Acid
Acetic Acid (CH3COOH): An Introduction
Acetic acid, known by its chemical formula CH3COOH, is a familiar and essential compound in both natural and industrial contexts. It is the main component of vinegar, providing its characteristic sour taste and pungent odor. Beyond its culinary significance, acetic acid finds applications in a range of industries, including food processing, pharmaceuticals, and chemical manufacturing.
Breaking Down the Molecular Weight
The molecular weight of any compound is determined by summing the atomic masses of all the atoms present in one molecule of that compound. To calculate the molecular weight of acetic acid (CH3COOH), we must consider the atomic masses of its constituent elements:
- Carbon (C): Carbon has an atomic mass of approximately 12.01 grams/mole (g/mol).
- Hydrogen (H): Hydrogen has an atomic mass of approximately 1.01 g/mol.
- Oxygen (O): Oxygen has an atomic mass of around 16.00 g/mol.
Let’s delve into the calculations:
Molecular Weight of CH3COOH = (Atomic Mass of C) + (2 * Atomic Mass of H) + (2 * Atomic Mass of O)
Molecular Weight of CH3COOH = (12.01 g/mol) + (2 * 1.01 g/mol) + (2 * 16.00 g/mol)
Molecular Weight of CH3COOH = 12.01 g/mol + 2.02 g/mol + 32.00 g/mol
Molecular Weight of CH3COOH ≈ 60.03 grams/mole
Therefore, the molecular weight of acetic acid (CH3COOH) is approximately 60.03 grams per mole.
Significance of Molecular Weight
Understanding the molecular weight of a compound is paramount in various scientific disciplines, especially in chemistry. It serves as a fundamental parameter for precise calculations in chemical reactions, stoichiometry, and determining concentrations. Molecular weight allows scientists to unravel the composition and properties of substances, offering critical insights into their behavior and reactions.
Applications of Acetic Acid
Acetic acid, with its molecular weight of approximately 60.03 g/mol, finds applications in diverse fields, including:
- Food Industry: It is used for flavoring, preserving, and pickling foods, particularly in the production of vinegar.
- Chemical Industry: It serves as a precursor for the manufacture of various chemicals, plastics, and solvents.
- Pharmaceuticals: Acetic acid is utilized in the synthesis of medications and pharmaceutical compounds.
- Laboratory Work: It is a common reagent for chemical experiments, titrations, and pH adjustments.
- In conclusion, the molecular weight of acetic acid (CH3COOH) provides valuable insights into its composition and behavior in chemical processes. With a molecular weight of approximately 60.03 g/mol, acetic acid stands as a versatile compound with profound importance in multiple industrial, scientific, and everyday applications. Its ability to impart flavor, preserve, and participate in chemical reactions showcases the intricate role of chemistry in our daily lives.
Read More
- Difference Between Torque And Power
- Difference Between Emission And Absorption Spectra
- Ionic Product Of Water
- Hunds Rule Of Maximum Multiplicity
- Thermodynamics class 11 physics
Frequently Asked Questions (FAQs) Molecular Weight Of Acetic Acid
1. What is the molecular weight of acetic acid (CH3COOH)?
The mol weight of acetic acid (CH3COOH) is approximately 60.03 grams per mole (g/mol).
2. How is the mol weight of acetic acid calculated?
The molecular weight of any compound is calculated by summing the atomic masses of all the atoms present in one molecule of that compound. For acetic acid (CH3COOH), you add up the atomic masses of carbon (C), hydrogen (H), and oxygen (O) as follows:
- Carbon (C): Atomic mass ≈ 12.01 g/mol
- Hydrogen (H): Atomic mass ≈ 1.01 g/mol
- Oxygen (O): Atomic mass ≈ 16.00 g/mol
The calculation is: Molecular Weight of CH3COOH = (Atomic Mass of C) + (2 * Atomic Mass of H) + (2 * Atomic Mass of O)
3. Why is knowing the molecular weight of acetic acid important in chemistry?
nderstanding the molecular weight of a compound is crucial in various scientific and industrial applications. It is fundamental for precise calculations in chemical reactions, stoichiometry, and determining concentrations. Molecular weight helps identify and analyze substances, predict their behavior in reactions, and design experiments.
4. What are the practical applications of acetic acid in everyday life?
Acetic acid (CH3COOH) has diverse applications, including:
- Food industry: It’s used for flavoring, preserving, and pickling foods, particularly in vinegar production.
- Chemical industry: It serves as a precursor for the production of various chemicals, plastics, and solvents.
- Pharmaceuticals: Acetic acid is used in the synthesis of medications and pharmaceutical compounds.
- Laboratory work: It’s a common reagent for chemical experiments, titrations, and pH adjustments.
5. How is acetic acid commonly encountered in household products?
- Acetic acid is often encountered in household products such as:
- Vinegar: Used for culinary purposes, cleaning, and as a natural household cleaner.
- Cleaning solutions: It’s used in cleaning products for its antimicrobial and degreasing properties.
- Fabric softeners: Acetic acid is sometimes used as a fabric softener and odor remover.

