Blog
Difference Between KVA And KW
Difference Between KVA And K. While both KVA and KW are utilized to quantify electrical power, there exists a clear distinction between the two. These units serve as power ratings in various measuring instruments to gauge current characteristics.
They are employed to denote power and are represented by the abbreviations “kilovolt amperes” (KVA) and “kilowatts” (KW). In this discussion, we will delve into the dissimilarities between KVA and KW.
kVA (Kilovolt-Ampere):
- kVA is a unit of apparent power in an electrical system.
- It represents the total power (real power and reactive power) consumed by a device.
- It considers both the active power (kW) and the reactive power (kVAR) components.
- Reactive power doesn’t perform useful work but is necessary for the operation of devices like motors and transformers.
- kVA is important for sizing equipment to handle the total power demand, including reactive power.
- It is used to determine the capacity of electrical systems, especially in situations where power factor correction is needed.
kW (Kilowatt):
- kW is a unit of real power in an electrical system.
- It represents the actual power that performs useful work or generates heat.
- kW is the power that is used to perform tasks, such as running appliances, lights, or machinery.
- It is a measure of the rate of energy consumption or production.
- kW is the primary factor in determining electricity bills, as it represents the energy used over time.
- Unlike kVA, kW doesn’t consider reactive power and only deals with the actual power being consumed or produced.
Key Differences:
-
Nature of Power:
- kVA includes both real power (kW) and reactive power (kVAR).
- kW represents only the real power that performs useful work.
-
Usefulness:
- kVA is important for sizing equipment and assessing the total power demand, including reactive power needs.
- kW is used to measure energy consumption or production for billing and evaluating the actual work performed.
-
Importance in Electrical Systems:
- kVA is crucial for determining the capacity of transformers, generators, and other equipment.
- kW is vital for understanding the actual power requirements and energy usage of devices.
-
Power Factor:
- kVA doesn’t consider power factor.
- kW is directly related to power factor, which indicates the efficiency of power usage.
-
Calculation:
- kVA is calculated using the formula: kVA = √(kW² + kVAR²).
- kW is calculated using the formula: kW = Voltage × Current × Power Factor.
-
Usage Examples:
- kVA is used in situations where power factor correction and equipment sizing are required.
- kW is used to assess energy consumption in homes, businesses, and industries.
In summary, while both kVA and kW are units of power, they represent different aspects of electrical systems. kVA considers total power, including reactive power, and is used for equipment sizing, while kW represents real power and is used to measure energy consumption and perform useful work.
Frequently Asked Question (FAQs)
What does kVA stand for?
kVA stands for Kilovolt-Ampere, a unit of apparent power in an electrical system.
What does kW stand for?
kW stands for Kilowatt, a unit of real power in an electrical system.
What is the main difference between kVA and kW?
The main difference lies in the components they represent. kVA includes both real power (kW) and reactive power (kVAR), while kW only represents the real power that performs useful work.
What is the purpose of kVA?
kVA is used to assess the total power demand, including reactive power requirements. It is important for sizing equipment and determining the capacity of transformers, generators, and other electrical devices.
What is the purpose of kW?
kW is used to measure the actual power that performs useful work or generates heat. It is crucial for understanding energy consumption, billing, and evaluating the efficiency of devices.
Changing States Of Matter
Changing States Of Matter The concept of changing states of matter revolves around the fascinating transformations substances undergo when transitioning from one physical state to another.
Matter, which can exist as solids, liquids, and gases, can shift between these states due to alterations in temperature and pressure. Understanding these transitions requires delving into the behavior of particles at the molecular level.

States of Matter:
- Solid State: In the solid state, particles are tightly packed and maintain a fixed arrangement. They vibrate in place due to their low energy levels. Solids have a defined shape and volume.
- Liquid State: In liquids, particles have more freedom to move. They are still relatively close together, allowing them to slide past each other. Liquids take the shape of their container and have a definite volume.
- Gaseous State: Gases have the highest energy levels among the three states. Particles in gases are widely spaced and move freely. Gases lack a fixed shape or volume and expand to fill their container.
Energy and Transitions:
Changing states of matter primarily involve the transfer of thermal energy. Adding energy to a substance causes its particles to gain kinetic energy, increasing their motion and interactions. Conversely, removing energy results in decreased motion and interactions.
Transitions Explained:
- Melting: When a solid is heated, it gains energy. At a specific temperature known as the melting point, the particles’ kinetic energy becomes strong enough to break the bonds that hold them in a fixed structure. The substance transitions from a solid to a liquid.
- Freezing: Conversely, when a liquid is cooled, it loses energy. As the particles lose kinetic energy, their motion slows down, allowing attractive forces between them to bring them closer together. The substance transitions from a liquid to a solid through freezing.
- Evaporation and Boiling: In a liquid, particles have varying kinetic energies. Some particles at the surface with higher energy can overcome the attractive forces and enter the gas phase. This process, called evaporation, gradually occurs at any temperature. Boiling, however, is rapid vaporization that happens at a specific temperature (boiling point) throughout the liquid.
- Condensation: When a gas loses energy (often through cooling), its particles slow down and come closer together. This allows attractive forces to take over, and the gas transitions into a liquid through condensation.
- Sublimation and Deposition: Sublimation involves the direct transition of a solid into a gas without passing through the liquid state. Deposition is the reverse process, where gas particles lose energy and transition directly to a solid.
Changes in States of Matter: A Deeper Exploration
The world around us is in a constant state of flux, and one of the most intriguing aspects is the changing states of matter. These transitions, from solid to liquid, liquid to gas, and vice versa, are not mere random occurrences but intricate processes driven by the interactions between particles and the energy they possess.
Kinetic Theory and Particle Behavior:
At the heart of understanding changing states of matter lies the kinetic theory. This theory explains the behavior of particles in different states:
Solids: In solids, particles are tightly packed and exhibit minimal movement. They vibrate in place due to thermal energy, but their positions remain relatively fixed.
Liquids: Liquid particles have more energy than their solid counterparts. They can move around, sliding past each other. This mobility allows liquids to flow and take the shape of their containers.
