Blog
Economics Class 10 Chapter 1 NCERT Solutions
Welcome to our comprehensive guide on “Economics Class 10 Chapter 1.” If you are a student gearing up to ace your economics studies or an eager learner seeking a deeper understanding of fundamental economic concepts, you’ve come to the right place.
In this article, we delve into the intricacies of Class 10 Economics Chapter 1, unlocking its valuable insights and practical applications. Economics plays a pivotal role in shaping the world around us, influencing decision-making, resource allocation, and overall societal well-being.

Join us as we demystify economic concepts, equip you with valuable knowledge, and empower you to make informed decisions in both academic and real-world scenarios. Let’s embark on this rewarding exploration of Economics Class 10 Chapter 1!
NCERT Economics Class 10 Chapter 1 Solution Explained
1. Development of a country can generally be determined by
- its per capita income
- its average literacy level
- health status of its people
- all of the above
Answer: d. all of the above
2. Which of the following neighbouring countries has better performance in terms of human development than India?
- Bangladesh
- Sri Lanka
- Nepal
- Pakistan
Answer: b. Sri Lanka
3. Assume there are four families in a country. The average per capita income of these families is Rs. 5000. If the income of three families is Rs. 4,000, Rs. 7,000 and Rs. 3,000, respectively, what is the income of the fourth family?
- Rs. 7,500
- Rs. 3,000
- Rs. 2,000
- Rs. 6,000
Answer: d. Rs. 6,000
(4000+7000+3000+x) ÷ 4 = 5000
14000+x = 5000 × 4
x = 20000-14000
x = 6000
4. What is the main criterion used by the World Bank in classifying different countries? What are the limitations of this criterion, if any?
The World Bank employs per capita income as a classification method for various countries. Per capita income is derived by dividing the total income of a country by its population. According to the World Bank, countries with a per capita income of US$955 or below for the year 2017 are classified as low-income countries.
However, this criterion has certain limitations:
1. Ignoring Key Factors: The classification solely relies on per capita income and overlooks critical indicators such as literacy rate, infant mortality rate, and healthcare facilities, which are crucial for assessing a country’s overall development and well-being.
2. Neglecting Income Inequality: The World Bank’s classification fails to consider information regarding the unequal distribution of income within a country. Inequality can significantly impact the socio-economic conditions of its citizens, influencing overall development.
3. Limited Scope: The per capita income criterion does not encompass the broader complexities of an economy and may not fully reflect a country’s development status. Economic development is influenced by numerous interconnected factors that extend beyond income levels alone.
To gain a comprehensive understanding of a country’s development, it is essential to consider a more inclusive approach that takes into account multiple indicators and factors affecting the well-being of its population.
5. In what respects is the criterion used by the UNDP for measuring development different from the one used by the World Bank?
The UNDP (United Nations Development Programme) employs a distinct criterion compared to the World Bank for country comparisons. Unlike the World Bank, which primarily uses per capita income to measure development, the UNDP takes a more comprehensive approach.
UNDP’s criterion considers three key indicators to assess development:
1. Educational Level: The educational level of the people within a country is a crucial factor in determining development. Education empowers individuals, enhances their skills, and contributes to overall human development.
2. Health Status: The health status of a population is another vital aspect of development. Factors such as life expectancy, infant mortality rate, and access to healthcare services play a significant role in assessing a country’s well-being.
3. Per Capita Income: While UNDP considers per capita income like the World Bank, it also integrates it with education and health indicators for a more holistic evaluation of development.
This approach by UNDP offers a more comprehensive and nuanced perspective on development, taking into account multiple dimensions that impact the quality of life and well-being of a country’s citizens. By considering education, health, and income together, UNDP’s criterion provides a more inclusive understanding of a country’s development status.
6. Why do we use averages? Are there any limitations to their use? Illustrate with your own examples related to development.
Calculating averages is a useful method for estimating and comparing different aspects across countries with varying populations. However, there are limitations to relying solely on averages, as they may not provide a complete picture of the income disparities within a country or state.
Let’s illustrate this with an example of two countries, A and B, each having 5 people:
In Country A, the incomes of the five individuals are Rs. 23,000, Rs. 22,000, Rs. 23,500, Rs. 28,000, and Rs. 25,000. The average income of Country A would be Rs. 24,300.
In Country B, the incomes of the five individuals are Rs. 1,50,000, Rs. 22,000, Rs. 50,000, Rs. 4,000, and Rs. 2,500. The average income of Country B would be Rs. 45,700.
While the average income of Country B appears higher than that of Country A, it doesn’t account for the significant disparity in income distribution within each country. In Country B, there seems to be a wide variation in incomes, with some individuals earning substantially higher than others. Conversely, Country A appears to have a more evenly distributed income among its population.
This example highlights that relying solely on average income figures may overlook the inequalities in income distribution within countries. To obtain a comprehensive understanding of economic conditions, it is crucial to consider additional factors, such as income distribution and the overall well-being of the population, to gain a more accurate assessment of a country’s economic situation.
7. Kerala, with lower per capita income, has a better human development ranking than Haryana. Hence, per capita income is not a useful criterion at all and should not be used to compare states. Do you agree? Discuss.
Despite having a lower per capita income, Kerala outperforms Haryana in terms of human development ranking. This indicates that per capita income alone is not a comprehensive criterion for comparing states. Kerala’s superior human development ranking can be attributed to factors like higher literacy rates, lower infant mortality rates, and better healthcare facilities compared to Haryana.
Per capita income merely calculates the average income of a state’s population without considering other crucial factors that contribute to overall development. In the case of Kerala and Haryana, the focus on per capita income alone fails to reflect the significant disparities in social indicators and human well-being.
To make accurate and meaningful comparisons between states, it is essential to consider a holistic set of indicators that encompass economic, social, and human development aspects. These include factors such as literacy rates, access to quality healthcare, education facilities, and infant mortality rates, which provide a more comprehensive picture of a state’s overall development and well-being.
8. Find out the present sources of energy that are used by the people in India. What could be the other possibilities fifty years from now?
Currently, people in India primarily rely on sources of energy such as firewood, coal, petroleum, crude oil, and natural gas. However, looking ahead to the next fifty years, there is significant potential for utilizing renewable sources like solar energy and wind energy to fulfill our diverse energy needs. Embracing these sustainable alternatives is crucial as the continued reliance on conventional energy sources could deplete precious natural resources, leaving future generations at a disadvantage. By transitioning towards renewable energy, we can ensure a greener and more sustainable future for all, preserving our planet’s resources for the well-being of generations to come.
9. Why is the issue of sustainability important for development?
Sustainable development is the responsible utilization of natural resources to meet the needs of both present and future generations. The concept of sustainability holds great significance for overall development because the careful management of natural resources ensures their availability for the well-being of future generations. Failing to use resources judiciously may lead to their depletion, hindering the progress and development of a country. Therefore, embracing sustainable practices is essential to ensure a harmonious balance between development and preservation, securing a prosperous and thriving future for all.
10. “The Earth has enough resources to meet the needs of all but not enough to satisfy the greed of even one person.” How is this statement relevant to the discussion of development? Discuss.
