Blog
Equivalent Weight Of H2so4
Equivalent Weight Of H2so4: The equivalent weight of a compound depends on its chemical formula and the specific reaction it is involved in. To calculate the equivalent weight of sulfuric acid (H₂SO₄) for a particular reaction, you need to know the reaction and the role of sulfuric acid in that reaction.
The equivalent weight is used in stoichiometry to represent the amount of a substance that provides or consumes one mole of electrons or reacts with one mole of a specific reagent. It is often used in acid-base reactions, redox reactions, and other chemical processes.
For sulfuric acid (H₂SO₄), it is essential to consider whether it is acting as a monoprotic, diprotic, or polyprotic acid in the reaction.

Equivalent Weight Of H2so4
1. Monoprotic Acid: If sulfuric acid is acting as a monoprotic acid (donating one hydrogen ion, H⁺), its equivalent weight is equal to its molar mass divided by the number of moles of H⁺ ions it donates. The molar mass of H₂SO₄ is approximately 98.08 g/mol.
Equivalent Weight (monoprotic H₂SO₄) = Molar Mass (H₂SO₄) / 1 (since it donates one H⁺ ion) Equivalent Weight (monoprotic H₂SO₄) ≈ 98.08 g/mol
2. Diprotic Acid: If sulfuric acid is acting as a diprotic acid (donating two hydrogen ions, H⁺), its equivalent weight is calculated by dividing its molar mass by the number of moles of H⁺ ions it donates, which is 2.
Equivalent Weight (diprotic H₂SO₄) = Molar Mass (H₂SO₄) / 2 (since it donates two H⁺ ions) Equivalent Weight (diprotic H₂SO₄) ≈ 98.08 g/mol / 2 ≈ 49.04 g/mol
In this case, the equivalent weight of diprotic sulfuric acid is approximately 49.04 g/mol.
3. Polyprotic Acid: Sulfuric acid can also act as a polyprotic acid in certain reactions, donating more than two hydrogen ions. In such cases, the equivalent weight would be adjusted accordingly based on the number of moles of H⁺ ions donated.
It’s important to note that the equivalent weight is a specific value for a particular reaction, and it may vary depending on the context of the chemical reaction in which sulfuric acid is involved. To determine the appropriate equivalent weight for a given reaction, you should consider the stoichiometry and the specific role of sulfuric acid in that reaction.
Read More
- Molecular Weight Of Carbon
- Molecular Weight Of Hydrogen
- Molecular Weight Of Urea
- Molecular Mass Of Urea
- Molecular Mass Of Hydrogen
Frequently Asked Questions (FAQs) Equivalent Weight Of H2so4
1. What is the equivalent weight of sulfuric acid (H₂SO₄)?
The equivalent weight of sulfuric acid (H₂SO₄) depends on the specific reaction and the number of moles of hydrogen ions (H⁺) it donates. For H₂SO₄ acting as a monoprotic acid, its equivalent weight is approximately 98.08 g/mol. However, it can vary based on the reaction and the role it plays.
2. What is the significance of the equivalent weight in chemistry?
The equivalent weight is a crucial concept in stoichiometry and is used to quantify the amount of a substance that provides or consumes one mole of electrons or reacts with one mole of a specific reagent. It simplifies chemical calculations and is particularly important in acid-base reactions, redox reactions, and titrations.
3. How is the equivalent weight of H₂SO₄ calculated when it acts as a monoprotic acid?
When H₂SO₄ acts as a monoprotic acid, its equivalent weight is determined by dividing its molar mass by the number of moles of hydrogen ions (H⁺) it donates, which is 1. The molar mass of H₂SO₄ is approximately 98.08 g/mol, so the equivalent weight for monoprotic H₂SO₄ is around 98.08 g/mol.
4. Can sulfuric acid act as a diprotic acid, and how is its equivalent weight calculated in such cases?
Yes, sulfuric acid can act as a diprotic acid, donating two hydrogen ions (H⁺) in certain reactions. In such cases, its equivalent weight is calculated by dividing its molar mass by the number of moles of H⁺ ions it donates, which is 2. The equivalent weight for diprotic H₂SO₄ is approximately 49.04 g/mol.
5. Does sulfuric acid ever act as a polyprotic acid, and how does that affect its equivalent weight?
Sulfuric acid can act as a polyprotic acid, donating more than two hydrogen ions (H⁺) in specific reactions. In polyprotic scenarios, the equivalent weight is adjusted based on the number of moles of H⁺ ions donated in that particular reaction. The equivalent weight varies depending on the reaction’s stoichiometry and the specific role of sulfuric acid.
Molecular Weight Of Carbon
Molecular Weight Of Carbon: Hydrogen, the first element on the periodic table, holds a special place in the world of chemistry and physics due to its simplicity and ubiquity in the universe. One of the key characteristics that defines hydrogen is its molecular weight, a fundamental property that influences its behavior, applications, and significance in various scientific fields.

Molecular Weight Of Carbon
The Atomic Weight of Hydrogen
Before delving into the molecular weight of hydrogen, it’s essential to understand the atomic weight of a single hydrogen atom. The atomic weight of an element is essentially a weighted average of its isotopes, and hydrogen has three isotopes: protium (¹H), deuterium (²H or D), and tritium (³H or T). However, the atomic weight commonly associated with hydrogen is approximately 1.008 amu (atomic mass units), which corresponds to the most abundant isotope, protium.
Molecular Weight of Hydrogen (H₂)
The molecular weight of hydrogen is calculated by considering a molecule of hydrogen gas, H₂, which consists of two hydrogen atoms. Since each hydrogen atom has an atomic weight of approximately 1.008 amu, the calculation is straightforward:
Molecular Weight of Hydrogen (H₂) = 2 × Atomic Weight of Hydrogen ≈ 2.016 g/mol
Hence, the molecular weight of hydrogen gas (H₂) is approximately 2.016 grams per mole (g/mol). This value is incredibly low, making hydrogen the lightest element and the lightest molecule known.
Significance of Hydrogen’s Low Molecular Weight
The low molecular weight of hydrogen is a defining characteristic that has profound implications across various scientific domains:
- Efficient Fuel: Hydrogen’s low molecular weight contributes to its role as an efficient fuel source. In combustion reactions, hydrogen releases a high amount of energy per unit mass, making it an ideal choice for rockets, fuel cells, and clean energy solutions.
- Buoyant Gas: Hydrogen’s low density relative to air gives it buoyancy. In the early days of aviation, hydrogen-filled airships, such as the infamous Hindenburg, were used for transportation. However, safety concerns due to hydrogen’s flammability led to the adoption of helium, a non-flammable alternative.
- Chemical Reactivity: The low molecular weight of hydrogen results in high chemical reactivity. It readily reacts with various elements, including oxygen, forming water (H₂O) in combustion reactions. Its reactivity is fundamental in numerous chemical processes and industrial applications.
Conclusion
The molecular weight of hydrogen, approximately 2.016 g/mol, is a fundamental property that defines the behavior and applications of this remarkable element. Its lightness, efficiency as a fuel, and widespread availability make it a subject of immense interest in scientific research and technological advancements. Understanding hydrogen’s molecular weight is essential for harnessing its potential as a clean energy source, propelling space exploration, and exploring new frontiers in chemistry and physics.
Read More
- Molecular Weight Of Hydrogen
- Molecular Weight Of Urea
- Molecular Mass Of Urea
- Molecular Mass Of Hydrogen
- Molecular Mass Of Nacl
Frequently Asked Questions (FAQs) Molecular Weight Of Carbon
1. What is the molecular weight of carbon?
The molecular weight of carbon is approximately 12.011 grams per mole (g/mol). This value represents the mass of one mole of carbon atoms and is used as a basis for calculating the molecular weights of various carbon-containing compounds.