Gases: Gas particles possess the most energy, leading to rapid and chaotic movement. They are widely spaced and have the freedom to move in all directions.
Energizing Transitions:
Melting and Freezing: When you heat a solid, you’re infusing energy into its particles. As they gain kinetic energy, they overcome their fixed positions, and the solid turns into a liquid in a process called melting. Conversely, cooling a liquid causes particles to lose energy. They slow down and arrange themselves in a fixed pattern, forming a solid through freezing.
Evaporation and Condensation: In a liquid, particles with higher energy are at the surface. Evaporation occurs when these energetic particles escape the attractive forces of the liquid and become a gas. Condensation, the reverse process, takes place when gas particles lose energy and revert to the liquid state.
Boiling: Boiling is an intense form of evaporation that happens throughout the liquid. It occurs when the vapor pressure of the liquid matches the atmospheric pressure.
Sublimation and Deposition: Some substances, like dry ice (solid carbon dioxide), can transition directly from a solid to a gas without passing through the liquid phase, a phenomenon known as sublimation. The reverse process, deposition, involves gas turning directly into a solid.
Energy Source: Thermal Energy:
In most cases, thermal energy in the form of heat drives these changes. The more thermal energy particles have, the faster they move and the farther they spread apart. This energy disrupts the attractive forces between particles, enabling them to change states.
Applications and Significance:
Understanding these state changes is essential in various fields. It’s the basis for weather phenomena like clouds and rain, industrial processes like distillation, and even our day-to-day experiences, like the feeling of warmth when wet clothes dry on a sunny day.
Read More
- Average Speed And Average Velocity Class 9
- Differences Between Acceleration And Velocity
- Matter In Our Surroundings Class 9 Summary
- Class 9th Chapter 2 Science Question Answer of NCERT
- NCERT Physics Class 9 Chapter 1 Solutions PDF Download
Conclusion:
Changing states of matter illustrate the dynamic nature of substances and the impact of energy on their behavior. These transitions play a pivotal role in our understanding of various natural phenomena and technological applications, from the water cycle to industrial processes.
Frequently Asked Question (FAQs)
What are the different states of matter?
Matter exists in three primary states: solid, liquid, and gas. These states are characterized by the arrangement and movement of particles within the substance.
How do substances change from one state to another?
Substances change from one state to another by gaining or losing energy. This energy exchange, often in the form of heat, affects the motion and interactions of particles, causing them to transition between states.
What happens during melting and freezing?
Melting is the process in which a solid substance gains enough energy to overcome the forces holding its particles together, resulting in the transition to a liquid state. Freezing, on the other hand, occurs when a liquid loses enough energy to slow down its particles, leading to a transition to a solid state.
What is evaporation, and how does it differ from boiling?
Evaporation is the gradual conversion of liquid particles into gas due to the high-energy particles at the surface escaping the liquid’s attractive forces. Boiling is a rapid form of evaporation that occurs throughout the liquid and requires the liquid’s vapor pressure to match the atmospheric pressure.
Can gases change directly into solids or vice versa?
Yes, this phenomenon is known as sublimation and deposition. Sublimation involves a solid changing directly into a gas without passing through the liquid state. Deposition is the reverse process, where gas particles lose energy and transition directly to a solid.
Difference Between LCD And LED
Difference Between Lcd And Led. LCD (Liquid Crystal Display) and LED (Light Emitting Diode) are two common types of display technologies used in various electronic devices. While they both serve the purpose of displaying visual content, they have distinct differences in how they function and produce images.

Technology:
- LCD: An LCD screen utilizes liquid crystal molecules to control the passage of light through the display. These molecules can change orientation when an electric current is applied, allowing or blocking light to create images.
- LED: An LED screen uses an array of light-emitting diodes to produce light directly. Each diode emits its own light, contributing to the overall illumination of the display.
Light Source:
- LCD: LCD screens require an external light source, often referred to as a backlight, to illuminate the liquid crystal display and make the images visible.
- LED: LED screens do not require a separate backlight, as the individual LEDs themselves emit light. This leads to better control over brightness and contrast.
Energy Efficiency:
- LCD: LCD screens are generally less energy-efficient compared to LED screens because the backlight needs to be on continuously, even in dark areas of the image.
- LED: LED screens are more energy-efficient since each LED can be independently controlled. This allows for dimming or turning off specific areas of the screen, reducing power consumption.
Thinness and Flexibility:
- LCD: Traditional LCD screens tend to be thicker and less flexible due to the need for a separate backlight layer.
- LED: LED screens can be thinner and more flexible since the LEDs themselves emit light, eliminating the need for a backlight.
Picture Quality:
- LCD: LCD screens can experience issues like motion blur and limited viewing angles due to the response time of liquid crystals and the need for a backlight.
- LED: LED screens generally offer better picture quality with improved contrast, brightness, and color accuracy. They also have faster response times and wider viewing angles.
Cost:
- LCD: LCD screens are often more cost-effective to manufacture, which has contributed to their widespread use in various devices.
- LED: LED screens were initially more expensive to produce due to the advanced technology involved, but their cost has decreased over time as technology has improved.
Types of LED Displays:
- LCD: LCD displays can also use LED backlighting, known as LED-LCD displays. This combines the liquid crystal technology of LCD with LED backlighting for improved energy efficiency and picture quality.
- LED: LED displays can refer to screens that use individual LEDs to emit light, often seen in large outdoor displays or signage.
In conclusion, LCD and LED are two distinct display technologies, each with its own advantages and disadvantages.
LED technology has largely become dominant due to its superior energy efficiency, picture quality, and flexibility, but LCD screens are still prevalent and can be found in many devices, especially when combined with LED backlighting.
Read More
- Electric Potential of a Point Charge Class 10
- Difference Between In Physics
- Electric Charge And Static Electricity Class 10
- Class 6 Maths Question Paper With Solutions NCERT PDF
- Sample Question Paper For Class 6 CBSE English Grammar
Frequently Asked Questions (FAQs)
What is the main difference between LCD and LED displays?
The main difference lies in the way they produce light. LCD (Liquid Crystal Display) screens use liquid crystals to control light passage, while LED (Light Emitting Diode) screens use arrays of light-emitting diodes to emit light directly.