Development relies not solely on a country’s economic factors but also on the availability of resources for its people. The saying, “The Earth has enough resources to meet the needs of all but not enough to satisfy the greed of even one person,” is highly pertinent to a country’s development as natural resources are finite. Responsible utilization of these resources is crucial to meet present needs without compromising the needs of future generations. It is incumbent upon society to use resources wisely, prioritizing needs over greed. Failure to do so could lead to the exhaustion of resources, ultimately hampering a country’s development. Embracing sustainable practices ensures a prosperous future for all.
11. List a few examples of environmental degradation that you may have observed around you.
Numerous instances of environmental degradation are evident in our surroundings, including:
1. Vehicle emissions and excessive fuel consumption leading to pollution
2. Improper disposal of industrial waste in residential areas and water bodies
3. Deforestation activities depleting forest cover
4. Mining operations affecting natural landscapes
5. Soil erosion impacting fertile land
6. Escalating pollution contributing to global warming, glacial melting, and deteriorating atmospheric conditions.
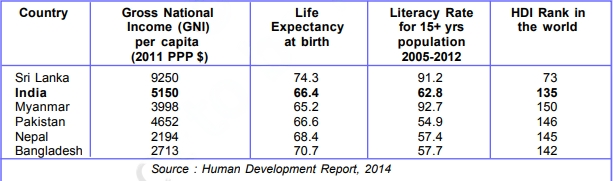
12. For each of the items given in Table 1.6, find out which country is at the top and which is at the bottom.
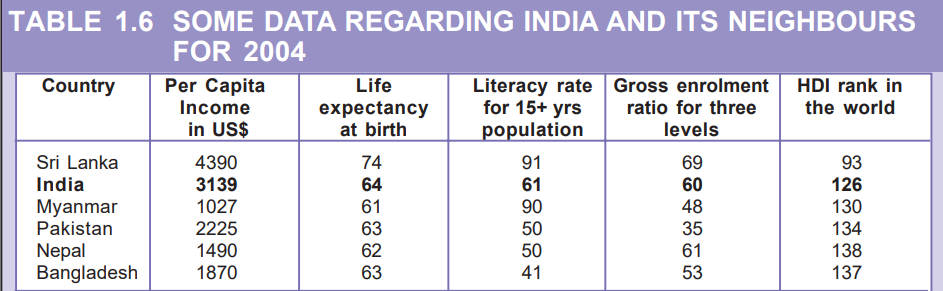
According to Table 1.6, Sri Lanka secures the top position in all four categories, namely Gross National Income, Life Expectancy at birth, mean years of schooling for individuals aged 25 and above, and HDI rank worldwide. On the other hand, Nepal holds the lowest Gross National Income among the listed countries. Pakistan has the least Life Expectancy at birth and ranks the lowest in HDI rank among the given countries. Finally, Myanmar and Nepal have the lowest mean years of schooling for individuals aged 25 and above.
13. The following table shows the proportion of adults (aged 15-49 years) whose BMI is below normal (BMI <18.5 kg/m2) in India. It is based on a survey of various states for the year 2015-16. Look at the table and answer the following questions.
- Compare the nutritional level of people in Kerala and Madhya Pradesh.
- Can you guess why around 40 per cent of people in the country are undernourished even though it is argued that there is enough food in the country? Describe in your own words.
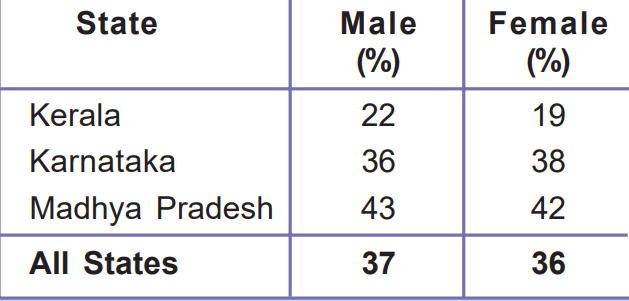
Ans 1. The nutritional level of people in Kerala surpasses that of people in Madhya Pradesh.
Ans 2. The argument supporting the presence of enough food in the country is based on the following reasons:
1. Disparity in PDS Distribution: The Public Distribution System (PDS) faces disparities in the distribution of food grains, leading to unequal access to food among the population.
2. Affordability of Nutritious Food: The poor population in the country may struggle to afford nutritious food, which impacts their overall food security.
3. Educational Backwardness and Unemployment: Educational backwardness and unemployment can lead to financial constraints, making it difficult for people to afford basic necessities like food.
4. Inadequate Ration Distribution: There are issues with the proper distribution of ration at fixed-price stores, hindering access to food for certain sections of the population.
Summery of Economics Class 10 Chapter 1
The summary of Economics Class 10 Chapter 1 provides an insight into the fundamental concepts of development and the importance of resources in a country’s progress. The chapter discusses various criteria used to measure development, such as per capita income, literacy rate, and healthcare facilities. It highlights the limitations of using only per capita income as a measure of development and emphasizes the significance of considering other socio-economic factors. The chapter also explores the concept of sustainable development, which involves using natural resources responsibly for the well-being of present and future generations. It emphasizes the need to strike a balance between economic growth and environmental preservation. Additionally, the chapter touches upon the impact of environmental degradation on the planet, emphasizing the urgency to adopt sustainable practices. Overall, this chapter serves as a foundational understanding of development and its broader implications for society and the environment.
Students will Get In Economics class 10 chapter 1:
1. Diverse development goals: The varied aspirations people have for development.
2. Purchasing power parity (PPP) concept: Understanding the comparative value of currencies.
3. Perspectives on development: Examining different viewpoints on progress.
4. Common indicators and measurement methods: Assessing development through various criteria.
5. Income and other aspirations: Identifying factors like salary and job security as development goals.
6. National development: Analyzing a nation’s ability to enhance its citizens’ living standards, considering per capita income, Gross Domestic Product (GDP), literacy rate, etc.
7. Comparison of countries or states based on per capita income due to population variations.
8. Calculation of per capita income and other criteria.
9. Access to public facilities: Understanding the availability of essential services.
10. Emphasis on sustainable development: Focusing on responsible resource use for future generations.
“Exploring Economic Development” is a crucial component of Class 10 SST Economics. For comprehensive solutions to the entire NCERT Class 10 Social Science syllabus, refer to the provided link.
Read Also:
Frequently Asked Question on Economics Class 10 Chapter 1
Q 1. What is the name of the book of Economics class 10?
Q 2. How many chapters are there in Economics Class 10 CBSE?
Five.
Q 3. How to pass in Economics?
Resources and Development Class 10 Notes of NCERT Geo. Ch. 1
Welcome to our exclusive and comprehensive guide on “Resources and Development Class 10 Notes.” If you’re a Class 10 student looking to excel in your geography studies or an eager learner seeking a deeper understanding of resources and their development, you’ve landed on the perfect page.
The subject of “Resources and Development” is a fundamental aspect of geography, encompassing the exploration, distribution, and sustainable use of various resources that shape our world. From understanding the significance of natural resources like water, minerals, and forests to analyzing the impact of human activities on the environment, this topic holds immense relevance in today’s context.
