Blog
Basic Properties Electrical Charge
Basic Properties Electrical Charge: Electrical charge is a fundamental concept in physics, and it plays a central role in understanding the behavior of matter and the functioning of electrical and electronic devices.
To comprehend electrical phenomena, it’s essential to grasp the basic properties of electrical charge. Here are the key properties of electrical charge:

Basic Properties Electrical Charge
1. Quantization:
Electrical charge is quantized, meaning it exists in discrete, indivisible units. The smallest unit of charge is the charge of an electron (e), which is approximately -1.602 x 10^-19 coulombs (C). Protons have an equal but opposite charge, +1.602 x 10^-19 C.
2. Polarity:
There are two types of electrical charge: positive and negative. Protons carry positive charge (+e), while electrons carry negative charge (-e). Like charges repel each other, and opposite charges attract.
3. Conservation:
The principle of conservation of charge states that the total electric charge in a closed system remains constant. Charge cannot be created or destroyed, only transferred from one object to another. This principle is a fundamental law of nature.
4. Additivity:
Electrical charge is additive. When multiple charges are present, the total charge is the algebraic sum of all individual charges. If you have a +2e charge and a -3e charge, the total charge is -e.
5. Coulomb’s Law:
Coulomb’s law describes the force between two charged objects. It states that the force (F) between two point charges is directly proportional to the product of their magnitudes (q1 and q2) and inversely proportional to the square of the distance (r) between them. Mathematically, it’s expressed as:
- F = k * |q1 * q2| / r^2
- Where k is Coulomb’s constant.
6. Induction:
Electrical charge can be induced in a neutral object by bringing a charged object near it without direct contact. This process is the basis for the function of many electrical devices, such as capacitors and electrometers.
7. Conduction:
Electrical charge can be transferred between objects through direct contact. When a charged object touches another object, charge can flow from one to the other, equalizing their charge distribution.
8. Repulsion and Attraction:
Objects with the same type of charge (either both positive or both negative) will repel each other, while objects with opposite charges will attract each other. This principle governs the behavior of charged particles.
9. Electrostatic Equilibrium:
In electrostatic equilibrium, there is no net movement of charge within an object. The electric field within the object is zero, and charges are distributed such that there is no repulsion or attraction between them.
10. Electrostatic Force:
The force between charged objects is mediated by the electromagnetic force, one of the four fundamental forces in nature. This force is responsible for all electrical and magnetic interactions.
Understanding these basic properties of electrical charge is fundamental to comprehending the behavior of matter, electricity, and electromagnetic phenomena. These principles are essential in fields such as physics, engineering, and electronics, where electrical charge plays a pivotal role in creating and controlling various technologies.
Read More
- Anatomy Of Flowering Plants
- Elastic Behaviour Of Materials
- Molecular Weight Of Oxalic Acid
- Structural Organisation In Animals
- CBSE Sample Papers for Class 11 English With Answers PDF
Frequently Asked Question (FAQs) Basic Properties Electrical Charge
1. What is electrical charge, and why is it important in physics?
Electrical charge is a fundamental property of matter, and it plays a crucial role in explaining the behavior of particles and the functioning of electrical and electronic devices. It is the basis for the study of electromagnetism in physics.
2. Are there different types of electrical charge?
There are two types of electrical charge: positive and negative. Protons carry positive charge, while electrons carry negative charge. Like charges repel each other, and opposite charges attract.
3. Can electrical charge be created or destroyed?
No, electrical charge is conserved. According to the principle of conservation of charge, the total electric charge in a closed system remains constant. Charge cannot be created or destroyed; it can only be transferred from one object to another.
4. How is electrical charge quantized?
Electrical charge is quantized, which means it exists in discrete, indivisible units. The elementary charge is the charge of an electron (e) and has a value of approximately -1.602 x 10^-19 coulombs (C).
5. What happens when you bring two charged objects close to each other?
When two charged objects are brought close to each other, they exert electrostatic forces on each other. Like charges will repel each other, while opposite charges will attract.
Animals Biology Science Lion Zoology
Animals Biology Science Lion Zoology: Lions, often referred to as the “King of the Jungle,” are majestic creatures that have captured the imagination of humans for centuries.
Their biology and behavior are a source of intrigue and admiration among zoologists and wildlife enthusiasts. In this article, we delve into the world of lion biology and zoology to uncover the secrets of these remarkable big cats.

Animals Biology Science Lion Zoology
Biology of Lions
1. Taxonomy:
Lions belong to the family Felidae and the genus Panthera. Their scientific name is Panthera leo.
2. Physical Characteristics:
Lions are known for their impressive size, with males (known as “males”) being larger than females (known as “lionesses”). They have a distinctive appearance, characterized by a tawny coat with a tufted tail and a prominent mane in males.
3. Habitat:
Lions are native to parts of Africa, primarily the grasslands, savannas, and open woodlands. They are known for their adaptability to various environments.
4. Diet:
Lions are carnivores and primarily prey on mammals, including zebras, wildebeests, and antelopes. Lionesses are usually the primary hunters in a pride.
5. Social Structure:
Lions are known for their social behavior and live in groups called prides. Prides consist of related lionesses, their offspring, and a dominant male or a coalition of males.
6. Reproduction:
Lionesses give birth to a litter of cubs after a gestation period of approximately 3.5 months. Cubs are typically raised communally within the pride.
7. Conservation Status:
Lions are classified as vulnerable by the International Union for Conservation of Nature (IUCN) due to habitat loss, human-wildlife conflict, and poaching.
Zoology of Lions
1. Behavior and Communication:
Lions are known for their vocalizations, including roars that can be heard from miles away. Roaring plays a crucial role in territory marking and communication within the pride.
2. Hunting Strategies:
Lions are often observed hunting in groups, using teamwork to take down large prey. Their collaborative hunting tactics increase their success rate.
3. Territorial Behavior:
Male lions defend their territory against rival males, and the size of their territory can vary based on factors such as prey availability and pride size.
4. Maternal Care:
Lionesses are attentive mothers and provide care and protection to their cubs. Cubs are introduced to the pride when they are a few months old.
5. Ecological Role:
Lions play a vital ecological role in their ecosystems by controlling herbivore populations, which helps maintain the balance of plant communities.
6. Cultural Significance:
Lions have been a symbol of strength, courage, and royalty in various cultures and religions throughout history. They are often featured in art, literature, and national emblems.
Lions continue to captivate the world with their remarkable biology and behavior. Their conservation is of paramount importance to ensure that future generations can appreciate the majesty of these iconic big cats in the wild.