2. How is the molecular weight of carbon calculated?
The mol weight of carbon is calculated based on the atomic weight of a carbon atom. Carbon has several isotopes, but the most common isotope is carbon-12 (¹²C), which has an atomic weight of exactly 12 atomic mass units (amu). Therefore, the molecular weight of carbon is equal to the atomic weight of carbon-12:
mol Weight of Carbon = Atomic Weight of Carbon-12 ≈ 12.011 g/mol
3. Why is the molecular weight of carbon important in chemistry?
The mol weight of carbon is a fundamental property that influences the mol weights of organic and inorganic compounds. It serves as a reference point for determining the mass of carbon atoms in various molecules, enabling chemists to calculate and compare the masses of different compounds.
4. Does the molecular weight of carbon vary for different isotopes of carbon?
Yes, the mol weight of carbon can vary slightly when considering different carbon isotopes. While the most common isotope, carbon-12 (¹²C), has an atomic weight of exactly 12 amu, other isotopes like carbon-13 (¹³C) and carbon-14 (¹⁴C) have slightly different atomic weights. However, in most chemical calculations and applications, the atomic weight of carbon-12 is used for simplicity.
5. How is the molecular weight of carbon relevant in organic chemistry?
In organic chemistry, carbon is the backbone of organic compounds. The mol weight of carbon is crucial for determining the molecular weights of organic molecules and calculating stoichiometry in chemical reactions. It plays a central role in understanding the composition and properties of organic compounds.
Molecular Weight Of Hydrogen
Molecular Weight Of Hydrogen: Hydrogen, the simplest and most abundant element in the universe, plays a fundamental role in the realm of chemistry and beyond.
To comprehend its properties and interactions, scientists employ various concepts, one of which is the molecular weight. In this article, we explore the molecular weight of hydrogen, shedding light on its significance and applications in the world of science.

Molecular Weight Of Hydrogen
1. Understanding Molecular Weight
Molecular weight, also known as molar mass, refers to the mass of a molecule expressed in atomic mass units (amu) or grams per mole (g/mol). It is a crucial concept in chemistry, enabling scientists to quantify the amount of a substance by mass rather than by the number of atoms or molecules, making it a valuable tool in chemical calculations.
2. The Hydrogen Molecule (H₂)
In the case of hydrogen, the molecular weight is calculated based on the diatomic molecule H₂. Each molecule consists of two hydrogen atoms (H), and the atomic weight of hydrogen is approximately 1.008 amu. To determine the molecular weight of H₂, we add the atomic weights of its constituent atoms:
Molecular Weight of H₂ = 2 × Atomic Weight of Hydrogen ≈ 2.016 g/mol
Therefore, the molecular weight of hydrogen gas (H₂) is approximately 2.016 grams per mole (g/mol).
3. The Significance of Hydrogen’s Low Molecular Weight
Hydrogen’s exceptionally low molecular weight is one of its defining characteristics. It is not only the lightest element but also the lightest molecule. This low molecular weight contributes to several remarkable properties and applications:
- Efficient Fuel: Hydrogen’s low molecular weight makes it an excellent fuel source for rockets and combustion engines. Its combustion produces a high amount of energy per unit mass, making it an ideal choice for space exploration and fuel cells.
- Buoyant Gas: Hydrogen is lighter than air, which makes it an efficient lifting gas. In the early days of aviation, hydrogen-filled airships (such as the Hindenburg) were used for transportation, although its flammability posed significant safety concerns.
- Chemical Reactivity: Due to its low molecular weight, hydrogen gas exhibits high chemical reactivity. It readily reacts with various elements, including oxygen, forming water (H₂O) in combustion reactions.
4. Hydrogen Isotope Variants
It’s essential to note that hydrogen has three isotopes, each with different atomic weights: protium (¹H), deuterium (²H or D), and tritium (³H or T). Protium is the most common and abundant isotope, making up approximately 99.98% of naturally occurring hydrogen. Deuterium is a stable isotope, while tritium is radioactive.
- The molecular weight of hydrogen gas (H₂) primarily accounts for the protium isotope. Deuterium, which is twice as heavy as protium, results in a slightly higher molecular weight when present in hydrogen molecules.
5. Applications Beyond Chemistry
The mol weight of hydrogen extends its importance beyond the realm of chemistry:
- Energy Production: Hydrogen holds promise as a clean and sustainable energy carrier. It can be used in fuel cells to generate electricity, producing water as the only byproduct.
- Space Exploration: Hydrogen is a critical component of rocket fuels used in space exploration due to its high energy content and low molecular weight.
- Alternative Fuel: In the context of addressing environmental concerns, hydrogen is being explored as a potential alternative to fossil fuels in transportation and industry.
6. Conclusion
The mol weight of hydrogen, approximately 2.016 g/mol, is a fundamental property that characterizes this element’s diatomic molecule, H₂. Its lightweight nature contributes to its versatile applications in energy production, space exploration, and emerging technologies aimed at a sustainable future. Understanding the mol weight of hydrogen is essential for harnessing its potential as an efficient and clean energy source and for advancing our understanding of the universe at its most basic level.
Read More
- Molecular Weight Of Hydrogen
- Molecular Weight Of Urea
- Molecular Mass Of Urea
- Molecular Mass Of Hydrogen
- Molecular Mass Of Nacl
Frequently Asked Questions (FAQs) Molecular Weight Of Hydrogen
1. What is the molecular weight of hydrogen?
The mol weight of hydrogen is approximately 2.016 grams per mole (g/mol). This value represents the mass of one mole of hydrogen molecules (H₂), with each molecule consisting of two hydrogen atoms.
2. How is the molecular weight of hydrogen calculated?
The mol weight of hydrogen is calculated by adding the atomic weights of its constituent atoms. The atomic weight of hydrogen is approximately 1.008 amu (atomic mass units). Since one molecule of hydrogen (H₂) contains two hydrogen atoms, you sum the atomic weights as follows: 1.008 amu + 1.008 amu ≈ 2.016 g/mol.
3. Why is hydrogen’s molecular weight important in chemistry?
Hydrogen’s low mol weight is significant because it affects its physical and chemical properties. It makes hydrogen an efficient fuel, a buoyant gas, and a highly reactive element in various chemical reactions. Understanding its molecular weight is crucial for applications in energy, space exploration, and chemistry.
4. How does hydrogen’s molecular weight compare to other elements?
Hydrogen has the lowest molecular weight of all elements. This low mass makes it exceptionally lightweight and has practical implications, such as its use in hydrogen fuel cells and its historical use in airships.
5. Is the molecular weight of hydrogen affected by its isotopes?
Yes, the mol weight of hydrogen can be affected by its isotopes. Hydrogen has three isotopes: protium (¹H), deuterium (²H or D), and tritium (³H or T). While the mol weight primarily considers the protium isotope, the presence of deuterium in hydrogen molecules (known as heavy hydrogen) can slightly increase the mol weight due to its higher atomic weight.
Ionic Product Of Water
Ionic Product Of Water: The Ionic Product of Water (also known as the Ionization Constant of Water) is a fundamental concept in chemistry that describes the extent of water’s self-ionization or autoionization in aqueous solutions.