In the article, “Resources and Development Class 10 Notes” we have meticulously curated notes and study materials that cater specifically to Class 10 students. Say goodbye to overwhelming textbooks and disjointed online resources; here, we present the information in a clear, concise, and student-friendly manner. Join us on this enlightening journey as we equip you with the knowledge and insights needed to conquer your exams and gain a deeper appreciation for the intricate workings of our planet. Let’s dive in!
Resources and Development Class 10 Notes

Topics in Resources and Development Class 10 Chapter 1
- Resources
- Classification of Resources
- On the basis of origin
- On the Basis of Exhaustibility
- On the Basis of Ownership
- On the Basis of the Status of Development
- Development of Resources
- Resource Planning
- Land Resources
- Land Resources in India
- Land Use Pattern in India
- Land Degradation and Conservation measures
- Soil as a Resource
- Classification of Soils
- Alluvial Soils
- Black Soil
- Red and Yellow Soils
- Laterite Soils
- Arid Soils
- Forest Soils
- Soil Erosion and Soil Conservation
Resources and Development Class 10 Notes
Resources
The term “Resource” encompasses everything in our environment that satisfies our needs, is technologically accessible, economically feasible, and culturally acceptable. Interestingly, human beings themselves are integral components of these resources.
They play a vital role in transforming materials available in the environment into valuable resources and subsequently utilize them for various purposes. This intricate process of conversion and utilization highlights the dynamic relationship between humanity and its surroundings, showcasing our ability to adapt and harness the elements of nature to meet our requirements effectively.
As we delve into the concept of resources, we begin to realize the profound impact our actions have on the environment, underlining the importance of sustainable practices to ensure a harmonious coexistence with the natural world.
Classification of Resources
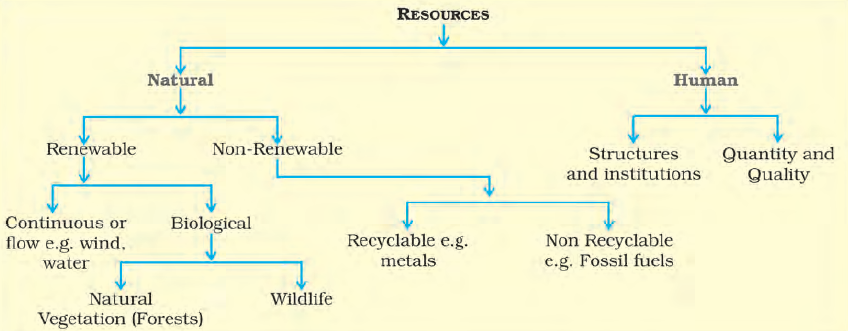
(a) Based on their Origin –
Biotic and Abiotic
Biotic Resources are derived from the biosphere and encompass all living organisms such as human beings, flora, fauna, fisheries, and livestock.
Example: Human populations, plant life, animals, aquatic life, etc.
Abiotic Resources, on the other hand, consist of non-living elements like rocks and metals.
Example: Minerals, ores, rocks, metals, etc.
(b) Based on Exhaustibility –
Renewable and Non-Renewable
Renewable or Replenishable Resources can be replenished or reproduced through natural processes, such as solar and wind energy, water, forests, and wildlife.
Example: Solar energy, wind power, water resources, etc.
Non-Renewable Resources take millions of years to form and cannot be readily replaced. Some non-renewable resources, like metals, are recyclable, while others, like fossil fuels, are exhausted with use.
Example: Fossil fuels (coal, oil, natural gas), minerals (iron, copper, gold), etc.
(c) Based on Ownership –
Individual, Community, National, and International
Individual Resources are privately owned by individuals and include lands, plots, houses, and other properties.
Example: Private estates, personal farms, etc.
Community Owned Resources are accessible to all members of a community and may include grazing grounds, public parks, and picnic spots.
Example: Village common lands, community parks, etc.
National Resources are owned by a nation or country, including minerals, water resources, forests, and land within its political boundaries.
Example: National parks, railways, etc.
International Resources are regulated by international institutions and encompass oceanic resources beyond 200 nautical miles of the Exclusive Economic Zone.
Example: Open ocean resources, international waters, etc.
(d) Based on the Status of Development –
Potential, Developed Stock, and Reserves
Potential Resources are available in a region but remain untapped or underutilized.
Example: Untapped wind and solar energy potential in certain regions.
Developed Resources have been surveyed, and their quantity and quality have been determined for utilization based on technology and feasibility.
Example: Extracted minerals, available water sources, etc.
Stocks are materials in the environment with potential to meet human needs, but current technology does not allow access.
Example: Hydrogen as a rich energy source without advanced utilization technology.
Reserves are a subset of stocks that can be utilized with existing technology but have not yet been put into use.
Example: Water stored in dams, untapped forest resources, etc.
Understanding these classifications helps us appreciate the diverse nature of resources and guides us in responsible and sustainable resource management for a better future.
Development of Resources
The indiscriminate use of resources by human beings has given rise to significant challenges, causing the following major problems:
1. Depletion of Resources: Many resources have been exploited without restraint to fulfill the greed of a few individuals. This uncontrolled consumption has led to the exhaustion of valuable resources, threatening the balance of ecosystems and natural cycles.
2. Unequal Distribution: As resources accumulate in the hands of a privileged few, societal divisions have intensified, creating a stark contrast between the wealthy and the impoverished. This economic disparity can perpetuate social inequalities and hinder overall progress.
3. Global Ecological Crises: The unregulated use of resources has triggered severe ecological crises on a global scale. Issues like global warming, ozone layer depletion, environmental pollution, and land degradation pose severe threats to the environment and all forms of life.
To address these challenges and ensure a sustainable future, resource planning becomes indispensable. Sustainable economic development aims to strike a harmonious balance between human progress and environmental preservation. It emphasizes the importance of developing without causing harm to the environment and ensuring that the needs of future generations are not compromised.
By implementing thoughtful resource management strategies, we can safeguard the well-being of both current and future generations while preserving the natural world for all life forms to thrive.
Resource Planning
In India, there exists a diverse landscape where some regions boast self-sufficiency in resources, while others face acute shortages of vital elements. To address this disparity, it becomes imperative to adopt a balanced resource planning approach at the national, state, regional, and local levels.
Resource Planning in India is a multifaceted process involving the following key steps:
(i) Identification and Inventory: An extensive survey, mapping, and qualitative as well as quantitative estimation of resources are conducted across the country’s various regions. This comprehensive assessment aids in understanding the resource distribution.
(ii) Planning Structure: A well-endowed planning structure is developed, incorporating appropriate technology, skills, and institutional setups to effectively implement resource development plans.
(iii) Alignment with National Development: Resource development plans are harmonized with the broader national development goals to ensure synergy and optimal utilization.
For resources to contribute effectively to development, it is crucial to accompany them with appropriate technological advancements and institutional changes. India has been actively pursuing resource planning since the inception of the First Five Year Plan after Independence, showcasing its commitment to sustainable growth.