Through the efforts of zoologists, wildlife organizations, and local communities, we can work together to protect the legacy of the lion, the King of the Jungle.
Read More
- Difference Between Voltage And Current
- Difference Between Two Stroke And Four Stroke
- Difference Between Ac And Dc
- Changing The Period Of A Pendulum
- Electromagnetic Spectrum Radio Waves
Frequently Asked Question (FAQs) Animals Biology Science Lion Zoology
1. What is the scientific classification of lions?
Lions belong to the family Felidae and the genus Panthera. Their scientific name is Panthera leo.
2. What is the primary habitat of lions in the wild?
Lions are native to parts of Africa and are commonly found in grasslands, savannas, and open woodlands.
3. How do male and female lions differ in terms of physical characteristics?
Male lions (males) are generally larger than female lions (lionesses) and have a prominent mane, which lionesses lack. Lionesses tend to be more streamlined in appearance.
4. What is the typical diet of lions?
Lions are carnivores and primarily prey on mammals, including zebras, wildebeests, and antelopes. They are known for their collaborative hunting tactics within a pride.
5. What is the role of the mane in male lions, and does it serve any specific functions?
The mane of male lions serves multiple purposes, including protection during fights with rival males and as a visual signal of their health and dominance.
Difference Between Voltage And Current
Difference Between Voltage And Current: Volt and current are fundamental electrical parameters that describe the behavior of electric circuits and are essential for understanding how electricity flows.
They represent different aspects of electrical phenomena and have distinct units of measurement. In this article, we will explore the key differences between voltage and current.

Difference Between Voltage And Current
1. Definition:
- Voltage: Volt, often referred to as electric potential difference, is the measure of electric potential energy per unit charge in an electric circuit. It is the force that drives electric charges (electrons) through a conductor. Voltage is measured in volts (V).
- Current: Current is the flow of electric charge in a circuit. It represents the rate at which electric charges (usually electrons) move through a conductor. Current is measured in amperes (A).
2. Symbol:
- Voltage: The symbol for voltage is “V.”
- Current: The symbol for current is “I.”
3. Unit:
- Voltage: Volt is measured in volts (V).
- Current: Current is measured in amperes (A).
4. Direction:
- Voltage: Volt is a scalar quantity, meaning it does not have a specific direction. It represents the electric potential difference between two points in a circuit.
- Current: Current is a vector quantity, meaning it has both magnitude and direction. It represents the flow of electric charge from one point to another in a specific direction.
5. Effect:
- Voltage: Voltage creates an electric field that exerts a force on electric charges, causing them to move in a certain direction. It provides the “push” or “pressure” that drives current.
- Current: Current is the actual flow of electric charges (usually electrons) through a conductor in response to the volt. It is the movement of these charges that constitutes an electric current.
6. Measurement Device:
- Voltage: Voltage is measured using a voltmeter, a device designed to measure the potential difference between two points in a circuit.
- Current: Current is measured using an ammeter, a device designed to measure the flow of electric charge in a circuit.
7. Example:
- Voltage: Imagine a water tank placed at a certain height above the ground. The height of the tank represents the volt. When a valve is opened, water (analogous to current) flows down from the tank due to the gravitational potential energy provided by the height (analogous to volt).
- Current: Think of the water flowing through a pipe. The rate at which water flows through the pipe is similar to electric current. The water pressure that pushes the water through the pipe is analogous to volt.
In summary, volt and current are distinct electrical parameters with different definitions, units of measurement, and effects in electrical circuits. Volt provides the potential energy that drives the flow of electric charges (current) through a conductor, and understanding both parameters is essential for comprehending the behavior of electrical systems and circuits.
Read More
- Difference Between Two Stroke And Four Stroke
- Difference Between Ac And Dc
- Changing The Period Of A Pendulum
- Electromagnetic Spectrum Radio Waves
- Behaviour Of Gas Molecules
Frequently Asked Questions (FAQs) Difference Between Voltage And Current
1. What is voltage, and how is it different from current?
Volt, measured in volts (V), represents the electric potential difference between two points in a circuit. It provides the force or pressure that drives the flow of electric charge (current) through a conductor.
2. What is current, and how does it relate to voltage?
Current, measured in amperes (A), is the flow of electric charge through a conductor. It is the result of volt applied to a circuit, as charges move in response to the electric field created by the volt.
3. Is voltage a scalar or vector quantity?
Volt is a scalar quantity, meaning it does not have a specific direction. It represents the electric potential difference between two points.
4. Is current a scalar or vector quantity?
Current is a vector quantity, meaning it has both magnitude and direction. It represents the flow of electric charge in a specific direction.
5. How do voltage and current interact in an electric circuit?
Volt provides the “push” or “pressure” that drives current through a conductor. In essence, volt creates an electric field that exerts a force on electric charges, causing them to move and establish a current.
Difference Between Two Stroke And Four Stroke
Difference Between Two Stroke And Four Stroke: Engines are the heart of machines that power our vehicles, equipment, and even some small appliances. Two-strokes and four-strokes engines are two common types used in various applications.
They differ in their design, operation, and performance characteristics. In this article, we will explore the key differences between two and four-strokes engines.