In simple terms, it quantifies the concentration of ions that are formed when water molecules spontaneously dissociate into hydroxide ions (OH⁻) and hydronium ions (H₃O⁺). This process is represented by the chemical equation:
H₂O(l) ⇌ H⁺(aq) + OH⁻(aq)
Here’s a detailed explanation of the Ionic Product of Water:

Ionic Product Of Water
1. Equilibrium Constant (Kw): The Ionic Product of Water is described by an equilibrium constant (Kw) that represents the ratio of the concentrations of the products (H⁺ and OH⁻ ions) to the concentration of the reactant (H₂O) when water reaches a dynamic equilibrium in an aqueous solution. Mathematically, it is expressed as:
Kw = [H⁺][OH⁻]
Where:
- [H⁺] represents the concentration of hydronium ions (H₃O⁺) in moles per liter (M).
- [OH⁻] represents the concentration of hydroxide ions (OH⁻) in moles per liter (M).
2. The Value of Kw: At a specific temperature, the value of Kw is constant and depends on the temperature of the water. At 25 degrees Celsius (25°C or 298.15 K), the accepted value of Kw is approximately 1.0 x 10⁻¹⁴ M². This means that, at this temperature, in pure water, the concentration of both H⁺ and OH⁻ ions is roughly 1.0 x 10⁻⁷ M.
3. Neutrality of Water: In pure water at 25°C, the concentration of H⁺ ions is equal to the concentration of OH⁻ ions, both being approximately 1.0 x 10⁻⁷ M. This leads to a neutral solution, as the concentrations of H⁺ and OH⁻ ions balance each other.
4. Acidic, Neutral, and Basic Solutions: Based on the values of [H⁺] and [OH⁻] in a solution, we can classify solutions as acidic, neutral, or basic:
- Acidic Solution: If [H⁺] > [OH⁻], the solution is acidic. This means there is an excess of H⁺ ions, resulting in a pH value below 7.
- Neutral Solution: In pure water or any solution where [H⁺] = [OH⁻], the solution is neutral with a pH of 7.
- Basic (Alkaline) Solution: If [OH⁻] > [H⁺], the solution is basic or alkaline. This indicates an excess of OH⁻ ions and results in a pH value above 7.
5. Temperature Dependence: The value of Kw is temperature-dependent, and as the temperature of water changes, so does the value of Kw. At higher temperatures, the self-ionization of water becomes more pronounced, leading to higher concentrations of both H⁺ and OH⁻ ions.
In summary, the Ionic Product of Water (Kw) is a fundamental constant that quantifies the extent of self-ionization in water. It helps us understand the nature of aqueous solutions, whether they are acidic, neutral, or basic, based on the concentrations of H⁺ and OH⁻ ions. This concept is essential in chemistry and is a cornerstone of understanding the pH scale and the behavior of solutions.
Read More
- Hunds Rule Of Maximum Multiplicity
- Thermodynamics class 11 physics
- Photosynthesis In Higher Plants
- Motion In A Plane
- Basic Properties Electrical Charge
Frequently Asked Questions (FAQs) Ionic Product Of Water
1. What is the Ionic Product of Water (Kw)?
The Ionic Product of Water, denoted as Kw, is a constant that represents the extent of self-ionization or autoionization of water in aqueous solutions. It quantifies the concentration of hydronium ions (H₃O⁺) and hydroxide ions (OH⁻) that are produced when water molecules dissociate.
2. How is the Ionic Product of Water related to the ionization of water?
The Ionic Product of Water is related to the ionization of water through the equilibrium expression: Kw = [H₃O⁺][OH⁻]. This equation describes the concentration of H₃O⁺ and OH⁻ ions produced when water undergoes self-ionization.
3. What is the value of Kw at 25 degrees Celsius (25°C)?
At 25°C, the accepted value of Kw is approximately 1.0 x 10⁻¹⁴ M². This means that, in pure water at this temperature, both [H₃O⁺] and [OH⁻] concentrations are approximately 1.0 x 10⁻⁷ M.
4. What does a neutral solution have to do with Kw?
A neutral solution, such as pure water, has equal concentrations of H₃O⁺ and OH⁻ ions, which are both approximately 1.0 x 10⁻⁷ M at 25°C. This equality of concentrations corresponds to the definition of neutrality and is a result of the Kw value for water.
5. How does the temperature affect the value of Kw?
The value of Kw is temperature-dependent. As the temperature increases, the self-ionization of water becomes more significant, leading to higher values of Kw. Conversely, at lower temperatures, Kw decreases. This temperature dependence is crucial when working with solutions at different temperatures.
Hunds Rule Of Maximum Multiplicity
Hunds Rule Of Maximum Multiplicity: In the intricate world of quantum mechanics, understanding the behavior and distribution of electrons within an atom is crucial to deciphering the properties and reactivity of chemical elements.
One fundamental principle that sheds light on this mysterious realm is Hund’s Rule of Maximum Multiplicity, often simply referred to as Hund’s Rule. This rule plays a pivotal role in explaining the electron configuration of elements and the filling of electron shells, guiding us through the fascinating world of atomic structure.
This comprehensive article delves deep into Hund’s Rule, exploring its historical context, its significance in atomic physics, and its practical applications in understanding the periodic table and chemical bonding. We will explore the intricacies of electron spin, the quantum numbers, and delve into real-world examples to illustrate the rule’s practical implications.
 Hunds Rule Of Maximum Multiplicity
Hunds Rule Of Maximum Multiplicity
1. Historical Perspective
Hund’s Rule of Maximum Multiplicity owes its name to the German physicist Friedrich Hund, who formulated it in the 1920s. Hund’s work, along with the contributions of other physicists, was instrumental in advancing our understanding of atomic and molecular structure during the early 20th century.
The period leading up to the formulation of Hund’s Rule was marked by significant advancements in quantum mechanics. The Bohr model, developed by Niels Bohr in 1913, laid the foundation for understanding the quantized energy levels of electrons in atoms. However, it was Werner Heisenberg’s matrix mechanics and Erwin Schrödinger’s wave mechanics that provided a more complete and mathematically rigorous description of the behavior of electrons in atoms.
Hund’s Rule emerged as a crucial component of the developing quantum theory, aiding in the interpretation of experimental observations and helping to bridge the gap between theory and reality. This rule was integral in refining our understanding of electron configurations, which describe the distribution of electrons within an atom’s energy levels or orbitals.
2. Principles of Hund’s Rule
Hund’s Rule can be summarized by two fundamental principles:
2.1. Electrons Fill Orbitals Singly Before Pairing Up When electrons are added to an atom’s electron configuration, they will first occupy available orbitals singly, rather than pairing up with another electron. This principle ensures that all orbitals within a subshell (such as s, p, d, or f) are singly occupied before any orbital receives a second electron with opposite spin.
2.2. Electrons in Singly Occupied Orbitals Have the Same Spin Electrons in singly occupied orbitals must have parallel spins, which means they have the same quantum spin number (either “up” or “down” as represented by ↑ or ↓). This results in the maximum possible value of electron spin, maximizing the overall electron spin of the subshell.
These two principles work together to establish the electron configuration of an atom in its lowest energy state, which corresponds to a more stable arrangement. Let’s explore each principle in more detail.
2.3. Maximizing Multiplicity and Stability To understand why Hund’s Rule promotes the filling of orbitals in this manner, it’s essential to consider the concept of multiplicity. Multiplicity refers to the number of unpaired electrons with parallel spins within a subshell. By filling orbitals singly before pairing up, Hund’s Rule maximizes the multiplicity of the subshell, leading to a lower overall energy and greater stability.