To combat irrational consumption and over-utilization of resources, conservation efforts at various levels play a pivotal role in preserving the environment and fostering a sustainable future. By embracing resource planning and conservation, India can pave the way towards a balanced and prosperous tomorrow for all its citizens.
Land Resources
Land stands as a paramount natural resource, bearing immense significance in its support for various aspects of life and development. It sustains natural vegetation, wildlife, human settlements, economic activities, as well as transportation and communication systems. India’s landscape comprises a diverse array of relief features, encompassing mountains, plateaus, plains, and islands, as illustrated below:
Land Utilisation
Land resources find application in the following purposes:
1. Forests: Land is allocated for the growth and preservation of forests, vital for ecological balance and biodiversity conservation.
2. Land not available for cultivation: This category includes two subtypes:
a) Barren and wasteland: Land that is unsuitable for agricultural activities due to its arid or unproductive nature.
b) Land put to non-agricultural uses: Land utilized for non-farming purposes such as residential, industrial, or infrastructural developments.
3. Fallow lands: These are lands that are temporarily left uncultivated to regain fertility or due to seasonal reasons.
4. Other uncultivated lands (excluding fallow land): Lands that are not cultivated, excluding those explicitly categorized as fallow.
5. Net sown area: The total land area under cultivation, which excludes the area under fallow and uncultivated land.
Understanding and efficiently utilizing land resources across these diverse purposes are essential for sustainable development and optimal land management.
Land Use Pattern in India
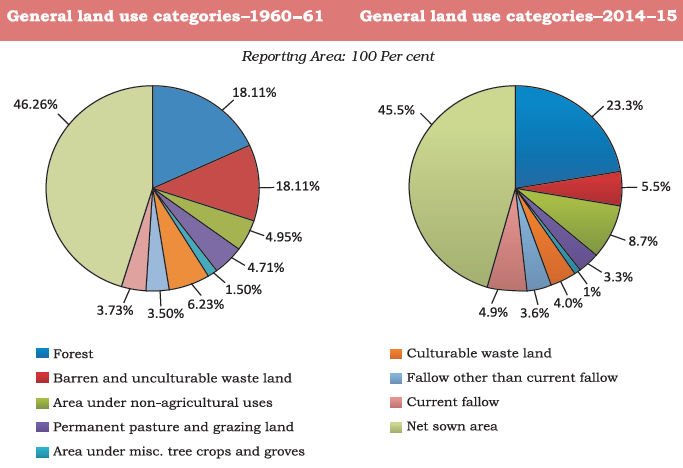
The utilization of land is influenced by two main sets of factors:
1. Physical Factors: These encompass various elements that are inherent to the land itself, such as topography (terrain features and elevation), climate (temperature, precipitation, etc.), and soil types (fertility, composition, etc.). These physical characteristics significantly impact the suitability and productivity of the land for various activities.
2. Human Factors: These factors relate to human interactions and decisions concerning land use. They include population density (the concentration of people in a specific area), technological capability (the level of technology available for land development and utilization), as well as culture and traditions, which influence the practices and preferences related to land use in a given society.
The interplay between these physical and human factors shapes how land is allocated and utilized for different purposes, ranging from agriculture and settlements to industrial and recreational activities. Understanding and balancing these factors are crucial for effective land management and sustainable development.
Wasteland refers to land that is utilized for non-agricultural purposes, encompassing areas characterized by rockiness, aridity, deserts, as well as spaces allocated for roads, railways, industries, and other non-farming activities. Unfortunately, the prolonged and unregulated use of land without implementing adequate conservation and management measures has led to the degradation of land resources.
As a consequence of this continuous usage, land degradation occurs, impacting the quality and fertility of the soil, reducing its ability to support vegetation and sustainable activities. It is essential to adopt responsible land management practices to counteract this degradation, ensuring the preservation and rejuvenation of our valuable land resources for future generations. Through proactive conservation efforts, we can combat the detrimental effects and promote sustainable land use, thus safeguarding the ecological balance and productivity of our lands.
Land Degradation and Conservation Measures
Land degradation is primarily driven by human activities, including deforestation, overgrazing, mining, and quarrying. These practices have left lasting impacts on the land, evident in deep scars and overburdened areas at mining sites. Additionally, industrial effluents have emerged as significant contributors to land and water pollution in various regions across the country.
Various soil conservation techniques are implemented to combat soil erosion and safeguard fertile land.
1. Afforestation and Grazing Management: Promoting afforestation initiatives and adopting proper grazing management practices help restore vegetation cover and prevent soil erosion.
2. Shelter Belt Planting: Creating shelter belts of plants can serve as protective barriers against wind and soil erosion, enhancing the stability of the land.
3. Sand Dune Stabilization: Growing thorny bushes on sand dunes aids in stabilizing them and curbing the encroachment of desertification.
4. Wasteland Management: Implementing comprehensive plans for the management of wastelands ensures their optimal utilization and potential for reclamation.
5. Control of Mining Activities: Regulating and controlling mining activities can minimize the adverse impacts on land, promoting sustainable extraction practices.
6. Industrial Effluent Treatment: Ensuring proper treatment and disposal of industrial effluents and wastes help mitigate land and water pollution, safeguarding the environment.
By implementing these proactive measures, we can combat land degradation and preserve the productivity and ecological health of our land resources. Emphasizing responsible land management practices is vital to secure a sustainable future for our environment and generations to come.
Soil as a Resource
Soil stands as the most crucial renewable natural resource, serving as the vital medium for plant growth and supporting diverse life forms on our planet.
The formation of soil is an incredibly slow process, taking millions of years to develop just a few centimeters in depth. Nature’s various forces, including temperature fluctuations, the actions of running water, wind, glaciers, and the activities of decomposers, all contribute to the gradual formation of soil.
Several key factors play significant roles in soil formation, such as the composition of the parent rock or bedrock, the prevailing climate, the types of vegetation, other living organisms, and the passage of time. Through a combination of chemical and organic changes, soil evolves, enriching its composition and supporting its essential functions.
Soil consists of both organic matter, commonly known as humus, and inorganic materials. This unique combination of components contributes to the soil’s fertility, making it an indispensable resource for sustaining life and fostering a wide array of ecosystems on Earth.
Classification of Soils
India’s diverse soils are classified based on factors such as soil formation, color, thickness, texture, age, and chemical and physical properties. Different types of soils found in India are:
1. Alluvial Soils:

– Found in the northern plains, as well as Rajasthan, Gujarat, and eastern coastal plains.
– Deposited by important Himalayan rivers like the Indus, Ganga, and Brahmaputra.
– Divided into Old Alluvial (Bangar) and New Alluvial (Khadar) based on age.
– Very fertile, ideal for growing crops like sugarcane, paddy, wheat, and pulses.
2. Black Soil:

– Also known as regur soil, found in Deccan trap (Basalt) regions.
– Ideal for cotton cultivation due to its moisture-retaining capacity.
– Rich in nutrients like calcium carbonate, magnesium, potash, and lime.
3. Red and Yellow Soils:

– Develop on crystalline igneous rocks in areas of low rainfall.