Difference Between Two Stroke And Four Stroke
1. Number of Strokes:
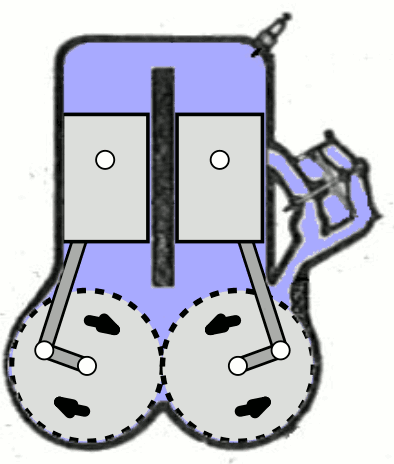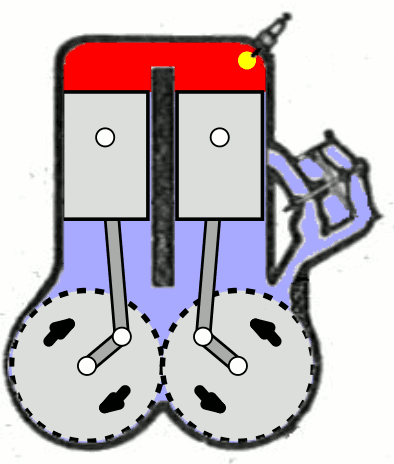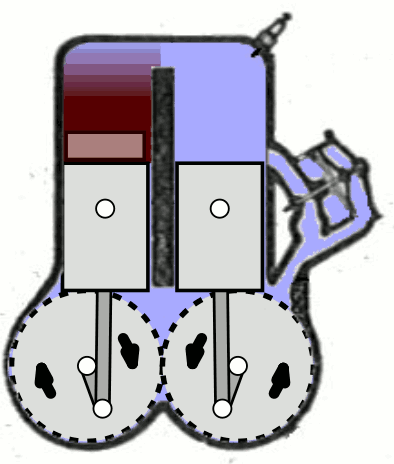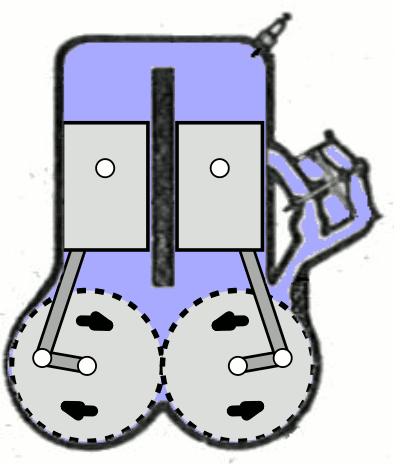
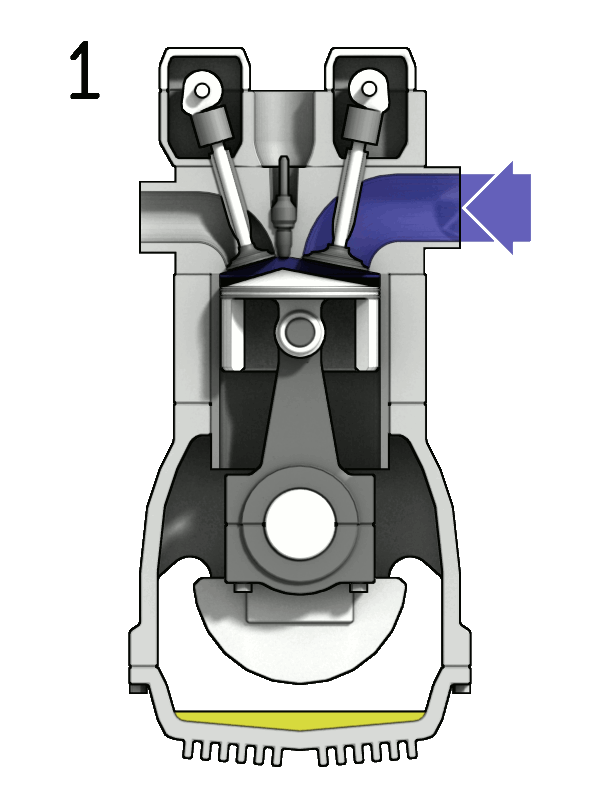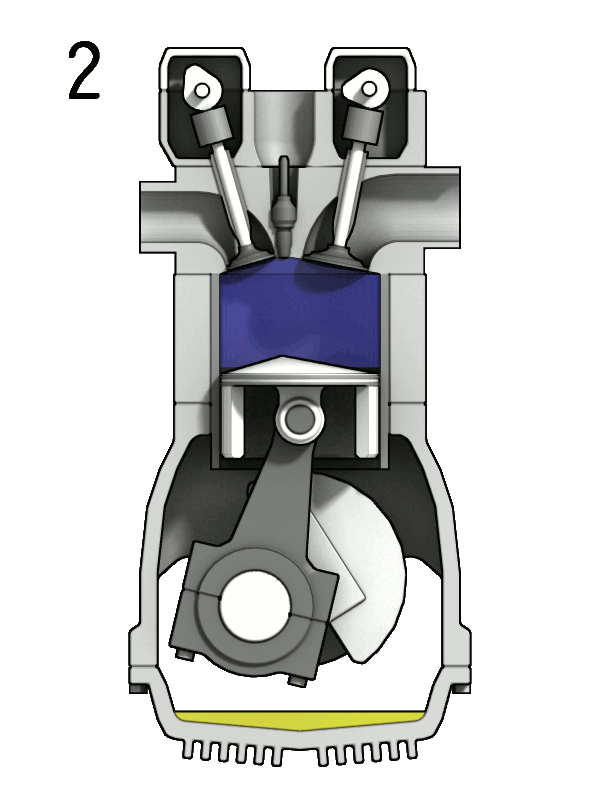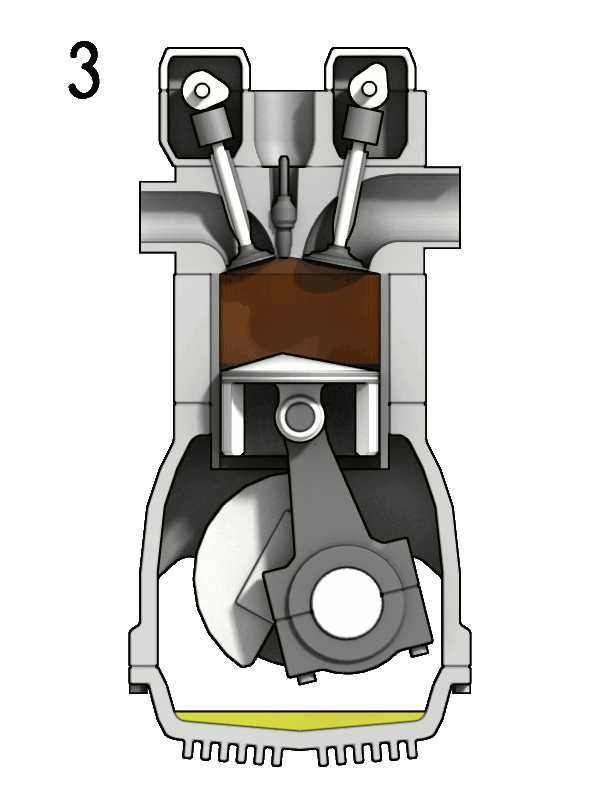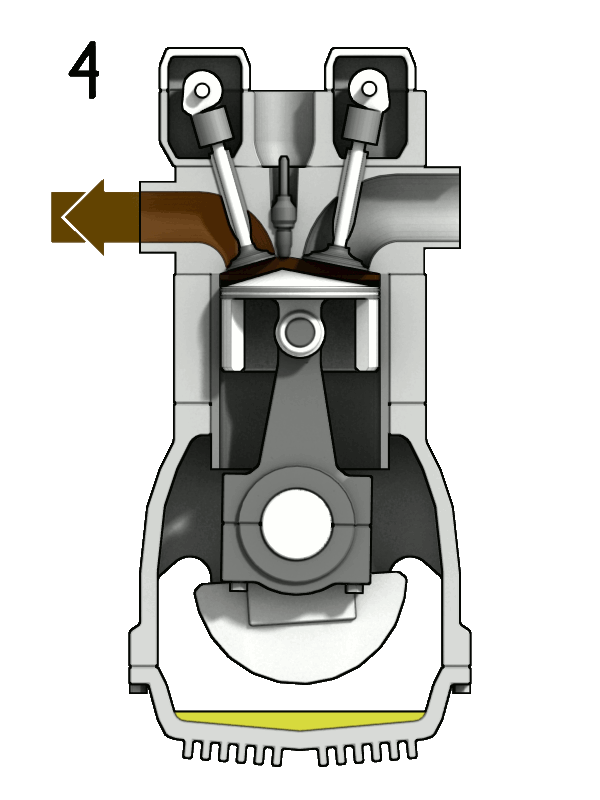
- Two-Stroke Engine: A two-strokes engine completes its cycle in two strokes of the piston—namely, the compression and power strokes. It combines the intake and exhaust processes in these two strokes.