Do LED displays have better picture quality than LCD displays?
Yes, generally LED displays offer better picture quality. (Light Emitting Diode) displays often have better contrast, brightness, color accuracy, and faster response times compared to traditional LCD displays.
Why are LED displays considered more energy-efficient?
LED displays are more energy-efficient because they can independently control each LED pixel’s light output. This allows for dimming or turning off specific areas of the screen, reducing overall power consumption.
Are all LED displays completely self-illuminating?
No, not all LED displays are self-illuminating. LED-LCD displays use LED backlighting to illuminate the liquid crystal display, which combines LED technology with the traditional LCD structure.
Are LED displays thinner than LCD displays?
(Light Emitting Diode) displays can be thinner compared to traditional (Liquid Crystal Display) displays because they don’t require a separate backlight layer. However, the thickness can also depend on the specific design and purpose of the display.
Average Speed And Average Velocity
Average Speed And Average Velocity In the realm of physics, both average speed and average velocity are concepts used to describe motion. Despite their similarities, they have distinct meanings and implications.

Average Speed:
Average speed is a scalar quantity that measures the overall rate at which an object covers distance during its entire journey. It is calculated by dividing the total distance traveled by the total time taken. The formula for average speed is:
Average Speed=Total Distance Total Time
Average speed provides an overview of how fast an object moves on average, without considering its direction. For instance, if a car travels 200 kilometers in 4 hours, its average speed would be 50 kilometers per hour (km/h).
Average Velocity:
Average velocity is a vector quantity that combines both the magnitude (speed) and direction of an object’s displacement over a specific time interval. It is the change in position divided by the change in time. The formula for average velocity is:
Average Velocity=Displacement Time Interval
Unlike average speed, average velocity considers the displacement of an object from its initial position to its final position. If a person moves 50 meters eastward and then returns 30 meters westward in 10 seconds, their average velocity would be 50 m−30 m10 s=2 m/s to the east.
Key Differences:
- Scalar vs. Vector: Average speed is a scalar quantity that only involves magnitude, while average velocity is a vector quantity that incorporates both magnitude and direction.
- Consideration of Direction: Average speed does not consider direction, whereas average velocity takes both direction and magnitude into account.
- Example: Imagine a car moving in a circular path. If the car returns to its starting point after some time, its average speed over that time might be significant, but its average velocity will be zero because the displacement is zero.
In conclusion, average speed characterizes the overall rate of motion without considering direction, while average velocity provides a more comprehensive picture by including both the direction and magnitude of displacement.
Both concepts are vital tools for describing and understanding the complexities of motion in physics.
Vector Nature:
The vector nature of average velocity makes it a more comprehensive representation of motion. It conveys not only how far something has traveled but also the path it took to get there. This is crucial when you’re interested in the object’s overall movement pattern.
Constant Speed vs. Changing Speed:
Average speed doesn’t differentiate between segments of motion with varying speeds. For example, if a car travels at 30 km/h for half the journey and 60 km/h for the other half, its average speed is 45 km/h (total distance divided by total time).
On the other hand, average velocity accounts for changes in speed and direction, giving you a better understanding of how the object moves overall.
In essence, while both average speed and average velocity offer insights into motion, their considerations of direction and displacement set them apart. The choice of which to use depends on the specific context and what aspects of motion you’re seeking to understand.
Read More
- Differences Between Acceleration And Velocity
- Matter In Our Surroundings Class 9 Summary
- Class 9th Chapter 2 Science Question Answer of NCERT
- NCERT Physics Class 9 Chapter 1 Solutions PDF Download
- CBSE Previous Year Question Papers Class 9 Science
Frequently Asked Questions – FAQs
What is the difference between average speed and average velocity?
Average speed is a scalar quantity that represents the total distance traveled divided by the total time taken, without regard to direction. Average velocity is a vector quantity that accounts for both the displacement and direction of an object’s motion over a specific time interval.
Can average speed be greater than average velocity?
Yes, average speed can be greater than average velocity. This can occur when an object covers a considerable distance in a given time interval, but its overall displacement is relatively small or zero due to changes in direction.
Is the magnitude of average velocity always equal to average speed?
Not necessarily. While the magnitude of average velocity (absolute value) can be equal to average speed in cases where motion is unidirectional, it can differ when there are changes in direction during the motion.
What does a negative average velocity signify?
A negative average velocity indicates that the object has moved in the opposite direction of its initial reference point. It signifies that the displacement has occurred in the negative direction of the coordinate system.
How is average velocity calculated when the direction changes?
When the direction changes, you need to consider the total displacement, not just the total distance traveled. Calculate the difference between the initial and final positions, and then divide it by the total time taken.
Electric Potential of a Point Charge Class 10
Electric Potential of a Point Charge Electric potential, also known as voltage, is a fundamental concept in electromagnetism that describes the amount of electric potential energy carried by a charged particle due to its position relative to other charges.

Electric Potential of a Point Charge Class 10
When dealing with a point charge, which is a theoretically charged particle with infinitesimal size, we can calculate its electric potential at a certain distance from it.
The electric potential at a distance from a point charge can be calculated using the formula:
Where:
- is the electric potential at the given point (in volts, V).
- is Coulomb’s constant (8.988×109 N m2/C2 in SI units).
- is the magnitude of the point charge (in coulombs, C).
- is the distance from the point charge to the point where electric potential is being calculated (in meters, m).
This formula tells us that the electric potential at a point decreases as we move farther away from the point charge. It is important to note that electric potential is a scalar quantity, meaning it only has magnitude and no direction.
The unit of electric potential is the volt (V), which is equivalent to one joule per coulomb (1 J/C). This unit reflects the fact that electric potential energy is measured in joules and charge is measured in coulombs.
In summary, the electric potential of a point charge is a measure of the electric potential energy carried by the charge at a specific distance from it. It provides insight into the behavior of charged particles in electric fields and plays a crucial role in understanding various electrical phenomena.
Calculating Electric Potential from a Point Charge:
To understand how the formula for electric potential of a point charge works, let’s break it down further.