– Get their reddish color from iron diffusion in crystalline rocks.
– Found in parts of Odisha, Chhattisgarh, and southern regions of the Deccan plateau.
4. Laterite Soil:

– Found in tropical and subtropical climates with alternating wet and dry seasons.
– Result of intense leaching due to heavy rain, making it acidic and nutrient-deficient.
– Suitable for tea and coffee cultivation and supports deciduous and evergreen forests.
5. Arid Soils:

– Ranging from red to brown in color, found in arid regions.
– Sandy texture with high saline content, lacking in humus and moisture.
– Kankar layer restricts water infiltration.
6. Forest Soils:
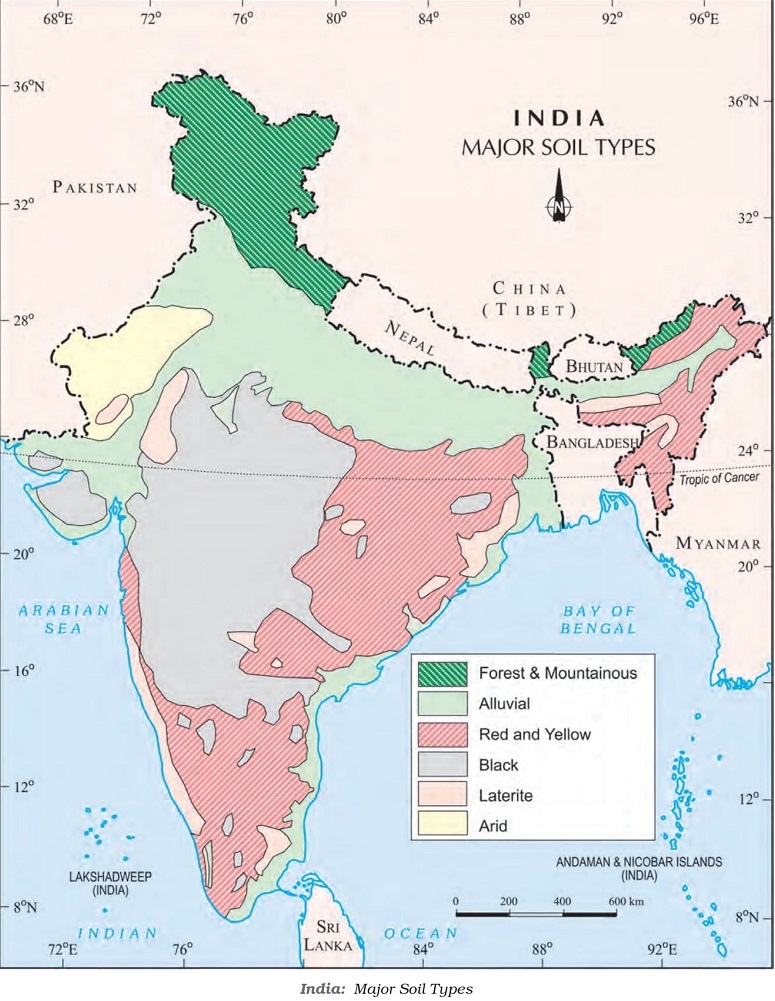
– Located in hilly and mountainous areas with varied textures based on elevation.
– Snow-covered areas in the Himalayas have acidic soils with low humus content.
– Fertile on river terraces and alluvial fans.
Each type of soil supports different ecosystems and is vital for agricultural productivity in its respective region. Understanding and managing these soils are crucial for sustainable land use and successful agricultural practices across the country.
Soil Erosion and Soil Conservation
Soil erosion, resulting from the denudation and subsequent washing down of the soil cover, poses a significant challenge. Deforestation, over-grazing, construction, and mining are the cause of erosion. Natural forces like wind, glaciers, and water. Defective farming methods also contribute to soil erosion.
Various forms of soil erosion occur depending on the terrain and circumstances. Running water carves deep channels known as gullies in clayey soils, rendering the land unsuitable for cultivation and termed as bad land.
To combat soil erosion and preserve fertile land, different soil conservation techniques are employed:
1. Contour Ploughing: Ploughing along the contour lines slows down water flow on slopes, reducing erosion.
2. Terrace Cultivation: Practiced in the Western and Central Himalayas, terrace cultivation helps control erosion.
3. Strip Cropping: Grass is grown between strips of large fields to break the force of the wind and minimize erosion.
4. Shelter Belts: Planting lines of trees to create shelter aids in stabilizing sand dunes and desert areas, particularly in western India.
By adopting these soil conservation methods, we can protect valuable topsoil, maintain fertile land for agriculture, and safeguard the environment from the adverse effects of soil erosion. Keep exploring and stay updated on CBSE and NCERT-related information. Access interactive Maths and Science videos through the BYJU’S App and subscribe to their YouTube channel for continuous learning.
Summery of the Article Resources and Development Class 10 Notes
The summary introduces an exclusive and comprehensive guide on “Resources and Development Class 10 Notes,” catering to Class 10 students seeking a deeper understanding of resources and their development. The article emphasizes the importance of resources for human survival, development, and progress.
The summary further delves into land resources, soil as a vital renewable natural resource, and the classification of soils in India. It concludes by addressing soil erosion and soil conservation measures.
Read Also:
Frequently Asked Questions on CBSE Resources and Development Class 10 Notes
Q 1. Importance of resources and development chapter for class 10 students?
The “Resources and Development” chapter is vital for Class 10 students as it introduces foundational knowledge about various resources, their distribution, and utilization.
It enhances environmental awareness, understanding the implications of human activities on the planet. Students learn about the economic significance of resources and their role in sustainable development. This knowledge fosters global perspective, encouraging responsible resource management.
Additionally, it equips students to analyze policies, participate in discussions, and advocate for environmentally conscious decisions. Mastering this chapter ensures excellent exam preparation while empowering students to become informed and proactive citizens, contributing to a sustainable future for our world.
Q 2. Importance of resources for humans?
Resources are of utmost importance for humans as they are essential for survival, development, and progress. Natural resources like water, air, soil, minerals, and energy sources provide the necessary elements for human sustenance, economic activities, and technological advancements.
They are vital for agriculture, industries, infrastructure, and daily living. Human civilization thrives on the availability and efficient utilization of resources, shaping societies, economies, and lifestyles.
Understanding and managing resources sustainably are crucial to ensure a better quality of life, economic prosperity, and environmental conservation. Recognizing the importance of resources empowers us to make responsible decisions, preserve nature’s gifts, and secure a better future for generations to come.
Q 3. How to protect natural resources?
Protecting natural resources requires a concerted effort and a collective commitment to sustainable practices. Some key measures include:
- Conservation and Preservation: Implementing conservation strategies like afforestation, reforestation, and protected areas helps maintain biodiversity and prevent resource depletion.
- Responsible Consumption: Promote responsible consumption habits, reduce waste, and practice recycling to minimize resource exploitation and environmental impact.
- Sustainable Agriculture: Adopt sustainable agricultural practices that preserve soil fertility, minimize chemical usage, and promote crop rotation for long-term resource preservation.