- Four-Stroke Engine: A four-strokes engine completes its cycle in four strokes of the piston: intake, compression, power, and exhaust. Each strokes has a distinct function.
2. Operation:
- Two-Stroke Engine: In a two-strokes engine, the combustion process occurs every revolution of the crankshaft, making it produce power more frequently. This design tends to be simpler but less fuel-efficient.
- Four-Stroke Engine: In a four-strokes engine, the combustion process occurs every two revolutions of the crankshaft. It has a more structured cycle, leading to greater fuel efficiency and lower emissions.
3. Lubrication:
- Two-Stroke Engine: Two-strokes engines require oil to be mixed with the fuel for lubrication. This oil is burnt during combustion, leading to some emissions and a characteristic blue smoke.
- Four-Stroke Engine: Four-strokes engines have a separate oil reservoir for lubrication, which results in cleaner exhaust emissions.
4. Power Output:
- Two-Stroke Engine: Two-strokes engines are known for their higher power-to-weight ratio, making them suitable for applications where lightweight and compactness are essential.
- Four-Stroke Engine: Four-strokes engines tend to be more fuel-efficient and offer better torque characteristics. They are often used in applications where fuel efficiency is crucial.
5. Exhaust Emissions:
- Two-Stroke Engine: Two-strokes engines are less environmentally friendly due to the incomplete combustion of oil in the fuel mixture, resulting in higher emissions of hydrocarbons and particulate matter.
- Four-Stroke Engine: Four-strokes engines produce fewer emissions and are more compliant with emission regulations, making them a preferred choice in many applications.
6. Maintenance:
- Two-Strokes Engine: Two-strokes engines are relatively simpler in design, requiring less maintenance. However, they may require more frequent attention due to their higher wear rates.
- Four-Stroke Engine: Four-strokes engines have more complex designs and may require more maintenance, but they often have longer service intervals.
7. Applications:
- Two-Strokes Engine: Two-strokes engines are commonly used in applications where weight and simplicity are prioritized, such as small motorcycles, chainsaws, and certain recreational vehicles.
- Four-Strokes Engine: Four-strokes engines are prevalent in automobiles, trucks, larger motorcycles, generators, and many industrial and marine applications.
8. Fuel Efficiency:
- Two-Strokes Engine: Two-strokes engines are generally less fuel-efficient compared to four-strokes engines due to their frequent combustion cycles.
- Four-Strokes Engine: Four-strokes engines offer better fuel efficiency, making them suitable for vehicles and equipment where economy is a key consideration.
In conclusion, the choice between a two-strokes and a four-strokes engine depends on the specific requirements of the application. Two-strokes offer simplicity and higher power output in a lightweight package, while four-strokes provide better fuel efficiency, lower emissions, and durability, making them the preferred choice for many modern vehicles and equipment.
Read More
- Electromagnetic Spectrum Radio Waves
- Behaviour Of Gas Molecules
- Molar Mass Of Oxalic Acid
- Types Of Chemical Bonding
- Chemistry In Everyday Life
Frequently Asked Questions (FAQs) Difference Between Two Strokes And Four Strokes
1. What is the fundamental difference between a two-strokes and a four-strokes engine?
The primary difference lies in the number of strokes required to complete one engine cycle. A two-strokes engine completes a cycle in two strokes (compression and power), while a four-strokes engine completes it in four strokes (intake, compression, power, and exhaust).
2. Which engine type is more fuel-efficient, a two-strokes, or a four-strokes engine?
Generally, four-strokes engines are more fuel-efficient than two-strokes engines. Four-strokes have a more structured combustion cycle, leading to better fuel economy.
3. Are two-strokes engines more powerful than four-strokes engines?
Two-strokes engines often have a higher power-to-weight ratio and can produce more power per unit of displacement. However, four-strokes engines can offer better torque characteristics and efficiency.
4. Do two-strokes engines require mixing oil with fuel for lubrication?
Yes, two-strokes engines require oil to be mixed with the fuel to provide lubrication. This oil is burned during combustion, leading to characteristic blue exhaust smoke.
5. Which type of engine produces fewer emissions, two-strokes, or four-strokes
Four-strokes engines generally produce fewer emissions and are more environmentally friendly due to their cleaner combustion process.
Difference Between Light Microscope And Electron Microscope
Difference Between Light Microscope And Electron Microscope: Microscopes are indispensable tools in the world of science and research, enabling us to explore the microscopic world and uncover intricate details of specimens.
Two primary types of microscopes used for this purpose are the light microscope (LM) and the electron microscope (EM).
Despite their common goal of magnifying objects, these microscopes differ significantly in their principles of operation, capabilities, and the types of specimens they can examine. Here, we highlight the key differences between light microscopes and electron microscopes.

Difference Between Light Microscope And Electron Microscope
1. Principle of Operation:
- Light Microscope (LM): LMs use visible light to illuminate and magnify specimens. They employ glass lenses to bend and focus light, revealing the specimen’s details. LMs are also known as optical or compound microscopes.
- Electron Microscope (EM): EMs use a beam of electrons instead of light to magnify specimens. Electrons have much shorter wavelengths than visible light, allowing for much higher magnification and resolution.