The stability gained through Hund’s Rule can be illustrated by comparing two hypothetical electron configurations for an atom. Consider an atom with three electrons to be distributed in the 2p subshell, which consists of three available p orbitals (px, py, and pz).
If we follow Hund’s Rule:
2p³ ⟶ ↑ ↓ ↑
In this scenario, we have maximized the multiplicity by distributing the three electrons with parallel spins in separate orbitals before pairing them up. This results in a more stable configuration.
In contrast, if we were to violate Hund’s Rule by pairing electrons prematurely:
2p³ ⟶ ↑ ↑ ↓
In this case, we have two electrons with opposite spins in the same orbital (px), violating Hund’s Rule. This configuration has lower multiplicity and, consequently, higher energy, making it less stable.
The principle of maximizing multiplicity through Hund’s Rule applies not only to the p subshell but also to all other subshells, including s, d, and f orbitals. It is a fundamental guideline for arranging electrons in atomic orbitals to achieve the most stable electron configuration.
3. Quantum Numbers and Hund’s Rule
To fully appreciate the application of Hund’s Rule, it’s essential to understand the role of quantum numbers in describing the distribution of electrons within atoms.
3.1. Principal Quantum Number (n) The principal quantum number (n) describes the energy level or shell in which an electron resides. It also determines the overall size and energy of an orbital. For any given electron configuration, the principal quantum number identifies the primary energy level, with higher values of n corresponding to higher energy levels.
In accordance with the Aufbau Principle, electrons fill lower energy levels before occupying higher ones. As electrons are added to the electron configuration, they move progressively outward from the nucleus to higher energy levels, in alignment with the values of n.
3.2. Azimuthal Quantum Number (l) The azimuthal quantum number (l) specifies the subshell or type of orbital within a particular energy level. It defines the orbital’s shape and can take on integer values ranging from 0 to (n-1). The values of l correspond to different subshells:
- l = 0 represents the s subshell.
- l = 1 represents the p subshell.
- l = 2 represents the d subshell.
- l = 3 represents the f subshell.
Hund’s Rule is most relevant when considering the distribution of electrons within a given subshell, where electrons must occupy orbitals singly before pairing up.
3.3. Magnetic Quantum Number (ml) The magnetic quantum number (ml) further specifies the orientation of an orbital within a subshell. It provides information about the spatial orientation of an orbital within a given subshell and can take on values from -l to +l in integer increments.
For example, within the p subshell (l = 1), there are three individual orbitals corresponding to ml values of -1, 0, and +1. These orbitals are oriented along the three mutually perpendicular axes (x, y, and z).
3.4. Spin Quantum Number (ms) The spin quantum number (ms) is perhaps the most crucial quantum number for understanding Hund’s Rule. It describes the intrinsic spin of an electron and can have only two values: +1/2 (spin “up”) or -1/2 (spin “down”). This quantum number accounts for the electron’s magnetic properties and is fundamental to the behavior of electrons in orbitals.
Hund’s Rule dictates that when electrons fill orbitals singly within a subshell, they must have parallel spins. This requirement ensures that electrons in singly occupied orbitals do not repel each other due to their like charges, leading to a more stable electron configuration.
4. Applying Hund’s Rule to the Periodic Table
Hund’s Rule finds practical application when determining the electron configurations of elements on the periodic table. The periodic table is organized in a way that reflects the filling of electron orbitals and follows the sequence of increasing atomic numbers.
Read More
- Thermodynamics class 11 physics
- Photosynthesis In Higher Plants
- Motion In A Plane
- Basic Properties Electrical Charge
- Anatomy Of Flowering Plants
Frequently Asked Questions (FAQs) Hunds Rule Of Maximum Multiplicity
1. What is Hund’s Rule of Maximum Multiplicity?
Hund’s Rule of Maximum Multiplicity is a principle in quantum mechanics that governs the arrangement of electrons in atomic orbitals. It specifies that electrons fill orbitals singly before pairing up and that electrons in singly occupied orbitals must have parallel spins. This rule helps determine the electron configuration of atoms in their lowest energy state, leading to greater stability.
2. Why is Hund’s Rule important in atomic physics?
Hund’s Rule is essential because it provides insights into how electrons are distributed in an atom’s orbitals, influencing the atom’s chemical properties and reactivity. By promoting the filling of orbitals with parallel spins before pairing up, it ensures that the electron configuration is as stable as possible.
3. What is the significance of maximizing multiplicity in Hund’s Rule?
Maximizing multiplicity means maximizing the number of unpaired electrons with parallel spins within a subshell. This leads to a lower overall energy and greater stability for the atom. Hund’s Rule achieves this by filling orbitals singly before pairing electrons, ensuring that electrons with like charges do not repel each other within the same orbital.
4. Can you explain Hund’s Rule with an example?
Certainly. Let’s consider the electron configuration of nitrogen (N, atomic number 7):
Nitrogen has seven electrons, and its electron configuration is 1s² 2s² 2p³. In the 2p subshell, we follow Hund’s Rule:
2p³ ⟶ ↑ ↓ ↑
Here, electrons are distributed with parallel spins in separate 2p orbitals before pairing up. This arrangement adheres to Hund’s Rule, maximizing multiplicity and stability.
5. Does Hund’s Rule apply to all subshells and elements?
Yes, Hund’s Rule applies to all subshells (s, p, d, and f) within atoms and is a fundamental principle for all elements. It governs the arrangement of electrons in subshells to achieve the lowest energy configuration. Whether it’s a small hydrogen atom or a large uranium atom, Hund’s Rule remains applicable.
Light Filtering Polaroid Films Polarization
Light Filtering Polaroid Films Polarization: Polarization is a fascinating phenomenon that plays a crucial role in various aspects of science and technology, particularly in the field of optics.
One of the practical applications of polarization is the use of Polaroid films to filter and manipulate light. In this article, we will explore the concept of polarization, how Polaroid films work, and their diverse applications in different industries.

Light Filtering Polaroid Films Polarization
Understanding Polarization
Light is composed of electromagnetic waves that oscillate in various directions perpendicular to the direction of propagation. Polarization refers to the orientation of these oscillations. When light waves oscillate in a specific direction, we say the light is polarized in that direction. Light from natural sources, such as the sun, is typically unpolarized, meaning its oscillations occur in all directions.
Polaroid Films and Polarization
Polaroid films, invented by Edwin Land in the 1930s, are specially designed materials that can selectively transmit or block polarized light. They consist of long-chain molecules that are aligned in a specific direction during the manufacturing process. These aligned molecules act as microscopic polarizing filters.
Here’s how Polaroid films work:
- Polarization by Absorption: Polaroid films are most commonly made by impregnating a transparent plastic sheet with iodine or another polarizing material. The long-chain molecules in the film selectively absorb light waves oscillating in one direction while allowing light waves oscillating perpendicular to that direction to pass through. This process essentially polarizes the transmitted light.
- Blocking Unwanted Light: When unpolarized light passes through a Polaroid film, it becomes polarized in the direction allowed by the film. Light waves oscillating in other directions are absorbed by the film, effectively blocking them.
Applications of Polaroid Films
Polaroid films have a wide range of practical applications across various fields:
- Sunglasses: Polarized sunglasses use Polaroid films to reduce glare from surfaces like water and roads. They block horizontally polarized light, which is often responsible for the blinding glare that can be uncomfortable and hazardous.
- Photography and Cameras: Polarizing filters can be attached to camera lenses to control reflections, enhance color saturation, and improve the contrast in photographs. They are particularly useful for landscape and outdoor photography.