- Efficient Energy Use: Promote energy efficiency, transition to renewable energy sources, and reduce reliance on fossil fuels to conserve natural resources.
- Water Conservation: Encourage responsible water usage, rainwater harvesting, and the protection of water bodies to preserve this vital resource.
By integrating these measures into our lifestyles and policies, we can safeguard natural resources, promote ecological harmony, and ensure a sustainable future for generations to come.
Trigonometry Class 10 Formulas List for NCERT Maths Students
Below are the provided trigonometry class 10 formulas. Trigonometry involves studying the relationships between angles, lengths, and heights of triangles. It encompasses ratios, functions, identities, and formulas used to solve problems, particularly concerning right-angled triangles.
The applications of trigonometry extend to various fields such as engineering, astronomy, physics, and architectural design. This chapter holds significant importance as it covers various topics, including Linear Algebra, Calculus, and Statistics.
In CBSE Class 10, students are introduced to trigonometry, a completely new and challenging chapter. It requires learning and mastering various formulas to apply them effectively. Below, you can find the tabulated Trigonometry Class 10 formulas.
Trigonometry class 10 formulas
Here are all the essential formulas introduced to Class 10 students. You can refer to these formulas at any time to learn and solve trigonometry-related problems effectively.
The trigonometric formulas for ratios predominantly rely on the three sides of a right-angled triangle, namely the adjacent side or base, perpendicular, and hypotenuse (as shown in the figure above). By applying the Pythagoras theorem to the given right-angled triangle, we get:
(Perpendicular)² + (Base)² = (Hypotenuse)²
⇒ (P)² + (B)² = (H)²
Now, let’s explore the formulas based on trigonometric ratios (sine, cosine, tangent, secant, cosecant, and cotangent).
Learn Trigonometry class 10 formulas
| SN. | Variable | Defination |
|---|---|---|
| 1. | Sin A | Perpendicular/Hypotenuse |
| 2. | Cos A | Base/Hypotenuse |
| 3. | Tan A | Perpendicular/Base |
| 4. | Cot A | Base/Perpendicular |
| 5. | Cosec A | Hypotenuse/Perpendicular |
| 6. | Sec A | Hypotenuse/Base |
Relation between different trigonometric Function
| SN. | Function | Realtion |
|---|---|---|
| 1. | tan A | sin A/cos A |
| 2. | Cos A | cos A/sin A |
| 3. | Cosec A | 1/sin A |
| 4. | Sec A | 1/cos A |
Trigonometric Sign Functions
- sin (-θ) = − sin θ
- cos (−θ) = cos θ
- tan (−θ) = − tan θ
- cosec (−θ) = − cosec θ
- sec (−θ) = sec θ
- cot (−θ) = − cot θ
Trigonometric Identities
- sin2A + cos2A = 1
- tan2A + 1 = sec2A
- cot2A + 1 = cosec2A
Periodic Identities
- sin(2nπ + θ ) = sin θ
- cos(2nπ + θ ) = cos θ
- tan(2nπ + θ ) = tan θ
- cot(2nπ + θ ) = cot θ
- sec(2nπ + θ ) = sec θ
- cosec(2nπ + θ ) = cosec θ
Complementary Ratios
1. Quadrant I
- sin(π/2 − θ) = cos θ
- cos(π/2 − θ) = sin θ
- tan(π/2 − θ) = cot θ
- cot(π/2 − θ) = tan θ
- sec(π/2 − θ) = cosec θ
- cosec(π/2 − θ) = sec θ
2. Quadrant II
- sin(π − θ) = sin θ
- cos(π − θ) = -cos θ
- tan(π − θ) = -tan θ
- cot(π − θ) = – cot θ
- sec(π − θ) = -sec θ
- cosec(π − θ) = cosec θ
3. Quadrant III
- sin(π + θ) = – sin θ
- cos(π + θ) = – cos θ
- tan(π + θ) = tan θ
- cot(π + θ) = cot θ
- sec(π + θ) = -sec θ
- cosec(π + θ) = -cosec θ
4. Quadrant IV
- sin(2π − θ) = – sin θ
- cos(2π − θ) = cos θ
- tan(2π − θ) = – tan θ
- cot(2π − θ) = – cot θ
- sec(2π − θ) = sec θ
- cosec(2π − θ) = -cosec θ
5. Sum and Difference of Two Angles
- sin (A + B) = sin A cos B + cos A sin B
- sin (A − B) = sin A cos B – cos A sin B
- cos (A + B) = cos A cos B – sin A sin B
- cos (A – B) = cos A cos B + sin A sin B
- tan(A + B) = [(tan A + tan B)/(1 – tan A tan B)]
- tan(A – B) = [(tan A – tan B)/(1 + tan A tan B)]
6. Double Angle Formulas
- sin 2A = 2 sin A cos A = [2 tan A /(1 + tan2A)]
- cos 2A = cos2A – sin2A = 1 – 2 sin2A = 2 cos2A – 1 = [(1 – tan2A)/(1 + tan2A)]
- tan 2A = (2 tan A)/(1 – tan2A)
7. Triple Angle Formulas
- sin 3A = 3 sinA – 4 sin3A
- cos 3A = 4 cos3A – 3 cos A
- tan 3A = [3 tan A – tan3A]/[1 − 3 tan2A]
What are Inverse Trigonometric Functions
Inverse trigonometric functions are mathematical functions that “undo” the effect of the regular trigonometric functions (sine, cosine, tangent, cosecant, secant, and cotangent). They are also known as “arc” functions or “antitrigonometric” functions. The inverse trigonometric functions allow us to find the angles associated with specific trigonometric ratios.
The inverse trigonometric functions are denoted by “arc” or “a” followed by the trigonometric function’s abbreviation. For example:
- Inverse Sine (arcsin or asin): The inverse of the sine function. It gives us the angle whose sine is a given value. Domain: [-1, 1] Range: [-π/2, π/2]
- Inverse Cosine (arccos or acos): The inverse of the cosine function. It gives us the angle whose cosine is a given value. Domain: [-1, 1] Range: [0, π]
- Inverse Tangent (arctan or atan): The inverse of the tangent function. It gives us the angle whose tangent is a given value. Domain: (-∞, ∞) Range: (-π/2, π/2)
- Inverse Cosecant (arccsc or acsc): The inverse of the cosecant function. It gives us the angle whose cosecant is a given value. Domain: (-∞, -1] ∪ [1, ∞) Range: [-π/2, 0) ∪ (0, π/2]
- Inverse Secant (arcsec or asec): The inverse of the secant function. It gives us the angle whose secant is a given value. Domain: (-∞, -1] ∪ [1, ∞) Range: [0, π/2) ∪ (π/2, π]
- Inverse Cotangent (arccot or acot): The inverse of the cotangent function. It gives us the angle whose cotangent is a given value. Domain: (-∞, ∞) Range: (0, π)
The inverse trigonometric functions are used to find angles in trigonometric equations and solve various real-world problems involving angles and trigonometric ratios. They are essential tools in calculus, engineering, physics, and other fields where trigonometry is applied.