2. Magnification:
- Light Microscope (LM): LMs typically offer magnifications of up to around 1,000 times the specimen’s actual size. They have limitations in achieving higher magnification due to the wavelength of visible light.
- Electron Microscope (EM): EMs can achieve magnifications exceeding 50,000 times, and some advanced EMs can go beyond 2,000,000 times. This exceptional magnification is due to the short wavelength of electrons.
3. Resolution:
- Light Microscope (LM): LMs have limited resolution, typically around 200 nanometers (nm). This means that two points closer than 200 nm will appear as a single point.
- Electron Microscope (EM): EMs offer much higher resolution, often reaching below 0.1 nm. This allows them to reveal fine details of specimens at the atomic and molecular level.
4. Specimen Type:
- Light Microscope (LM): LMs are suitable for viewing living and non-living specimens such as cells, tissues, bacteria, and larger structures. They can observe specimens in their natural, hydrated state.
- Electron Microscope (EM): EMs are used to examine non-living specimens, as the process requires a vacuum. They are ideal for studying ultra-thin sections of cells, viruses, nanomaterials, and inorganic structures.
5. Sample Preparation:
- Light Microscope (LM): Sample preparation for LMs is relatively simple. Specimens are typically mounted on glass slides, often stained to enhance contrast.
- Electron Microscope (EM): EMs require more complex sample preparation, including fixation, dehydration, embedding in resin, and ultra-thin sectioning. Samples must withstand the vacuum and electron beam.
6. Color:
- Light Microscope (LM): LMs provide color images as they use visible light. Stains and dyes can enhance contrast and highlight specific structures.
- Electron Microscope (EM): EMs produce grayscale images since they use electrons. Different shades of gray represent variations in electron density within the specimen.
7. Cost and Accessibility:
- Light Microscope (LM): LMs are generally more affordable and widely accessible in educational and research settings.
- Electron Microscope (EM): EMs are complex and expensive instruments, often found in specialized research institutions and laboratories.
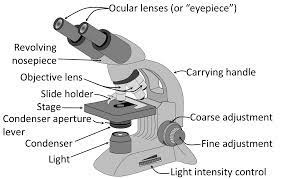

In summary, light microscopes are versatile tools suitable for studying a wide range of biological and non-biological specimens in their natural state, whereas electron microscopes provide unparalleled resolution and are indispensable for detailed examinations of nanoscale structures but require more extensive sample preparation and are primarily used for non-living specimens. The choice between the two depends on the specific research objectives and the nature of the specimens being studied.
Read More
- Molar Mass Of Aluminium
- Molecular Mass Of Glucose
- Latent Heat Of Water
- Difference Between Work And Power
- Difference Between Gravitation And Gravity
Frequently Asked Questions (FAQs) Difference Between Light Microscope And Electron Microscope
1. What is the main difference between a light microscope and an electron microscope?
The primary difference is in the type of radiation used for imaging. Light microscopes use visible light, while electron microscopes use a beam of electrons.
2. How do light microscopes and electron microscopes differ in terms of magnification
Light microscopes typically offer magnifications of up to around 1,000 times the specimen’s actual size, while electron microscopes can achieve magnifications exceeding 50,000 times and even up to 2,000,000 times.
3. What is the resolution difference between these microscopes?
Light microscopes have limited resolution, typically around 200 nanometers (nm), whereas electron microscopes offer much higher resolution, often below 0.1 nm.
4. Can light microscopes be used for studying living specimens?
Yes, light microscopes are suitable for observing living specimens such as cells, tissues, and bacteria in their natural, hydrated state.
5. Are electron microscopes suitable for studying living specimens?
No, electron microscopes require a vacuum environment, making them unsuitable for living specimens. They are primarily used for non-living specimens.
Molar Mass Of Aluminium
Molar Mass Of Aluminium: Aluminium, with its remarkable combination of strength, lightness, and versatility, holds a prominent place in various industrial, technological, and everyday applications.
Understanding the molar mass of Al is essential in chemistry and materials science as it serves as a fundamental parameter for numerous calculations and applications. In this article, we delve into the concept of the molar mass of Al, its significance, and its diverse uses.

Molar Mass Of Aluminium
The Elemental Beauty of Aluminium
Before we explore the molar mass of aluminium, let’s appreciate its elemental properties. Aluminium, represented by the chemical symbol Al, is a silvery-white, non-ferrous metal. It is the third most abundant element in Earth’s crust, making up approximately 8.23% of the Earth’s mass. Despite its prevalence, extracting pure Al from its ores was once a complex and energy-intensive process.
Calculating the Molar Mass of Aluminium
The molar mass of Al is the mass of one mole of Al atoms. To calculate it, we consider the atomic mass of Al, which is approximately 26.98 atomic mass units (amu). One mole of any element contains Avogadro’s number of atoms, which is approximately 6.022 x 10^23 atoms.
Molar Mass of Aluminium = Atomic Mass of Aluminium x Avogadro’s Number
Molar Mass of Al ≈ 26.98 amu x 6.022 x 10^23 atoms/mol
Molar Mass of Al ≈ 26.98 grams per mole (g/mol)
Thus, the molar mass of aluminium is approximately 26.98 g/mol, which means that one mole of Al atoms has a mass of 26.98 grams.
The Significance of Molar Mass
The molar mass of Al is a vital concept in chemistry and materials science, carrying significant implications:
1. Chemical Reactions: Molar mass is used to calculate the amount of Al required or produced in chemical reactions, allowing for precise stoichiometric calculations.
2. Materials Science: Understanding the molar mass helps engineers and materials scientists design and analyze Al alloys and composites for various applications, such as aerospace, automotive, and construction.
3. Aluminum Production: In the Al industry, the determination of molar mass is crucial for process optimization and quality control during smelting and refining.
4. Everyday Life: Aluminium’s lightness and resistance to corrosion make it a popular choice for a wide range of products, from beverage cans to window frames and aircraft parts.
5. Energy Efficiency: The use of aluminium in transportation and construction contributes to energy-efficient designs due to its lightweight properties.
6. Environmental Impact: Recycling aluminium saves energy and resources, reducing the environmental footprint associated with its production.
Conclusion
The molar mass of aluminium, approximately 26.98 g/mol, is a foundational concept in chemistry and materials science. It plays a pivotal role in chemical calculations, materials engineering, and the practical applications of aluminium in modern life.
Whether you’re studying chemical reactions, designing advanced materials, or appreciating the lightweight strength of aluminium in everyday products, a grasp of its molar mass is key to unlocking the full potential of this versatile metal.