- Liquid Crystal Displays (LCDs): LCD screens, including those in TVs, computer monitors, and smartphones, use polarization to control the passage of light and create images with vibrant colors and high contrast.
- 3D Glasses: Polaroid films are used in 3D glasses to present different images to each eye, creating the illusion of depth in 3D movies and games.
- Microscopy: Polarized light microscopy is used in scientific research and materials analysis to study the birefringence of specimens, revealing structural and compositional information.
- Communication Devices: Polarization is employed in various optical and communication devices, such as optical filters, signal modulators, and polarizing beam splitters.
Conclusion
Polarization and Polaroid films are essential components in optics and light management. They allow us to manipulate light waves, reduce glare, enhance image quality, and explore the properties of materials. Whether in everyday sunglasses, high-tech LCD displays, or advanced scientific instruments, the power of polarization through Polaroid films continues to shape the way we interact with and understand light.
Read More
- Animals Biology Science Lion Zoology
- Difference Between Voltage And Current
- Difference Between Two Stroke And Four Stroke
- Difference Between Ac And Dc
- Changing The Period Of A Pendulum
Frequently Asked Questions (FAQs) Light Filtering Polaroid Films Polarization
What is polarization in the context of light?
Polarization refers to the orientation of oscillations of light waves. It indicates the direction in which the electromagnetic waves oscillate as they propagate through space.
How do Polaroid films work to filter light?
Polaroid films consist of long-chain molecules that are aligned during manufacturing. These aligned molecules selectively absorb light waves oscillating in one direction while allowing light waves oscillating perpendicular to that direction to pass through, effectively polarizing the transmitted light.
What are some common applications of Polaroid films?
Polaroid films are used in sunglasses to reduce glare, in photography to control reflections and enhance image quality, in LCD screens to create vibrant and high-contrast images, in 3D glasses to provide the illusion of depth, and in scientific research for microscopy and materials analysis, among other applications.
How do Polaroid sunglasses reduce glare?
Polarized sunglasses block horizontally polarized light, which is often responsible for glare from surfaces like water, roads, and glass. By selectively allowing vertically polarized light to pass through, they reduce the blinding effect of glare.
Can Polaroid films be used to improve the visibility of LCD screens?
Yes, Polaroid films are used in LCD screens to control the passage of light, enhance color saturation, and improve contrast, resulting in brighter and more vibrant displays.
Difference Between Watts And Volts
Difference Between Watts And Volts: Understanding the Difference Between Watts and Volts
Watts And Volts
Introduction
In the realm of electricity and power, two fundamental concepts that often lead to confusion are watts and volts. While they both play critical roles in the world of electrical systems, they represent different aspects of electricity. This article aims to clarify the distinctions between watts and volts, helping you better comprehend their significance and practical applications.
Watts (W) – The Measure of Power
Watts, denoted by the symbol ‘W,’ are the units used to measure power in an electrical circuit. Power is essentially the rate at which energy is transferred, converted, or used. In simpler terms, it tells us how quickly work is being done in an electrical system. The relationship between watts, voltage (volts), and current (amperes) is described by the formula:
In this equation:
- P represents power in watts.
- V represents voltage in volts.
- I represents current in amperes.
In practical terms, watts are a measure of how much energy is consumed or produced per unit of time. For instance, a 100-watt light bulb consumes 100 watts of electrical power while it’s illuminated. Similarly, a 1,000-watt (1 kilowatt) space heater consumes 1 kilowatt-hour of energy in one hour.
Volts (V) – The Measure of Electrical Potential
Volts, denoted by the symbol ‘V,’ represent electrical potential or voltage. Voltage is a measure of the electric potential energy per unit charge at a given point in an electrical circuit. In simpler terms, it tells us the force that drives electric charge through a conductor. A higher voltage means that electrons have more potential energy and will move with more force through the circuit.
Voltage is often compared to water pressure in a plumbing system. Just as higher water pressure causes water to flow more forcefully through pipes, higher voltage causes electric current to flow more forcefully through wires.
Practical Examples
To better understand the difference between watts and volts, consider the following examples:
- Light Bulb: A 100-watt light bulb requires 100 watts of power to produce light. However, it operates at a standard household voltage of 120 volts in the United States. To calculate the current flowing through it, you can use the formula I = P / V, which in this case is I = 100W / 120V ≈ 0.83 amperes.
- Battery: A common AA battery typically supplies 1.5 volts. The voltage represents the potential difference between the battery’s positive and negative terminals, which drives the flow of electric current. The power (in watts) the battery can deliver depends on the device it’s connected to and the current it draws.
- Electric Kettle: A 1,500-watt electric kettle heats water quickly. When you plug it into a standard 120-volt outlet, it draws approximately 12.5 amperes of current to produce that 1,500 watts of power, creating the heat needed to boil water.
Conclusion
In summary, watts and volts are distinct but interrelated concepts in the world of electricity. Watts measure power, which is the rate of energy transfer or consumption, while volts measure voltage, which is the electrical potential that drives the flow of electric current.
Understanding these differences is crucial for effectively managing and using electrical systems, from household appliances to industrial machinery. Whether you’re troubleshooting electrical issues at home or designing complex electrical circuits, a clear grasp of the distinction between watts and volts is essential for safe and efficient operation.
Read More
- Molar Mass Of Sulphur
- Electronics In Daily Life
- Molecular Weight Of Hcl
- Asteroid And Comet Difference
- Molecular Weight Of Nitrogen
Frequently Asked Questions (FAQs) Watts And Volts
What is the fundamental difference between watts and volts?
Watts measure power, which is the rate of energy transfer or consumption, while volts measure voltage, which is the electrical potential that drives the flow of electric current.
What do watts and volts represent in electrical terms?
Watts represent the amount of work done or the rate of energy transfer in an electrical circuit. Volts represent the electrical potential difference that drives the flow of electric charge.
How are watts and volts related in an electrical circuit?
Watts can be calculated by multiplying volts by amperes (W = V × I), where (V) represent the voltage and amperes (I) represent the current.
Can you provide a practical example illustrating the difference between W and V ?
Sure, consider a 100-W light bulb. The 100 watts (W) represent the power it consumes to produce light, while the standard household voltage of 120 V provides the electrical potential needed for the bulb to function.
What happens if you increase the voltage while keeping the wattage constant in an electrical circuit?
Increasing the voltage while keeping the wattage constant will result in an increase in the current (amperes). This can lead to higher power consumption and may affect the operation of electrical devices.
Examples Of Rotational Motion
Examples Of Rotational Motion: Rotational motion refers to the movement of an object as it spins or rotates around an axis. It is a common phenomenon in our everyday lives and can be observed in various contexts. Here are some examples of rotational motion:
Examples Of Rotational Motion
- Spinning Top: When a spinning top is set in motion, it rotates around its central axis, demonstrating rotational motion. The top’s stability depends on the conservation of angular momentum.
- Earth’s Rotation: The Earth rotates on its axis, causing day and night cycles. This is a fundamental example of rotational motion that affects our daily lives.
- Rolling Wheel: When a wheel rolls, it rotates around its central axis as it moves forward. This is a combination of translational and rotational motion. For instance, a car’s wheels rotate as the car moves forward.
- Rotating Fan: The blades of a fan rotate around a central hub when the fan is turned on. This circular motion creates airflow and cools the room.
- Spinning Gyroscope: A gyroscope is a device used in navigation and stabilization systems. When a gyroscope is spun, it exhibits stable rotational motion, resisting changes in orientation.