Consider a positive point charge located at the origin (0, 0, 0) in a three-dimensional Cartesian coordinate system. We want to find the electric potential at a point located at coordinates .
The electric potential at point due to the point charge is given by:
Where is the distance between point charge and point , given by:
![]()
Substituting this value of back into the electric potential formula, we get:
This formula shows that the electric potential at point is inversely proportional to the distance from the point charge . As the distance increases, the electric potential decreases.
Significance of Electric Potential:
The concept of electric potential is valuable in various practical applications and theoretical understandings:
- Work Done by Electric Forces: The electric potential energy of a charge in an electric field is related to the work done by the electric force when the charge is moved between two points. Specifically, the work done on a charge when moving it from point to point is given by , where and are the electric potentials at points and respectively.
- Equipotential Surfaces: Points that have the same electric potential form an equipotential surface. No work is required to move a charge along an equipotential surface since the potential remains constant. Electric field lines are always perpendicular to equipotential surfaces.
- Capacitors and Circuits: The concept of electric potential is essential in understanding how capacitors store electric energy and how circuits function. The potential difference across a capacitor determines the stored charge.
- Voltage Sources: In electronics, voltage sources such as batteries provide a potential difference that drives the movement of charges through a circuit, enabling the flow of electric current.
- Electrostatic Potential Energy: Electric potential energy is closely related to electric potential. The change in potential energy of a charge moving in an electric field is the product of its charge and the change in electric potential.
In essence, the electric potential of a point charge is a foundational concept in electromagnetism, serving as a key component in explaining and predicting the behavior of charges and electric fields in various contexts.
Relationship Between Electric Potential and Electric Field
The relationship between electric potential and electric field is a fundamental concept in the study of electromagnetism. Understanding this relationship helps explain how charges interact and how electrical phenomena occur.
Here’s an overview of the relationship between electric potential (V) and electric field (E):
The electric field and electric potential are related by the gradient (spatial derivative) of the electric potential. Mathematically, this relationship is expressed as: ,
where:
- is the electric field vector,
- is the gradient of the electric potential.
This equation indicates that the electric field points in the direction of the steepest decrease of the electric potential. In other words, charges tend to move from higher to lower electric potential regions.
Read More
- Difference Between In Physics
- Electric Charge And Static Electricity Class 10
- Class 6 Maths Question Paper With Solutions NCERT PDF
- Sample Question Paper For Class 6 CBSE English Grammar
- CBSE Class 8 Science Sample Paper Set 1 Solution
frequently asked questions (FAQ)
What is electric potential?
Electric potential, also known as voltage, is a scalar quantity that describes the electric potential energy per unit charge at a certain point in an electric field. It is a measure of the work done to move a positive test charge from infinity to that point, divided by the magnitude of the test charge.
What is a point charge?
A point charge is a hypothetical charged particle with no physical size, meaning its charge is concentrated at a single point. It serves as a simplified model to understand how electric charges interact in certain situations.
How is the electric potential of a point charge calculated?
The electric potential () at a distance () from a PC () is calculated using the formula: Where is Coulomb’s constant and is approximately 8.988×109 N m2/C2.
What is Coulomb’s constant?
Coulomb’s constant () is a proportionality constant that appears in Coulomb’s law, which describes the force between two point charges. It is used in various electrostatic calculations and is related to the permittivity of free space.
How does electric potential change with distance from a point charge?
Electric potential decreases as you move farther away from a point charge. This is because the electric potential energy decreases with increasing distance, and the potential is the potential energy per unit charge.
Difference Between In Physics: Comparisons Article
Difference Between In Physics Physics, the fundamental science that seeks to understand the natural world and its underlying principles, often involves comparing and contrasting various concepts to uncover their distinctions. Here are some notable differences between key concepts in physics:
Difference Between In Physics
Distance vs. Displacement:
- Distance refers to the total length of the path traveled by an object. It is a scalar quantity and is always positive.
- Displacement is the change in position of an object from its initial point to its final point, considering direction. It is a vector quantity and can be positive, negative, or zero.
Speed vs. Velocity:
- Speed is the rate of change of distance traveled by an object. It is a scalar quantity and only considers magnitude.
- Velocity is the rate of change of displacement. It is a vector quantity that includes both magnitude and direction.
Mass vs. Weight:
- Mass is the amount of matter in an object and remains constant regardless of location. It is measured in kilograms.
- Weight is the force exerted on an object due to gravity. It depends on both the mass of the object and the gravitational acceleration and is measured in newtons.
Accuracy vs. Precision:
- Accuracy refers to how close a measured value is to the true or accepted value.
- Precision relates to the consistency or reproducibility of repeated measurements. It indicates how closely measurements agree with each other.
Kinetic Energy vs. Potential Energy:
- Kinetic Energy is the energy an object possesses due to its motion. It depends on the mass and velocity of the object.
- Potential Energy is the energy stored in an object due to its position or state. It includes gravitational potential energy, elastic potential energy, etc.
Conduction vs. Convection vs. Radiation:
- Conduction is the transfer of heat through direct contact between particles of a substance.
- Convection is the transfer of heat through the movement of fluids (liquids or gases).
- Radiation is the transfer of heat through electromagnetic waves, such as infrared radiation.
AC (Alternating Current) vs. DC (Direct Current):
- AC is an electric current that periodically reverses direction. It is commonly used in household electricity and power transmission.
- DC is an electric current that flows in one direction. It is often used in batteries and electronic devices.
Wave vs. Particle Duality:
- The wave-particle duality concept in quantum phy states that particles like electrons and photons can exhibit both wave-like and particle-like behavior, depending on the experimental setup.
Understanding these differences is crucial for grasping the nuances of various physical phenomena and their applications in the real world.
Difference between Physics and Applied Physics
Phy and applied physics are related fields, but they differ in their focus, scope, and practical applications. Here’s a breakdown of the distinctions between these two disciplines:
1. Nature of Study:
- Physics: Physics is the fundamental science that seeks to understand the fundamental laws and principles governing the universe. It aims to uncover the underlying theoretical concepts and phenomena.
- Applied Physics: Applied physics, on the other hand, takes the principles and theories of phy and applies them to practical real-world problems and technological advancements.