From the aerospace industry to your household, aluminium continues to shape our world with its unique combination of properties, making it an indispensable element in the realm of materials science and technology.
Read More
- Molecular Mass Of Glucose
- Latent Heat Of Water
- Difference Between Work And Power
- Difference Between Gravitation And Gravity
- Molecular Weight Of H2SO4
Frequently Asked Questions (FAQs) Molar Mass Of Aluminium
What is the molar mass of aluminium?
The molar mass of Al is approximately 26.98 grams per mole (g/mol). This value represents the mass of one mole of aluminium atoms.
Why is the molar mass of aluminium important in chemistry?
The molar mass of Al is crucial for various chemical calculations, including stoichiometry, determining the amount of Al in compounds, and balancing chemical equations.
How is the molar mass of aluminium calculated?
The molar mass of Al is calculated by considering the atomic mass of Al (approximately 26.98 atomic mass units) and multiplying it by Avogadro’s number, which is approximately 6.022 x 10^23.
What is the significance of aluminium in materials science?
Al is a versatile metal widely used in materials science and engineering due to its lightweight, corrosion resistance, and strength. Its molar mass is crucial for designing and analyzing aluminium alloys and composites for various applications.
How is aluminium produced and refined in the aluminium industry?
The aluminium production process involves extracting Al from bauxite ore through a series of steps, including refining and smelting. The determination of molar mass is essential for process optimization and quality control in Al production.
Anatomy Of Flowering Plants
Anatomy Of Flowering Plants: The anatomy of flowering plants is a fascinating field of study that delves into the inner workings and structural complexity of these botanical wonders.
Flowering plants, also known as angiosperms, comprise the majority of plant species on Earth and play essential roles in ecosystems, agriculture, and our daily lives. In this article, we embark on a journey to explore the anatomy of flowering plants, from root to flower, uncovering the intricate structures that make these plants thrive and reproduce.
 Anatomy Of Flowering Plants
Anatomy Of Flowering Plants
The Root System
1. Roots: Roots anchor the plant in the soil and absorb water and nutrients. They often have a central taproot (primary root) with lateral roots branching off.
2. Root Cap: The root cap protects the delicate apical meristem (growing tip) as it pushes through the soil.
3. Root Hairs: Tiny hair-like structures on roots increase the surface area for water and nutrient absorption.
The Stem
4. Stem: The stem provides support for leaves, flowers, and fruits, and it conducts water, nutrients, and sugars throughout the plant.
5. Node: The node is the point on the stem where leaves, branches, or flowers are attached.
6. Internode: The internode is the stem segment between two nodes.
The Leaf
7. Leaf Blade: The broad, flat part of the leaf where photosynthesis occurs.
8. Petiole: The petiole is the leaf stalk that attaches the leaf blade to the stem.
9. Veins: Veins transport water, nutrients, and sugars within the leaf.
10. Leaf Margin: The leaf margin is the outer edge of the leaf, which can be smooth, serrated, or lobed.
The Flower
11. Flower: The reproductive structure of angiosperms, responsible for producing seeds. It typically consists of sepals, petals, stamens, and carpels.
12. Sepals: Sepals are the outermost floral parts, usually green, that protect the developing flower bud.
13. Petals: Petals are often colorful and serve to attract pollinators, such as bees and butterflies.
14. Stamens: Stamens are the male reproductive organs, consisting of anthers (where pollen is produced) and filaments.
15. Carpels: Carpels are the female reproductive organs, consisting of the stigma (where pollen is received), style, and ovary (containing ovules).
The Fruit
16. Fruit: The mature ovary of the flower, which contains seeds. Fruits serve as a means of seed dispersal.
17. Pericarp: The pericarp is the fruit wall, which can be fleshy (e.g., apple) or dry (e.g., bean pod).
The Vascular Tissues
18. Xylem: Xylem conducts water and minerals from the roots to the rest of the plant.
19. Phloem: Phloem transports sugars produced during photosynthesis from the leaves to other parts of the plant.
Specialized Structures
20. Meristems: Meristems are regions of undifferentiated cells where growth occurs. They are found at the tips of roots and stems.
21. Parenchyma, Collenchyma, and Sclerenchyma: These are different types of plant cells with various structural and support functions.
Understanding the anatomy of flowering plants is vital for horticulture, agriculture, and ecological studies. It allows us to cultivate crops, improve plant health, and appreciate the beauty and complexity of the natural world.
Whether you’re an aspiring botanist or simply an admirer of nature, exploring the intricate structures of flowering plants offers a deeper connection to the diverse plant life that surrounds us.
Read More
- Elastic Behaviour Of Materials
- Molecular Weight Of Oxalic Acid
- Structural Organisation In Animals
- CBSE Sample Papers for Class 11 English With Answers PDF
Frequently Asked Questions (FAQs) Anatomy Of Flowering Plants
What is the anatomy of a flowering plant?
The anatomy of a flowering plant encompasses its structural features, including roots, stems, leaves, flowers, and reproductive organs. It also involves specialized tissues like xylem and phloem for transport and meristems for growth.
What is the function of the root system in a plant?
The root system of a plant anchors it in the soil, absorbs water and nutrients, and stores reserve food materials.
How do leaves contribute to a plant’s survival?
Leaves are the primary sites for photosynthesis, where plants produce sugars using sunlight, carbon dioxide, and water. They also regulate transpiration, helping to maintain the plant’s water balance.
What role do stems play in a plant’s growth and development?
Stems provide structural support for leaves, flowers, and fruits. They also transport water, nutrients, and sugars throughout the plant.
What are the essential parts of a flower and their functions?
Flowers consist of sepals (protective outermost layer), petals (often colorful and attracting pollinators), stamens (male reproductive organs), and carpels (female reproductive organs). Their functions include reproduction and attracting pollinators.
Molar Mass Of Sulphur
Molar Mass Of Sulphur: Sulfur, represented by the chemical symbol S, is a fundamental element in the periodic table, known for its diverse chemical properties and essential roles in various natural and industrial processes.
Understanding the molar mass of sulfur is a crucial aspect of chemistry, providing insight into its atomic weight and its applications in a wide range of scientific and industrial fields. In this article, we delve into the concept of the molar mass of sulfur, its significance, and practical implications.
 Molar Mass Of Sulphur
Molar Mass Of Sulphur
The Atomic Structure of Sulphur
Before exploring the molar mass of sulfur, it’s essential to appreciate its atomic structure. A sulfur atom has 16 electrons, 16 protons, and varying numbers of neutrons, depending on its isotope.