Tips to Memorize Trigonometry Class 10 Formulas
- Understand the Concepts: Before memorizing, make sure you understand the basic concepts and principles behind each formula. This will make it easier to remember and apply them.
- Create Acronyms or Mnemonics: Associate each formula with a short acronym or mnemonic that represents the formula’s variables or terms. This can help you recall the formula quickly during exams.
- Practice Regularly: Practice solving trigonometry problems using the formulas frequently. Repetition will reinforce your memory.
- Use Flashcards: Write the formulas on flashcards and review them regularly. This technique enhances memory retention.
- Break Down Formulas: Break complex formulas into smaller parts and focus on memorizing each part separately. Then, combine the parts to remember the entire formula.
- Relate Formulas to Real-Life Applications: Connect the trigonometry formulas to real-life scenarios or applications. It will make them more relatable and easier to remember.
- Visualize: Create visual aids like diagrams or charts to represent the formulas. Visual cues can enhance memory.
- Teach Someone Else: Explaining the formulas to someone else helps reinforce your understanding and memorization.
- Use Online Resources: There are many online platforms and mobile apps that offer interactive quizzes and games to help memorize formulas effectively.
- Stay Consistent: Dedicate regular study time to review and practice the formulas. Consistency is key to long-term retention.
Remember, practice and understanding are crucial for memorizing trigonometry formulas effectively. With consistent effort, you’ll master these formulas in no time!
Applications of Trigonometry Class 10 Formulas
Trigonometry formulas from Class 10 have various practical applications in everyday life as well as in various fields of science, engineering, and technology. Some of the key applications include:
- Architecture and Construction: Trigonometry is used in architectural designs, surveying, and construction to calculate angles, distances, and heights of buildings and structures.
- Engineering: Engineers use trigonometry to design and analyze various mechanical, electrical, and civil engineering projects, such as bridges, roads, and buildings.
- Astronomy and Navigation: Trigonometry is crucial in astronomy to calculate the positions of celestial objects and navigate using tools like compasses and GPS.
- Geography and Cartography: Trigonometry is used in mapmaking, cartography, and land surveying to measure distances and map features accurately.
- Computer Graphics and Animation: In computer graphics and animation, trigonometry is essential for calculating angles and positioning objects in 2D and 3D space.
- Physics: Trigonometry is used extensively in physics to analyze forces, motion, and wave phenomena.
- Music and Sound Engineering: Trigonometry plays a role in analyzing sound waves and designing musical instruments.
- Optics: Trigonometry is used in optics to calculate angles of incidence and refraction in lenses and mirrors.
- Medicine: Medical imaging techniques like X-rays and CT scans use trigonometry to reconstruct images of the internal body.
- Mechanical Engineering: Trigonometry is used to analyze mechanical systems, such as gears, pulleys, and levers.
These are just a few examples of how trigonometry formulas are applied in various fields. Trigonometry is a fundamental branch of mathematics with wide-ranging applications, making it essential knowledge for students in Class 10 and beyond.
Read More:
- RD Sharma Class 10 Book Pdf Free Download Without Solutions
- Sample Question Paper for Class 10 CBSE Maths With Solutions
- Median Formula Class 10th For Grouped and Ungrouped Data
Frequently Asked Questions – FAQs
Q 1. How to solve trigonometry?
Ans: To solve trigonometry problems, follow these steps:
- Understand the problem and identify the given information and what needs to be found.
- Label the triangle and use standard labels like “opposite,” “adjacent,” and “hypotenuse” for right-angled triangles.
- Choose the appropriate trigonometric ratio (sin, cos, tan) based on what needs to be found.
- Apply the trigonometric ratio by substituting known values and setting up the equation.
- Use the Pythagoras theorem for right-angled triangles if necessary (a² + b² = c²).
- Check your calculator mode (degrees or radians) before using trigonometric functions.
- Use inverse trigonometric functions (sin⁻¹, cos⁻¹, tan⁻¹) to find angle measures.
- Check for extraneous solutions that may not fit the problem’s requirements.
- Practice regularly and review trigonometric identities for more complex problem-solving.
- Seek help from teachers or online resources if needed. Practice is crucial in mastering trigonometry.
Q 2. How can I learn trigonometry fast?
Ans: To learn trigonometry fast, master basic geometry concepts, understand trigonometric ratios, and memorize key formulas. Regularly practice solving diverse problems, use flashcards, and visualize concepts with diagrams. Watch video tutorials, engage in group study, and teach others to reinforce learning.
Set goals, track progress, and practice under time constraints to improve speed and accuracy. Seek help when needed and stay organized with notes and formulas. Be patient and persistent, as learning trigonometry effectively is a gradual process. With dedication, you can quickly grasp concepts and become proficient in trigonometry problem-solving.
Q 3. What is the formula for a 30 60 90 triangle?
Ans: The formula for the sides of a 30-60-90 triangle is as follows:
- Shortest side (opposite 30-degree angle): a
- Side opposite the 60-degree angle: b = a√3
- Hypotenuse: c = 2a
Remember, this formula is applicable to all 30-60-90 triangles, regardless of their size. If you know the length of one side, you can use these ratios to find the lengths of the other sides. Conversely, if you know the lengths of two sides, you can calculate the third side using these ratios.
Q 4. What angle is depression?
Ans: The term “angle of depression” refers to the angle formed between a horizontal line and the line of sight when an observer looks downward to view an object or point that is at a lower level. In other words, it is the angle measured from the horizontal line to the line of sight directed downward.
The angle of depression is commonly used in various fields, such as surveying, architecture, and physics, to determine the height or distance of an object or point below the observer’s viewpoint. It is the complement of the angle of elevation, which is the angle formed when an observer looks upward to view an object or point that is at a higher level.
Q 5. What is angle of sight?
Ans: The term “angle of sight” is not a standard or widely used trigonometric term. It might be a colloquial or informal expression to refer to either the “angle of elevation” or the “angle of depression,” depending on the context.
1. Angle of Elevation: The angle of elevation is the angle formed between a horizontal line and the line of sight when an observer looks upward to view an object or point that is at a higher level. It is used to determine the height or distance of an object above the observer’s viewpoint.
2. Angle of Depression: The angle of depression, as mentioned earlier, is the angle formed between a horizontal line and the line of sight when an observer looks downward to view an object or point that is at a lower level. It helps determine the height or distance of an object below the observer’s viewpoint.
If you encounter the term “angle of sight” in a specific context, it would be best to clarify with additional context or refer to the specific trigonometric concept it represents, which is either the angle of elevation or the angle of depression.
Class 10th Political Parties Notes of NCERT Civics Chapter 4
Class 10th Political Parties: In Class 10th, students delve into Chapter 6: Political Parties, an essential topic in the Social Science curriculum. This chapter introduces young minds to the world of political parties, their significance in a democratic nation like India, and their role in shaping the country’s governance.
By comprehending the inner workings of political parties, students gain insights into the democratic backbone of India and the process by which leaders and representatives are elected to govern the nation. Let’s embark on a journey to understand the fundamentals of political parties in India and their impact on the nation’s progress.