2. Focus:
- Physics: Physics focuses on studying the fundamental aspects of nature, such as the behavior of matter, forces, energy, and the fundamental particles that make up the universe.
- Applied Physics: Applied physics focuses on using the insights gained from physics to solve specific technological challenges and develop new technologies.
3. Research and Theory:
- Physics: Physics research often delves into theoretical concepts, mathematical models, and fundamental discoveries. It seeks to expand our understanding of the universe’s basic nature.
- Applied Physics: Applied physics research aims to bridge the gap between theory and practical applications. It involves using physics concepts to develop new devices, materials, and technologies.
4. Practical Applications:
- Physics: While physics has led to numerous technological advancements, its primary focus is not necessarily on immediate practical applications but rather on understanding the fundamental laws of nature.
- Applied Physics: Applied physics directly contributes to the development of technologies that have tangible applications, such as lasers, semiconductors, medical imaging devices, and renewable energy technologies.
5. Career Paths:
- Physics: Physics graduates often pursue careers in academia, research institutions, or theoretical research, contributing to the advancement of knowledge.
- Applied Physics: Graduates in applied physics typically find employment in industries such as engineering, electronics, optics, materials science, and other technology-driven sectors.
Advantages of Differences & Comparisons Articles in Physics:
Questions involving distinctions and comparisons are frequently encountered in most physics examinations. Presenting these variances and parallels in a tabular format significantly enhances comprehension, facilitating more effective learning for students.
These articles play a pivotal role in providing students with a comprehensive grasp of the respective subjects. The tabular arrangement provided herein also offers students a more adept way to retain key points, promoting enhanced and efficient understanding.
Read More
- Electric Charge And Static Electricity Class 10
- Class 6 Maths Question Paper With Solutions NCERT PDF
- Sample Question Paper For Class 6 CBSE English Grammar
- CBSE Class 8 Science Sample Paper Set 1 Solution
Frequently Asked Questions (FAQs)
What is the difference between distance and displacement in phy ?
- Distance is the total length of the path traveled by an object, measured in scalar units (e.g., meters). It is always positive and doesn’t consider direction.
- Displacement is the change in position of an object from its initial point to its final point, including direction. It is a vector quantity and can be positive, negative, or zero.
How do speed and velocity differ?
Speed is the rate of change of distance and is a scalar quantity, only indicating magnitude.
Velocity is the rate of change of displacement and is a vector quantity, considering both magnitude and direction.
What distinguishes mass from weight in physics?
Mass is the amount of matter in an object and remains constant regardless of location. It is measured in kilograms (kg).
Weight is the force exerted on an object due to gravity and varies with location. It is measured in newtons (N) and depends on both mass and gravitational acceleration.
What is the difference between accuracy and precision?
Accuracy relates to how close a measured value is to the true or accepted value.
Precision refers to the consistency of repeated measurements and how closely they agree with each other.
How do kinetic energy and potential energy differ?
Kinetic Energy is the energy an object possesses due to its motion and depends on mass and velocity.
Potential Energy is the energy stored in an object due to its position or state, including gravitational potential energy and elastic potential energy.
Electric Charge And Static Electricity Class 10
Electric Charge And Static Electricity: Certainly, I’d be happy to provide a more detailed explanation of electric charge and static electricity.

Electric Charge And Static Electricity
Definition of Electric Charge:
The Electric charge is a fundamental property of subatomic particles, such as electrons and protons. It is a property that gives rise to electromagnetic interactions between particles. There are two types of electric charge:
Positive Charge:
This is the type of charge that protons possess. Protons have a positive electric charge.
Negative Charge:
This is the type of charge that electrons possess. Electrons have a negative electric charge.
Electric charges interact with each other through electromagnetic forces. Like charges repel each other (positive repels positive or negative repels negative), and opposite charges attract each other (positive attracts negative).
Static Electricity:
Static electricity is a phenomenon that occurs when there is an imbalance of electric charge on the surface of an object.
This imbalance can happen due to various processes, including friction, contact, and induction. Here’s how it works:
What is Friction in Electric Charge And Static Electricity:
When two materials rub against each other, electrons can be transferred from one material to the other.
The material that gains electrons becomes negatively charged, and the material that loses electrons becomes positively charged.
For example, when you rub a balloon against your hair, electrons from your hair are transferred to the balloon, leaving your hair with a positive charge and the balloon with a negative charge.
Contact:
When two objects with different charges come into contact, electrons can move from one object to the other until their charges become more balanced.
This can result in one object becoming positively charged and the other becoming negatively charged.
Induction:
Induction involves the redistribution of charges within an object without direct contact with another charged object.
When a charged object is brought near a neutral object, the charges within the neutral object can shift, causing one side to become more positively charged and the other side more negatively charged.
Static electricity is more noticeable in certain conditions, such as when the air is dry, because dry air is a poor conductor of electricity. This means that electric charges do not easily flow through the air, allowing them to accumulate on the surface of objects.
Common examples of Electric Charge And Static Electricity include:
- Getting a shock when you touch a doorknob after walking across a carpet.
- Seeing your hair stand on end when you rub a balloon against it.
- Observing sparks when you touch a metal object after shuffling your feet on a rug.
Static electricity has various applications and implications, from industrial processes to everyday phenomena like lightning during storms.
It’s important to manage static electricity in certain contexts to prevent hazards, such as the ignition of flammable materials.
Read more.
- CBSE Class 10 Basic Maths Question Paper 2020 With Solutions
- CBSE Social Science Class 10 Question Paper 2020 With Answers
- 10th Class All Subject Books Hindi Medium NCERT PDF Download
- Class 10 All Subjects Books NCERT PDF Download in English
Frequency Question FAQ Electric Charge And Static Electricity
What is electric charge?
Electric charge is a fundamental property of subatomic particles, such as electrons and protons. It is responsible for electromagnetic interactions between particles and is categorized as positive or negative.
What are positive and negative charges?
Positive charges are associated with protons, while negative charges are associated with electrons. Like charges repel each other, and opposite charges attract each other.
What is static electricity in Electric Charge And Static Electricity?