The most common naturally occurring isotope, sulfur-32 (32S), has 16 neutrons. Sulfur typically forms stable molecules consisting of eight atoms, resulting in molecules with the chemical formula S8.
Calculating the Molar Mass of Sulphur
The molar mass of sulfur is the mass of one mole of sulfur atoms or molecules. To calculate it, we consider the atomic masses of the constituent atoms. The atomic mass of sulfur, as listed in the periodic table, is approximately 32.06 atomic mass units (amu).
One mole of sulfur consists of Avogadro’s number of sulfur atoms, which is approximately 6.022 x 10^23 atoms. Therefore, to find the molar mass of sulfur, we use the atomic mass as follows:
Molar Mass of Sulfur = Atomic Mass of Sulfur x Avogadro’s Number
Molar Mass of Sulfur ≈ 32.06 amu x 6.022 x 10^23 atoms/mol
Molar Mass of Sulfur ≈ 32.06 grams per mole (g/mol)
Thus, the molar mass of sulfur is approximately 32.06 g/mol, which means that one mole of sulfur atoms has a mass of 32.06 grams.
Significance and Applications
The molar mass of sulfur holds significant importance in various scientific and industrial contexts:
1. Chemical Reactions: In chemistry, the molar mass of sulfur is used in stoichiometric calculations to determine the amount of sulfur required or produced in chemical reactions. It is essential for balancing chemical equations.
2. Industry: Sulfur finds extensive use in the chemical industry, including the production of sulfuric acid, fertilizers, and various chemicals. Understanding its molar mass is crucial in manufacturing processes.
3. Agriculture: Sulfur is an essential nutrient for plant growth, and sulfur-containing fertilizers are used to enhance crop yields. Accurate molar mass information aids in fertilizer formulation.
4. Environmental Monitoring: Monitoring sulfur emissions, such as sulfur dioxide (SO2) from industrial processes and volcanic eruptions, is critical for assessing air quality and environmental impact.
5. Pharmaceuticals: Sulfur compounds are employed in pharmaceuticals, and the molar mass of sulfur is essential for precise dosing and formulation.
6. Petrochemicals: Sulfur content in petroleum products can affect their properties and environmental impact. Determining sulfur molar mass is crucial for quality control in the petroleum industry.
Conclusion
The molar mass of sulfur, approximately 32.06 g/mol, is a fundamental parameter in chemistry and various industrial sectors. It guides chemical reactions, facilitates manufacturing processes, and plays a pivotal role in agriculture, environmental monitoring, and pharmaceuticals. As a versatile element with diverse applications, sulfur’s molar mass is a cornerstone in our understanding of chemical processes and their practical implications, contributing to advancements in science and industry alike.
Read More
- Difference Between Diode And Rectifier
- Difference Between AM And FM
- Dielectric Material And Dipole Moment
- Difference Between Centre Of Gravity And Centroid
- Kinetic Gas Equation Derivation
Frequently Asked Questions (FAQs) Molar Mass Of Sulphur
What is the molar mass of sulfur?
The molar mass of sulfur is approximately 32.06 grams per mole (g/mol). This value represents the mass of one mole of sulfur atoms or molecules.
Why is the molar mass of sulfur important?
The molar mass of sulfur is essential for various chemical calculations, including stoichiometry, determining the amount of sulfur in compounds, and balancing chemical equations. It is also crucial in industrial applications and environmental monitoring.
How is the molar mass of sulfur calculated?
The molar mass of sulfur is calculated by considering the atomic mass of sulfur (approximately 32.06 atomic mass units) and multiplying it by Avogadro’s number, which is approximately 6.022 x 10^23.
What is the significance of sulfur in chemistry and industry?
Sulfur is a versatile element used in various chemical processes and industries. It is a key component in the production of sulfuric acid, fertilizers, and various chemicals. Sulfur compounds also have applications in pharmaceuticals and petrochemicals.
How is sulfur used in agriculture?
Sulfur is an essential nutrient for plant growth. Sulfur-containing fertilizers are used to provide plants with the necessary sulfur for healthy development and improved crop yields.
Molecular Mass Of Glucose
Molecular Mass Of Glucose: Glucose, often referred to as the “fuel” of life, is a fundamental molecule in biology and biochemistry.
Its molecular mass plays a pivotal role in various scientific and practical applications, ranging from nutrition and energy metabolism to pharmaceuticals and clinical diagnostics. In this article, we delve into the molecular mass of C6H12O6, its significance, and its diverse applications.

Molecular Mass Of Glucose
Molecular Formula of Glucose
Before we explore the molecular mass of C6H12O6, it’s essential to understand its molecular formula. Glucose has the chemical formula C6H12O6. This formula represents the number of carbon (C), hydrogen (H), and oxygen (O) atoms in a single glucose molecule.
Calculating Molecular Mass
The molecular mass of a compound is the sum of the atomic masses of all the constituent atoms in its molecular formula. To calculate the molecular mass of C6H12O6, we add the atomic masses of the individual elements:
- Carbon (C) has an atomic mass of approximately 12.01 atomic mass units (amu).
- Hydrogen (H) has an atomic mass of approximately 1.01 amu.
- Oxygen (O) has an atomic mass of approximately 16.00 amu.
Now, let’s calculate the molecular mass of C6H12O6:
Molecular mass of glucose = (6 × C) + (12 × H) + (6 × O) Molecular mass of C6H12O6 ≈ (6 × 12.01 amu) + (12 × 1.01 amu) + (6 × 16.00 amu) Molecular mass of glucose ≈ 72.06 amu + 12.12 amu + 96.00 amu Molecular mass of glucose ≈ 180.18 amu
Rounded to two decimal places, the molecular mass of C6H12O6 is approximately 180.18 amu or 180.18 g/mol (grams per mole).
Significance of Molecular Mass
The molecular mass of glucose holds immense significance in various fields:
1. Nutrition: Glucose is a primary source of energy for the human body. Its molecular mass is used to calculate the calorie content in foods and beverages, providing vital nutritional information.
2. Biochemistry: In cellular metabolism, glucose undergoes glycolysis and other biochemical processes. Understanding its molecular mass is crucial for studying these metabolic pathways.
3. Pharmaceutical and Medical Applications: Glucose serves as a key component in various medications, intravenous solutions, and diagnostic tests. Knowledge of its molecular mass is essential for formulation and dosage calculations.
4. Chemical Reactions: In chemistry, the molecular mass of glucose is used in stoichiometric calculations, chemical reactions, and the determination of molar concentrations in solutions.