- Planet and Moon Rotation: Celestial bodies like planets (e.g., Jupiter) and moons (e.g., our Moon) rotate around their own axes. Observing these bodies through telescopes can provide evidence of rotational motion.
- Rotating Drill Bit: When using a power drill, the bit at the end rotates rapidly to drill holes in materials like wood, metal, or plastic. This is a practical application of rotational motion.
- Turbines: Wind turbines and water turbines harness the power of rotational motion to generate electricity. Wind turbines have large blades that rotate in the wind, while water turbines have blades turned by flowing water.
- Record Player: In a vinyl record player, the record rotates around a central axis, and a needle or stylus follows the grooves on the record, producing sound. This demonstrates rotational motion in entertainment technology.
- Ballet Pirouette: During a ballet pirouette, a dancer spins around one foot while maintaining a fixed axis. This is a graceful and controlled example of rotational motion in performing arts.
- Roller Coaster Loops: In a roller coaster ride, when the car goes through loops or corkscrews, it experiences rotational motion as it spins around different axes, providing thrill and excitement to riders.
These examples illustrate the diverse ways in which rotational motion is encountered in the natural world, technology, and everyday activities.
Read More
- Addition And Subtraction Of Fractions
- Difference Between Asteroid And Meteoroid
- Class 6 Maths Question Paper With Solutions NCERT PDF
- Sample Question Paper For Class 6 CBSE English Grammar
- NCERT Maths Book Class 6 Solutions PDF Free Download
Frequently Asked Questions (FAQs) Examples Of Rotational Motion
What is rotational motion?
Rotational motion is the movement of an object as it spins or rotates around a fixed axis. It involves the circular motion of an object’s parts around a central point.
What is the difference between rotational and translational motion?
Translational motion involves the linear movement of an object from one point to another, while rotational motion involves the spinning or rotating of an object around an axis without changing its position.
What are some real-world examples of rotational motion?
Examples of rotational motion include spinning tops, the Earth’s rotation causing day and night, rolling wheels, rotating fans, and spinning gyroscopes, among others.
How does rotational motion apply to machinery and technology?
Rotational motion is essential in machinery such as engines, drills, and turbines. Engines use pistons that rotate crankshafts, drills have rotating bits, and turbines harness rotational motion to generate electricity.
What is angular momentum in rotational motion?
Angular momentum is a property of a rotating object and depends on its moment of inertia and angular velocity. It is conserved in the absence of external torques, and changes in angular momentum can affect an object’s rotational motion.
Thermodynamics class 11 physics
Thermodynamics: Certainly, here is a more detailed explanation of the topics typically covered in a Class 11 Physics Thermodynamics course, with additional information and examples. This comprehensive guide should provide you with a deeper understanding of thermodynamics:

Thermodynamics
Introduction to Thermodynamics
Thermodynamics is a branch of physics that deals with the study of heat, work, and energy transfer. It provides essential insights into the behavior of matter and energy in various physical systems. Thermodynamics plays a crucial role in understanding natural processes, from engines and refrigerators to chemical reactions and the behavior of gases.
System and Surroundings
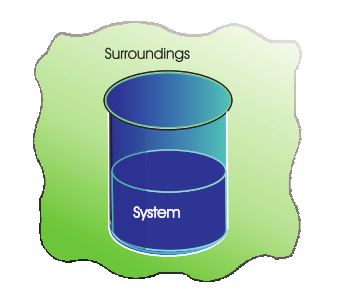
System
In thermodynamics, we define a system as a specific region of space or a quantity of matter under consideration. It is the part of the universe we are interested in studying. The surroundings are everything outside the system. We often use the terms “system” and “surroundings” to describe the boundaries and interactions in thermodynamic processes.
Types of Systems
- Open System: A system that can exchange both matter and energy with its surroundings. For example, an open cup of coffee where heat is lost to the surroundings, and you can add or remove coffee.
- Closed System: A system that can exchange energy but not matter with its surroundings. A sealed thermos containing hot coffee is an example.
- Isolated System: A system that cannot exchange either matter or energy with its surroundings. The universe itself is often considered an isolated system for some thermodynamic analyses.
Thermodynamic Processes
Thermodynamic processes describe how a system changes from one state to another. Several common processes include:
Isothermal Process
An isothermal process is one in which the temperature of the system remains constant throughout the process. For example, when a gas in a piston-cylinder system expands while being in contact with a hot reservoir, keeping its temperature constant.
Adiabatic Process
An adiabatic process is one in which there is no heat exchange between the system and its surroundings. In such processes, any change in the internal energy of the system is solely due to work done on or by the system. A rapid compression of a gas is an example of an adiabatic process.
Isobaric Process
An isobaric process is one in which the pressure of the system remains constant while other parameters like volume and temperature may change. Boiling of water at a constant atmospheric pressure is an isobaric process.
Isochoric Process
An isochoric process is one in which the volume of the system remains constant. It typically results in changes in pressure and temperature. Heating a sealed container with a fixed volume is an example of an isochoric process.
Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics states that if two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. In simpler terms, if two objects are at the same temperature as a third object, they are also at the same temperature as each other. This law allows us to define temperature scales.
First Law of Thermodynamics
The First Law of Thermodynamics, often called the Law of Conservation of Energy, states that energy cannot be created or destroyed in an isolated system. Instead, it can only change forms. Mathematically, it can be expressed as:
ΔU = Q – W
Where:
- ΔU represents the change in the internal energy of the system.
- Q represents the heat added to the system.
- W represents the work done by the system.
This law is fundamental and is applied to various processes and systems.
Internal Energy
Internal energy (U) is the sum of the kinetic and potential energies of the particles within a system. It depends on the state of the system and is a state function.
Heat Capacity and Specific Heat
Heat capacity (C) is the amount of heat required to raise the temperature of a substance by one degree Celsius (or one Kelvin). The specific heat (c) is the heat capacity per unit mass. Mathematically:
C = mc
Where:
- C is the heat capacity.
- m is the mass.
- c is the specific heat.
Work and Heat Transfer
Work Done
In thermodynamics, work is defined as the energy transferred to or from a system as a result of a force acting on a boundary and causing a displacement in the direction of the force. Work done on a system is considered positive, while work done by a system is considered negative. The formula for work in a piston-cylinder system is:
W = PΔV
Where:
- W is the work done.
- P is the pressure.
- ΔV is the change in volume.
Heat Transfer
Heat transfer is the process of energy transfer due to temperature differences. There are three primary modes of heat transfer:
- Conduction: Heat transfer through direct contact between particles in a solid.
- Convection: Heat transfer through the movement of fluids (liquids or gases).
- Radiation: Heat transfer through electromagnetic waves (e.g., sunlight).
Applications of the First Law
Calorimetry
Calorimetry is the science of measuring heat. A calorimeter is a device used to measure the heat transfer in various processes. Calorimetry is often employed to determine specific heat capacities and heat of reaction in chemical reactions.
Enthalpy
Enthalpy (H) is a thermodynamic quantity used to describe the heat content of a system at constant pressure. It is defined as:
H = U + PV
Where:
- H is enthalpy.
- U is internal energy.
- P is pressure.
- V is volume.
The change in enthalpy (ΔH) is often used to calculate the heat exchanged in processes at constant pressure.
Second Law of Thermodynamics
The Second Law of Thermodynamics states that heat naturally flows from a region of higher temperature to a region of lower temperature. This law is often associated with the concept of entropy.
Heat Engines
A heat engine is a device that converts thermal energy into mechanical work. The efficiency of a heat engine is given by:
Efficiency = (Useful Work Output) / (Heat Input)
The most efficient heat engine theoretically possible is the Carnot engine, which operates between two heat reservoirs.