Static electricity refers to the accumulation of electric charge on the surface of an object due to processes like friction, contact, or induction. It is characterized by an imbalance of charges, resulting in attractive or repulsive forces.
How does static electricity occur?
Static electricity can occur through processes like friction, where materials rub against each other and exchange electrons. Contact between objects with different charges can also lead to static buildup. Induction involves redistributing charges within an object without direct contact.
Why is static electricity more noticeable in dry conditions?
Dry air is a poor conductor of electricity, so electric charges do not easily dissipate into the environment. This allows charges to accumulate on the surfaces of objects, leading to a greater buildup of static electricity.
Differences Between Acceleration And Velocity
Differences Between Acceleration And Velocity: Physics is a field that describes and explains the fundamental principles governing the behavior of the universe.

In the realm of mechanics, two key concepts are often discussed: acceleration and velocity. While they both relate to the motion of objects, they represent distinct aspects of an object’s movement and carry different meanings and implications.
This article aims to elucidate the differences between acceleration and velocity, highlighting their definitions, units, calculations, and real-world applications.
Differences Between Acceleration And Velocity
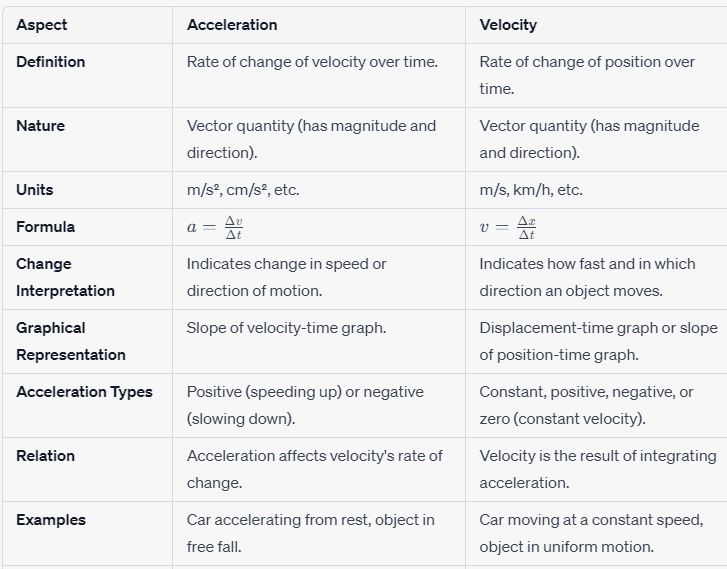
1. Definition and Nature:
Velocity refers to the rate of change of an object’s position concerning time. It is a vector quantity, which means it has both magnitude (numerical value) and direction. In simple terms, velocity informs us how fast an object is moving and in which direction it is headed.
Acceleration, on the other hand, signifies the rate of change of an object’s velocity concerning time. It is also a vector quantity, encompassing magnitude and direction. Acceleration portrays the change in an object’s speed or the alteration in its direction of motion.
2. Units:
Velocity is typically measured in units of distance divided by units of time, such as meters per second (m/s), kilometers per hour (km/h), or even feet per second (ft/s) depending on the context.
Acceleration is measured in units of change in velocity divided by units of time. Common units include meters per second squared (m/s²) and centimeters per second squared (cm/s²).
3. Calculations:
To calculate velocity, you can use the following formula:
Velocity(v) = Change in position(Δx)/Change in time(Δt)
For acceleration, the formula is:
Acceleration(a)=Change in velocity(Δv)/Change in time(Δt)
4. Relationship:
Velocity and acceleration are related in the sense that acceleration affects the change in velocity. When an object undergoes a constant acceleration, its velocity changes uniformly over time. If an object is accelerating in the same direction as its initial velocity, it speeds up. If it’s accelerating in the opposite direction, it slows down.
5. Real-World Examples:
Imagine a car accelerating from a standstill at a traffic light. Initially, the car’s velocity is zero because it’s not moving. As the car accelerates, its velocity increases in the direction of motion. This increase in velocity signifies positive acceleration.
Now, consider a car braking to come to a stop. As the driver presses the brakes, the car’s velocity decreases until it comes to a complete halt. This decrease in velocity denotes negative acceleration, often referred to as deceleration.
6. Graphical Representation:
Velocity and acceleration can be graphically represented over time. A velocity-time graph would show a slope corresponding to the acceleration. A constant slope indicates constant acceleration, while a changing slope signifies changing acceleration.
An acceleration-time graph would depict the rate of change of velocity over time. The slope of this graph represents the actual acceleration.
Newton’s Second Law of Motion
Newton’s Second Law of Motion is a fundamental principle in classical mechanics that establishes the relationship between the net force acting on an object, its mass, and the resulting acceleration. It states that the accel. of an object is directly proportional to the net force applied to it and inversely proportional to its mass. Mathematically, this law is expressed as:
Where:
- represents the net force applied to the object.
- is the mass of the object.
- signifies the resulting acceleration.
This law highlights that when an external force is applied to an object, it causes the object to accelerate in the direction of the force. Additionally, the greater the force applied, the greater the resulting acceleration, provided the mass remains constant. Conversely, if the mass increases while the force remains constant, the acceleration decreases.
Newton’s Second Law also encapsulates the concept of inertia, which is an object’s resistance to changes in its state of motion.
Objects with larger masses exhibit greater inertia, requiring more force to produce the same acceleration as objects with smaller masses.
This law provides a foundational framework for understanding and predicting the behavior of objects in motion under the influence of external forces.
Conclusion:
In the realm of physics and mechanics, the concepts of velocity and acceleration play crucial roles in describing and understanding the motion of objects.
While velocity reveals the speed and direction of an object’s movement, acceleration reveals how that velocity is changing over time.
These distinctions are vital for comprehending the complexities of motion and form the foundation for various scientific and technological advancements.
Whether you’re observing a rocket launch, calculating the motion of planets, or designing safer vehicles, a clear grasp of the differences between acce. and velocity is essential.
Read Also
- Matter In Our Surroundings Class 9 Summary
- Class 9th Chapter 2 Science Question Answer of NCERT
- NCERT Physics Class 9 Chapter 1 Solutions PDF Download
- CBSE Previous Year Question Papers Class 9 Science
Frequently Asked Question – FAQs on Differences Between Acceleration And Velocity
What is the fundamental difference between acceleration and velocity?