5. Industrial Processes: Glucose is utilized in the food industry for sweetening and in fermentation processes for biofuel and alcohol production. Its molecular mass is relevant in industrial calculations.
Glucose in the Human Body
In the human body, glucose is transported through the bloodstream and taken up by cells. It is a primary substrate for cellular respiration, where it is oxidized to produce adenosine triphosphate (ATP), the energy currency of cells. Proper regulation of blood glucose levels is essential for maintaining health.
Conclusion
The molecular mass of glucose, approximately 180.18 g/mol, is a fundamental parameter in nutrition, biochemistry, pharmaceuticals, and numerous scientific disciplines. It underscores the essential role glucose plays as an energy source and its widespread applications in various industries.
Understanding the molecular mass of glucose allows scientists, healthcare professionals, and researchers to make precise calculations and advances in their respective fields, ultimately contributing to our knowledge of life processes and the practical applications of this vital molecule.
Read More
- Latent Heat Of Water
- Difference Between Work And Power
- Difference Between Gravitation And Gravity
- Molecular Weight Of H2SO4
- Distance Time Velocity Time Graph
Frequently Asked Questions (FAQs) Molecular Mass Of Glucose
What is the molecular mass of glucose?
The molecular mass of C6H12O6, also known as the molar mass, is approximately 180.16 grams per mole (g/mol).
What is the molecular formula of glucose?
The molecular formula of glucose is C6H12O6. This formula represents the number of carbon (C), hydrogen (H), and oxygen (O) atoms in a glucose molecule.
How is the molecular mass of glucose calculated?
The molecular mass of C6H12O6 is calculated by summing the atomic masses of all the constituent atoms in its molecular formula. You add the atomic masses of 6 carbon atoms (C), 12 hydrogen atoms (H), and 6 oxygen atoms (O).
Why is the molecular mass of glucose important?
The molecular mass of C6H12O6 is important in chemistry and biology for various calculations, including stoichiometry, chemical reactions, and determining the concentration of C6H12O6 solutions.
How is the molecular mass of a compound used in chemistry?
The molecular mass is used to calculate the number of moles of a substance, which is important in stoichiometric calculations, balancing chemical equations, and understanding the relationships between reactants and products in chemical reactions.
Latent Heat Of Water
Latent Heat Of Water: The latent heat of water is a fundamental concept in thermodynamics and describes the amount of heat energy required to change the phase of water from one state to another while keeping the temperature constant.

Latent Heat Of Water
Phases of Water
- Solid (Ice): In the solid phase, water molecules are arranged in a regular, crystalline structure. Heat must be added to convert ice into liquid water.
- Liquid (Water): In the liquid phase, water molecules are loosely arranged, allowing them to flow. Heat is absorbed when ice melts to become liquid water.
- Gas (Water Vapor): In the gas phase, water molecules are highly disordered and move freely. Heat is required to change liquid water into water vapor (evaporation), and heat is released when water vapor condenses back into liquid water.
Latent Heat of Fusion
The latent heat of fusion () is the amount of heat energy required to change one unit mass of a substance from a solid to a liquid phase (or vice versa) at a constant temperature. For water, the latent heat of fusion is approximately 334,000 joules per kilogram (J/kg). This means that to melt 1 kilogram of ice at 0°C into liquid water at 0°C, approximately 334,000 joules of heat energy must be added.
Latent Heat of Vaporization
The latent heat of vaporization () is the amount of heat energy required to change one unit mass of a substance from a liquid to a gas phase (or vice versa) at a constant temperature. For water, the latent heat of vaporization is approximately 2,257,000 joules per kilogram (J/kg). This means that to vaporize 1 kilogram of liquid water at 100°C into water vapor at 100°C, approximately 2,257,000 joules of heat energy must be added.
Significance and Applications
Understanding the latent heat of water is crucial in various natural and practical scenarios:
- Weather and Climate: Latent heat plays a significant role in weather phenomena. The release of latent heat during the condensation of water vapor in the atmosphere is responsible for cloud formation, precipitation, and the release of energy in thunderstorms.
- Cooking: Latent heat is involved in cooking processes, such as boiling and steaming. Heat is continuously supplied to convert liquid water into water vapor, which cooks food.
- Heating and Cooling Systems: In heating and cooling systems, heat pumps utilize the latent heat of vaporization and fusion to transfer heat efficiently.
- Thermal Comfort: Sweating is a cooling mechanism in humans. When sweat evaporates from the skin, it absorbs latent heat from the body, providing a cooling effect.
- Energy Storage: Latent heat storage systems are used in some renewable energy applications to store excess energy for later use. For example, phase-change materials (PCMs) store and release energy during phase transitions.
- Cryogenics: In cryogenic applications, understanding the latent heat of vaporization is crucial for the liquefaction and storage of gases like nitrogen and oxygen.
In summary, the latent heat of water is a fundamental property that governs phase changes in water and plays a pivotal role in various natural phenomena and practical applications, from weather patterns to everyday cooking and energy storage. It is a key concept in thermodynamics and heat transfer.
Read More
- Difference Between Work And Power
- Difference Between Gravitation And Gravity
- Molecular Weight Of H2SO4
- Distance Time Velocity Time Graph
- Difference Between Kinetics And Kinematics
Frequently Asked Questions (FAQs) Latent Heat Of Water
What is latent heat?
Latent heat is the amount of heat energy absorbed or released during a phase change of a substance (solid to liquid, liquid to gas, etc.) at a constant temperature, without a change in temperature.
What is the latent heat of water?
The latent heats of water refers to the amount of heat energy required or released during phase changes involving water. Specifically, it includes the latent heat of fusion (melting or freezing) and the latent heat of vaporization (evaporation or condensation).
What is the latent heat of fusion for water, and why is it important?
The latent heats of fusion for water is approximately 334,000 joules per kilogram (J/kg). It’s the energy required to change 1 kilogram of ice at 0°C into 1 kilogram of liquid water at 0°C. This process is fundamental in weather patterns, ice melting, and heat transfer in cooling systems.
What is the latent heat of vaporization for water, and why is it significant?
The latent heats of vaporization for water is approximately 2,257,000 joules per kilogram (J/kg). It’s the energy required to change 1 kilogram of liquid water at 100°C into 1 kilogram of water vapor at 100°C. This process is crucial in weather phenomena, cooking, and many industrial processes.
How does latent heat affect weather and climate?
Latent heat is involved in the formation of clouds, precipitation (rain, snow, etc.), and the release of energy during thunderstorms. It plays a vital role in Earth’s climate system by redistributing heat in the atmosphere and oceans.