Entropy
Entropy (S) is a measure of the amount of disorder or randomness in a system. The Second Law of Thermodynamics can be expressed as the increase in entropy in an isolated system over time. It is given by:
ΔS ≥ Q/T
Where:
- ΔS is the change in entropy.
- Q is the heat added to the system.
- T is the absolute temperature in Kelvin.
Entropy is a state function and is used to analyze the direction of spontaneous processes.
Reversible and Irreversible Processes
In thermodynamics, a reversible process is one that can be reversed by an infinitesimal change in a property, such as temperature or pressure. In contrast, an irreversible process is one that cannot be reversed without some external influence. Real-world processes are often irreversible, and reversible processes are idealized for analysis.
Third Law of Thermodynamics
The Third Law of Thermodynamics states that as the temperature of a system approaches absolute zero (0 Kelvin), the entropy of the system approaches a minimum value. This law helps explain the behavior of matter at extremely low temperatures and is often used in the study of materials at cryogenic temperatures.
Entropy and Probability
Entropy can be interpreted in terms of probability. It is related to the number of microscopic configurations (ways particles can arrange themselves) consistent with the macroscopic properties of the system. In simple terms, systems tend to evolve toward states with higher entropy, which correspond to greater disorder and randomness.
Heat Transfer
Fourier’s Law of Heat Conduction
Fourier’s law describes the heat conduction in a material. It states that the rate of heat transfer through a material is directly proportional to the temperature gradient (change in temperature over distance) and is inversely proportional to the thermal conductivity of the material.
Newton’s Law of Cooling
Newton’s law of cooling describes the rate at which an object cools when placed in a cooler environment. It states that the rate of change of temperature of an object is directly proportional to the temperature difference between the object and its surroundings.
Carnot Cycle
The Carnot cycle is a theoretical thermodynamic cycle that represents the most efficient engine possible operating between two heat reservoirs. It consists of four reversible processes: two isothermal processes and two adiabatic processes. The Carnot engine is often used as an idealized model for the maximum efficiency of real heat engines.
Carnot’s Theorem
Carnot’s theorem states that no engine operating between two heat reservoirs can be more efficient than a Carnot engine operating between the same reservoirs. This theorem establishes an upper limit on the efficiency of real heat engines, and it highlights the importance of reversible processes in achieving maximum efficiency.
Heat Pumps and Refrigerators
In addition to heat engines, thermodynamics also applies to heat pumps and refrigerators. These devices transfer heat from a lower-temperature reservoir to a higher-temperature reservoir. The key difference between a heat pump and a refrigerator lies in their primary objectives:
- A heat pump is designed to heat a space or provide hot water by extracting heat from a colder environment and transferring it to a warmer one. Heat pumps are commonly used for home heating and cooling.
- A refrigerator is designed to remove heat from the interior of the refrigerator and expel it to the room, keeping the contents of the refrigerator cool.
Both heat pumps and refrigerators operate using a cycle similar to the Carnot cycle but with certain modifications.
Phase Transitions
Thermodynamics also includes the study of phase transitions, which are changes in the state of matter. Common phase transitions include:
- Melting: The transition from a solid to a liquid state.
- Freezing: The transition from a liquid to a solid state.
- Vaporization: The transition from a liquid to a gas state, which includes both boiling (at a specific temperature and pressure) and evaporation (at any temperature).
- Condensation: The transition from a gas to a liquid state.
- Sublimation: The transition from a solid directly to a gas state without passing through the liquid state.
- Deposition: The transition from a gas directly to a solid state without passing through the liquid state.
The energy changes associated with these phase transitions are fundamental to understanding various physical processes, including the operation of refrigeration systems and the behavior of substances under different conditions.
State Functions
In thermodynamics, certain properties are defined as state functions because their values depend only on the current state of the system, not on how the system arrived at that state. Examples of state functions include internal energy (U), enthalpy (H), entropy (S), and pressure (P). Path functions, on the other hand, depend on the path taken to reach a particular state and include work (W) and heat (Q).
Maxwell’s Relations
Maxwell’s relations are a set of equations that relate the partial derivatives of the state functions, providing valuable relationships between thermodynamic properties. These relations are derived from the basic thermodynamic laws and are used to simplify calculations and analyze complex thermodynamic systems.
Thermodynamic Diagrams
In thermodynamics, graphical representations are often used to visualize and analyze processes. Two common diagrams include:
- P-V Diagram: A pressure-volume diagram represents the changes in pressure and volume of a system during a thermodynamic process. It helps in understanding processes like expansion, compression, and heat exchange.
- T-S Diagram (Temperature-Entropy Diagram): A temperature-entropy diagram illustrates changes in temperature and entropy during a thermodynamic process. It is particularly useful in analyzing the performance of heat engines and refrigeration cycles.
Applications of Thermodynamics
Thermodynamics finds applications in various fields, including:
- Chemical Engineering: Thermodynamics is crucial in chemical reactions, industrial processes, and the design of chemical reactors.
- Aerospace Engineering: It plays a pivotal role in the design and analysis of propulsion systems, such as jet engines and rockets.
- Environmental Science: Thermodynamics is used to study energy transfer in ecosystems and climate systems.
- Materials Science: It helps understand the behavior of materials under different conditions, aiding in material selection and design.
- Biological Systems: Thermodynamics is applied to analyze energy transfer and metabolic processes in biological systems.
Gibbs Free Energy
Gibbs free energy (G) is a thermodynamic potential that combines enthalpy and entropy to predict whether a chemical reaction will be spontaneous or non-spontaneous under constant temperature and pressure conditions. The Gibbs free energy change (ΔG) of a reaction is related to its spontaneity:
- If ΔG < 0, the reaction is spontaneous (exergonic).
- If ΔG > 0, the reaction is non-spontaneous (endergonic).
- If ΔG = 0, the system is at equilibrium.
The concept of Gibbs free energy is particularly important in chemistry and biochemistry to predict reaction outcomes.
Thermodynamic Equilibrium
Thermodynamic equilibrium occurs when a system’s properties (such as temperature, pressure, and chemical composition) no longer change with time because there is no net exchange of energy or matter with its surroundings. Different types of equilibrium include thermal, mechanical, and chemical equilibrium.
Thermodynamic Laws in Chemistry
In chemistry, thermodynamics plays a crucial role in understanding chemical reactions and equilibria. The following concepts are significant:
- Chemical Potential: The chemical potential (μ) represents the change in Gibbs free energy with respect to changes in the number of particles (e.g., moles of a substance). It is essential for understanding phase equilibria and chemical reactions.
- Equilibrium Constants: Thermodynamics provides the foundation for understanding and calculating equilibrium constants (K) for chemical reactions. The equilibrium constant expresses the ratio of product concetrations to reactant concentrations at equilibrium.
- Le Chatelier’s Principle: This principle states that if a system at equilibrium is subjected to an external change (e.g., temperature, pressure, or concentration), the system will adjust to partially counteract that change and restore equilibrium. Thermodynamics helps explain how and why these adjustments occur.
- Thermodynamics and Climate Science
- Thermodynamics plays a vital role in climate science and the study of Earth’s climate system. Concepts such as heat transfer, greenhouse gas effects, and phase changes are essential for understanding climate patterns, climate change, and the impact of human activities on the environment.
- Thermodynamics in Engineering
- Thermodynamics is a cornerstone of engineering disciplines, including:
- Mechanical Engineering: It is essential for the design and analysis of engines, turbines, and HVAC (heating, ventilation, and air conditioning) systems.