The fundamental difference lies in their definitions. Velocity is the rate of change of an object’s position concerning time and includes both speed and direction. Acceleration is the rate of change of an object’s vel. over time, indicating how much an object’s speed or direction of motion is changing.
Are velocity and acceleration scalar or vector quantities?
Both velocity and acceleration are vector quantities. This means they have both magnitude (numerical value) and direction.
How are velocity and acceleration units measured?
Velocity is measured in units of distance divided by units of time, such as meters per second (m/s) or kilometers per hour (km/h). Acceler. is measured in units of change in vel. divided by units of time, such as meters per second squared (m/s²).
Can an object have constant velocity and still be accelerating?
No, constant velocity implies that an object is moving at a steady speed in a straight line without changing direction. Acce. indicates a change in velocity, either in terms of speed or direction.
What does positive acceleration mean?
Positive acceleration occurs when an object’s vel. is increasing over time. It could mean the object is speeding up in the same direction as its initial motion or slowing down in the opposite direction.
Compound Lenses Thin Lenses In Contact
Compound Lenses Thin Lenses In Contact: In a basic lens system, a solitary lens is employed, in contrast to the compound lens system where the utilization of multiple lenses aligned along a shared axis is possible. The most straightforward form of such a compound lens setup involves the alignment of two thin lenses in direct contact. This article aims to delve into the realm of compound lenses, examining their distinctive properties.

The frequently utilized types of lenses encompass:
1. Convex Lenses (Converging Lenses)
2. Concave Lenses (Diverging Lenses)
As you may recall, we have extensively covered the characteristics of convex and concave lenses in previous sessions. Now, our focus shifts towards comprehending the intricacies of compound lenses.
Compound Lenses
The illustration below demonstrates a compound lens arrangement. Compound lenses are characterized by the presence of two thin lenses positioned on a shared axis, often in close proximity or bonded together.
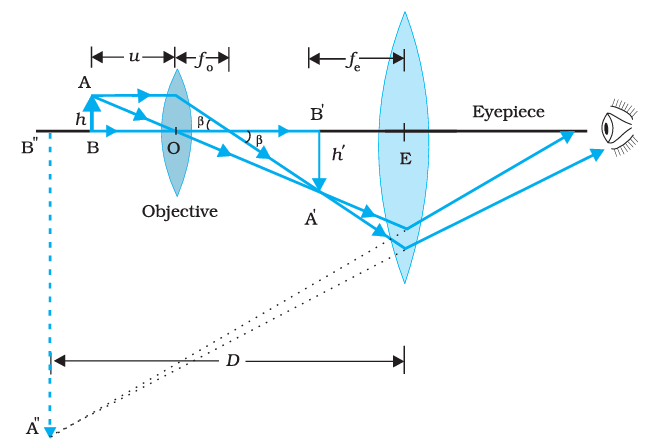
Compound Lens Configuration
In cases where two thin lenses sharing an axis are positioned in contact with each other, the common focal length for the system can be determined using the following formula:
Where:
- is the combined focal length
- is the focal length of the first lens
- is the focal length of the second lens
Given that power is the reciprocal of focal length, a noticeable observation arises in this scenario. For thin lenses in contact, it becomes apparent that the combined power of the system results from the summation of the powers of the individual lenses.
However, if the lenses are not in direct contact but separated by a distance , the combined focal length can be computed using the subsequent formula:
Now, let’s put your understanding to the test with a simple problem: A bi-convex lens with a focal length of 10 cm and a bi-concave lens with a focal length of 20 cm are positioned in contact. Both lenses share the same refractive index. Calculate the combined focal length.
When delving into the realm of lens combinations, you may encounter the following terminology.
A Focal System
When the separation distance (d) between the two lenses equals the sum of their individual focal lengths (f1 + f2), the resulting combined focal length becomes infinite. This configuration causes the refracted light rays to become parallel to one another.
Achromatic Doublet
The arrangement of the lenses is designed to align such that two specific wavelengths, usually the extremes of red and violet light, converge to a focused point. This utilization represents an instance of employing a compound lens system to rectify chromatic aberrations, a phenomenon that can occur with single lenses.
Application of Compound Lenses
Compounded lenses are employed in telescopes and microscopes, combining two or more lenses to achieve the following objectives:
1. Minimize aberrations and flaws resulting from single lens usage.
2. Obtain an upright image of the object.
3. Enhance image magnification.
I trust you now have a clear comprehension of compound lenses.
The Purpose and Benefits
The application of compound lenses, particularly with thin lenses in contact, is multifaceted and impactful. Let’s explore the primary reasons behind their adoption:
- Aberration Correction: Single lenses often suffer from optical aberrations, leading to distortion, blurring, and color fringing in images. Compound lenses tackle this issue by carefully aligning and combining thin lenses. This alignment aids in reducing aberrations, resulting in clearer, sharper images.
- Chromatic Aberration Mitigation: The dispersion of light into different colors, known as chromatic aberration, is a challenge that can affect the quality of images produced by single lenses. Compound lenses help alleviate this problem by ensuring that light of varying colors converges more effectively onto a single focal point.
- Erect Image Formation: Erecting images – producing images that are not inverted – is crucial in various optical devices, such as microscopes and telescopes. Compound lenses, when configured correctly, can generate upright images, enhancing the usability of such devices.
- Magnification Enhancement: The integration of thin lenses in contact can lead to increased magnification capabilities, which is vital for applications requiring close examination of intricate details.
Read More
Frequently Asked Questions – FAQs
What are compound lenses composed of?
Compound lenses are made up of two or more thin lenses positioned in contact with each other along a common axis.
What is the purpose of combining thin lenses in contact?
Combining thin lenses in contact helps to correct optical aberrations and improve the quality of images formed.
What advantage do compound lenses offer over single lenses?
Compound lenses help to correct chromatic aberrations that can occur with single lenses, leading to improved image quality.
Where are compound lenses commonly used?
Compound lenses are frequently used in telescopes and microscopes to achieve various optical benefits, including increased magnification and reduced defects.