- Chemical Engineering: Thermodynamics is used to design and optimize chemical processes, including reactions, separations, and heat exchangers.
- Electrical Engineering: It is applied to the design of power generation systems, such as thermoelectric generators and power plants.
- Materials Science: Understanding thermodynamic principles helps in material selection, phase diagrams, and heat treatment processes.
- Thermodynamics in Biology
- Thermodynamics is also relevant in biology, particularly in the study of living organisms and biochemical processes. The principles of thermodynamics help explain how cells obtain and utilize energy, how enzymes function, and how metabolic pathways operate.
- Modern Advances in Thermodynamics
- Modern physics and engineering continue to push the boundaries of thermodynamics. Concepts like non-equilibrium thermodynamics explore systems that are far from equilibrium, such as those involving turbulence, fluid dynamics, and biological systems. Additionally, quantum thermodynamics extends thermodynamic principles to the quantum scale, providing insights into the behavior of very small systems.
Conclusion
Thermodynamics is a broad and fundamental field with applications spanning various scientific and engineering disciplines. Its principles help us understand and predict the behavior of energy and matter in diverse systems, from the macroscopic to the microscopic, and from natural processes to industrial applications. A strong foundation in thermodynamics is essential for scientists and engineers working in fields where energy transfer, heat, and work are critical components of their research and designs.
Read More
- Photosynthesis In Higher Plants
- Motion In A Plane
- Basic Properties Electrical Charge
- Anatomy Of Flowering Plants
- Elastic Behaviour Of Materials
Frequently Asked Question (FAQs) Thermodynamics
1. What is thermodynamics?
Thermodynamics is a branch of physics that deals with the study of heat, work, and energy transfer in various physical systems. It provides fundamental principles for understanding the behavior of matter and energy.
2. What are the key laws of thermodynamics?
The key laws of thermodynamics are:
- Zeroth Law: If two systems are in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
- First Law: Energy cannot be created or destroyed; it can only change forms. This law is also known as the law of conservation of energy.
- Second Law: Heat naturally flows from a region of higher temperature to a region of lower temperature. This law is associated with the concept of entropy.
- Third Law: As temperature approaches absolute zero (0 Kelvin), the entropy of a perfect crystal approaches a minimum value.
3. What are the different types of thermodynamic systems?
Thermodynamic systems can be categorized into three types:
- Open System: Allows the exchange of both matter and energy with the surroundings.
- Closed System: Allows the exchange of energy but not matter with the surroundings.
- Isolated System: Does not allow the exchange of either matter or energy with the surroundings.
4. What are thermodynamic processes?
Thermodynamic processes describe how a system changes from one state to another. Common processes include isothermal, adiabatic, isobaric, and isochoric processes, each with specific characteristics.
5. How is heat related to thermodynamics?
Heat is a form of energy transfer that plays a central role in thermodynamics. It can enter or leave a system during various processes and is typically represented by the symbol Q.
Molecular Weight Of Sulphur
Molecular Weight Of Sulphur: Sulphur, a non-metal element with the chemical symbol “S” and atomic number 16, is an essential element in the periodic table. It plays various critical roles in chemistry, industry, and biology.
One fundamental property of any chemical element is its molecular weight, which provides insight into its behavior and significance. In this article, we will delve into the molecular weight of sulphur and its implications in different contexts.

Molecular Weight Of Sulphur
Understanding Molecular Weight
Molecular weight, also known as mol mass or molar mass, is a fundamental concept in chemistry. It represents the mass of a molecule or an element expressed in atomic mass units (u) or unified atomic mass units (AMU). Molecular weight is calculated by summing the atomic masses of all the atoms in a molecule.
The Atomic Mass of Sulphur
Before calculating the mol weight of sulphur, let’s consider the atomic mass of a sulphur atom. The atomic mass of sulphur (S) is approximately 32.06 atomic mass units (u). This value is an average of the isotopes of sulphur found in nature, which have slightly different masses due to variations in the number of neutrons.
Calculating the Molecular Weight of Sulphur
Sulphur typically exists as S8 molecules, where eight sulphur atoms are chemically bonded together to form a molecule. To calculate the mol weight of sulphur (S8), we simply multiply the atomic mass of sulphur by the number of sulphur atoms in a molecule:
mol Weight of Sulphur (S8) = Atomic Mass of Sulphur (S) × Number of Sulphur Atoms
mol Weight of Sulphur (S8) = 32.06 u × 8
mol Weight of Sulphur (S8) = 256.48 u
Rounded to two decimal places, the mol weight of sulphur (S8) is approximately 256.48 atomic mass units (u).
Significance of Sulphur’s Molecular Weight
Understanding the molecular weight of sulphur is important in various scientific and industrial applications:
- Chemical Reactions: Sulphur is involved in many chemical reactions and forms compounds with other elements. Knowledge of its molecular weight is essential for stoichiometry calculations and determining reaction products.
- Industrial Processes: Sulphur is used in various industrial processes, including the production of sulfuric acid, fertilizers, and rubber vulcanization. Its molecular weight is critical for quality control and process optimization.
- Environmental Impact: Sulphur compounds contribute to environmental issues such as acid rain and air pollution. Monitoring the molecular weight of sulphur-containing compounds helps assess their impact.
- Geological Exploration: Sulphur deposits are important resources in mining and geology. Accurate measurements of sulphur’s molecular weight aid in resource assessment and extraction.
- Biological Functions: Sulphur is a vital element in biological molecules like amino acids and vitamins. Its molecular weight is relevant in understanding biochemical processes.
- Materials Science: Sulphur is used in materials science for applications such as vulcanizing rubber and creating sulfur polymers. Molecular weight is a critical factor in material properties.
In conclusion, the mol weight of sulphur, approximately 256.48 atomic mass units (u) for S8 molecules, is a fundamental property of this element. It plays a pivotal role in various scientific, industrial, and environmental contexts, reflecting the versatility and importance of sulphur in our world.
Read More
- Molecular Weight Of Urea
- Molecular Mass Of Urea
- Molecular Mass Of Hydrogen
- Molecular Mass Of Nacl
- Molecular Mass Of Naoh
Frequently Asked Question (FAQs) Molecular Weight Of Sulphur
1. What is the molecular weight of sulphur?
The mol weight of sulphur (S) is approximately 32.06 atomic mass units (u) or unified atomic mass units (AMU). This value represents the average atomic mass of sulphur isotopes found in nature.
2. How is the molecular weight of sulphur calculated?
The mol weight of sulphur is calculated by summing the atomic masses of all the sulphur atoms in a molecule. Sulphur typically exists as S8 molecules, so the molecular weight of sulphur (S8) is 8 times the atomic mass of a sulphur atom.
3. Why is the molecular weight of sulphur important in chemistry?
The mol weight of sulphur is a fundamental property used in stoichiometry calculations, chemical reactions, and determining the composition of sulphur-containing compounds. It helps chemists understand the behavior of sulphur in various contexts.
4. How is sulphur used in industrial processes, and why does its molecular weight matter?
Sulphur is used in industrial processes, such as the production of sulfuric acid and fertilizers. Its mol weight is crucial for process optimization, quality control, and determining the amount of sulphur required in reactions.
5. What is the environmental impact of sulphur compounds, and how does molecular weight relate to it?
Sulphur compounds can contribute to environmental issues like acid rain and air pollution. Monitoring the mol weight of sulphur-containing compounds helps assess their environmental impact and regulation.