Can compound lenses be used for erect image formation?
Yes, by properly arranging the thin lenses, compound lens systems can produce an erect image of an object.
What is the significance of correcting chromatic aberrations?
Correcting chromatic aberrations ensures that different colors of light converge to the same focal point, resulting in sharper and clearer images.
Matter In Our Surroundings Class 9 Summary

Matter In Our Surroundings Class 9 Summary: Matter is all around us; it’s the substance that occupies space and has mass. In the realm of science, understanding matter and its various properties is crucial.
The study of matter begins with the class of substances known as “Matter in Our Surroundings.” This topic, covered in Class 9 of the CBSE curriculum (and equivalent standards), provides a fundamental understanding of the nature and behavior of matter in different states.
“Matter in Our Surroundings” is a chapter from the Class 9 Science curriculum that focuses on introducing students to the concept of matter, its various states, and the properties associated with these states. Here’s a summary of the key points covered in this chapter:
Matter In Our Surroundings Class 9 Summary
Following topics are covered in class 9 chapter 1 Matter and Surroundings
States of Matter:
-
- Introduction to the three primary states of matter: solid, liquid, and gas.
- Explanation of how the arrangement and movement of particles differ in each state.
Kinetic Theory of Matter:
-
-
- Definition and explanation of the Kinetic Theory of Matter.
- Understanding that particles are in constant motion, and their kinetic energy increases with temperature.
- Relationship between temperature and the state of matter.
-
Change of State:
- Explanation of various processes that involve changing the state of matter:
- Melting: Solid to liquid.
- Freezing: Liquid to solid.
- Evaporation: Liquid to gas.
- Condensation: Gas to liquid.
- Sublimation: Solid to gas.
- Deposition: Gas to solid.
- Description of how energy is gained or lost during these state changes.
Effect of Pressure and Temperature:
- Discussion of the influence of pressure and temperature on the states of matter.
- Explanation of how changes in pressure and temperature can lead to changes in state.
Diffusion:
- Definition and explanation of diffusion as the movement of particles from higher to lower concentration.
- Factors affecting the rate of diffusion, including temperature, particle size, and intermolecular forces.
Brownian Motion:
- Introduction to Brownian motion as the random movement of microscopic particles suspended in a fluid.
- Significance of Brownian motion in demonstrating the kinetic behavior of particles.
Importance in Daily Life:
- Illustration of the practical applications of understanding matter and its behavior in everyday situations.
- Examples of how knowledge of matter’s properties and states contributes to various fields and activities.
Matter In Our Surroundings Class 9 Summary
States of Matter:
Matter exists in three primary states: solid, liquid, and gas. These states are defined by the arrangement and movement of particles within a substance.
In solids, particles are tightly packed and have a fixed shape and volume. Liquids have particles that are close together but can move past each other, allowing them to take the shape of their container.
Gases have particles that are widely spaced and move freely, leading to indefinite shape and volume.
Kinetic Theory of Matter:
The Kinetic Theory of Matter explains the behavior of particles in different states. It posits that particles are in constant motion, with their kinetic energy increasing as temperature rises.
When heated, particles gain energy and move more vigorously, leading to changes in state. Cooling reduces particle movement, resulting in a state change as well.
Change of State:
Substances can change from one state to another through processes like melting, freezing, evaporation, condensation, sublimation, and deposition.
Melting is the transition from solid to liquid, while freezing is the reverse. Evaporation involves the conversion from liquid to gas, and condensation is the opposite process.
Sublimation occurs when a solid changes directly into a gas, bypassing the liquid state, and deposition is the reverse of sublimation.
Effect of Pressure and Temperature: Matter In Our Surroundings Class 9 Summary
Both pressure and temperature have a significant impact on the states of matter. Increasing pressure can force gases to condense into liquids and liquids to freeze into solids.
On the other hand, higher temperatures can cause solids to melt into liquids and liquids to evaporate into gases. This relationship is best described by phase diagrams, which show the conditions at which a substance exists in a specific state.
Diffusion:
Diffusion is the process of particles moving from regions of higher concentration to lower concentration until a uniform distribution is achieved.
It is a common phenomenon in gases and liquids, driven by the random motion of particles. Factors such as temperature, particle size, and intermolecular forces influence the rate of diffusion.
Brownian Motion:
Brownian motion is the erratic, random movement of microscopic particles suspended in a fluid (liquid or gas).
It was first observed by Robert Brown and serves as direct evidence of the kinetic behavior of particles in fluids.
Importance in Daily Life:
Understanding matter and its behavior has practical applications in our daily lives. From cooking to transportation, from weather changes to medical advancements, knowledge of the properties of matter and its various states helps us comprehend and manipulate the world around us.
Read Also
- Class 9th Chapter 2 Science Question Answer of NCERT
- NCERT Physics Class 9 Chapter 1 Solutions PDF Download
- CBSE Previous Year Question Papers Class 9 Science
- NCERT Science Book Class 9 Solutions Pdf Free Download
Frequently Asked Questions – FAQs on Matter In Our Surroundings Class 9 Summary
What is the main focus of the topic “Matter in Our Surroundings” in Class 9?
The main focus of this topic is to introduce students to the concept of matter, its various states (solid, liquid, gas), the kinetic theory explaining the behavior of particles in these states, and the processes of changing states due to factors like temperature and pressure.
What are the three primary states of matter?
The three primary states of matter are solid, liquid, and gas. These states are defined by the arrangement and movement of particles within a substance.
What does the Kinetic Theory of Matter explain?
The Kinetic Theory of Matter explains that particles in all states of matter are in constant motion. It states that as temperature increases, the kinetic energy of particles also increases, leading to changes in state.
What are the processes involved in changing the state of matter?
The processes of changing the state of matter are:
-
- Melting: Solid to liquid.
- Freezing: Liquid to solid.
- Evaporation: Liquid to gas.
- Condensation: Gas to liquid.
- Sublimation: Solid to gas.
- Deposition: Gas to solid.
How does pressure affect the states of matter?
Increasing pressure can cause gases to condense into liquids and liquids to freeze into solids. Decreasing pressure can lead to the opposite changes in state.