Class 10th Political Parties
The Importance of Political Parties in a Democracy
In a democratic nation, political parties play a pivotal role in representing the diverse interests and aspirations of the citizens. These organizations are essential for a well-functioning democracy, as they provide a platform for people to participate in governance actively. Here’s why political parties are crucial in a democratic setup:
- Representation of People: Political parties act as intermediaries between the government and the citizens. They voice the concerns and demands of the people, making sure their needs are taken into account in policymaking.
- Formulation and Articulation of Policies: Parties develop and present different policies and ideologies to address societal issues. Through healthy debates, they propose solutions that cater to various sections of the population.
- Electoral Process: Political parties contest elections to fill legislative positions, including the Parliament and state assemblies. These elections offer citizens the opportunity to choose their representatives and participate in the decision-making process.
- Government Formation: Political parties with majority support in the elections form the government, and the party’s leader becomes the Prime Minister or Chief Minister. The party in power is responsible for implementing policies and running the country or state effectively.
- Accountability: Political parties hold elected representatives accountable for their actions and decisions. They act as watchdogs, ensuring that the government works in the best interest of the citizens.
Evolution of Political Parties in India
India’s political landscape has witnessed significant transformations over the years. Understanding the evolution of political parties is crucial to grasp their current impact on the nation’s governance. Here’s a brief overview of the development of political parties in India:
| SN. | Party Name | Foundation Year |
|---|---|---|
| 1. | Indian National Congress | 1885 |
| 2. | Communist Party of India | 1925 |
| 3. | Bhartiya Janta Party | 1980 |
| 4. | Bahujan Samaj Party | 1984 |
| 5. | All India Trinamool Congress | 1998 |
| 6. | Nationalist Congress Party | 1999 |
| 7. | Aam Adami Party | 2012 |
| 8. | National People Party | 2013 |
- Pre-Independence Era: Before India gained independence from British rule in 1947, the political scene was characterized by the Indian National Congress (INC) and a few regional parties. The INC, led by prominent leaders like Mahatma Gandhi and Jawaharlal Nehru, spearheaded the freedom struggle.
- Post-Independence: After independence, the Indian National Congress emerged as the dominant political party. It played a critical role in shaping India’s early democratic institutions and policies.
- Emergence of Regional Parties: Over time, regional parties gained prominence as they addressed specific issues of regional importance. These parties represented the diverse cultural and linguistic identities of various states.
- Era of Coalition Politics: In the 1990s, India witnessed a shift towards coalition politics, where no single party could secure a clear majority. This led to the formation of coalition governments at the central level.
- Rise of Ideology-Based Parties: Alongside regional parties, ideology-based parties like the Bharatiya Janata Party (BJP) and the Communist Party of India (Marxist) (CPI-M) emerged, attracting followers with distinct ideologies.
- Current Scenario: Present-day India features a multi-party system, with a mix of national and regional parties competing in elections. Coalition governments have become more common, requiring parties to form alliances for stability.
The Role of Political Parties in Indian Democracy
Political parties play a crucial role in Indian democracy, serving as the primary means through which citizens participate in the political process. They act as bridges between the government and the people, representing diverse interests and ideologies. Here are some key roles of political parties in Indian democracy:
Representation:
Political parties represent the interests and aspirations of various sections of society. They bring together like-minded individuals and groups who share common goals, providing a platform for their voices to be heard.
Formation of Government:
In a parliamentary democracy like India, the political party or coalition with the majority in the legislative body forms the government. The party with the most seats in the Lok Sabha (House of the People) elects the Prime Minister, who becomes the head of the government.
Political Stability:
Political parties contribute to stability by providing continuity in governance. They establish stable governments, reducing the likelihood of frequent political crises or changes.
Policy Formulation:
Political parties present their visions and policies to the public during elections. Once in power, they implement these policies, shaping the country’s direction and development.
Accountability:
Opposition parties act as watchdogs, holding the ruling party accountable for its actions and decisions. They provide checks and balances, ensuring transparency and preventing misuse of power.
Participation:
Political parties encourage citizen participation in the democratic process. They mobilize people to vote, engage in political debates, and exercise their right to choose their representatives.
Social Transformation:
Political parties promote social change and inclusivity. They advocate for the rights of marginalized communities and work towards a more equitable society.
Regional Representation:
India’s diversity is reflected in the existence of regional parties that represent the interests of specific states or regions. These parties address local issues and concerns, fostering a federal structure.
Coalition Building:
In a multi-party system, alliances and coalitions are formed to gain a majority in the government. This promotes cooperation and consensus-building among different parties.
Promotion of Democratic Values:
Political parties are instrumental in upholding democratic values, such as freedom of speech, expression, and association. They play a vital role in safeguarding the democratic fabric of the country.
However, challenges such as money power, political dynasties, and lack of internal democracy in some parties can undermine the democratic process. Despite these challenges, political parties remain central to the functioning of Indian democracy, shaping its course and reflecting the collective will of the people.
Challenges Faced by Political Parties in India
Despite their significant role in Indian democracy, political parties face various challenges that impact their functioning. Some of the prominent challenges include:
- Corruption: Corruption is a prevalent issue that affects several political parties. It erodes public trust and undermines the democratic process.
- Communalism and Caste-Based Politics: Some parties indulge in communal or caste-based politics to garner votes, leading to social divisions and polarization.
- Money Power: The influence of money in elections and party financing is a concern that needs addressing. It can lead to unequal representation and affect the fairness of the electoral process.
- Lack of Internal Democracy: Some parties lack internal democracy, with decision-making concentrated in the hands of a few leaders.
- Dynastic Politics: The prevalence of dynastic politics, where party leadership is based on family lineage, can hinder meritocracy and limit opportunities for new leaders to emerge.
- Regionalism and Fragmentation: The proliferation of regional parties and fragmented mandates in elections can result in unstable coalition governments.
Role of Election commission of India
Following are the work of Election Commission:
- Independent Constitutional Body overseeing elections.
- Comprises Chief Election Commissioner and two Election Commissioners.
- Conducts elections for Lok Sabha, State Legislative Assemblies, President, and Vice-President.
- Responsible for delimitation of constituencies.
- Prepares and updates electoral rolls.
- Enforces the Model Code of Conduct during elections.
- Conducts voter education and awareness programs.
- Introduced Electronic Voting Machines (EVMs) for secure voting.
- Handles election disputes and complaints.
- Engages in international cooperation and election observation programs.
Summery of Class 10th Political Parties
Political Parties in Class 10th offers students valuable insights into the functioning and significance of political parties in India’s democratic system.
By understanding their evolution, roles, and challenges, students can appreciate the dynamic nature of India’s political landscape.
Political parties remain integral to the nation’s governance, providing citizens with a voice and shaping policies that impact the lives of millions.
As future citizens, the knowledge gained from this chapter empowers students to actively engage in the democratic process, fostering a stronger and more inclusive India.
Read Also:
- Power Sharing Notes Class 10th of NCERT Civic Chapter 1
- Outcomes of Democracy Class 10 Notes of NCERT Civics Ch. 7
Frequently Asked Question – FAQs: Class 10th Political Parties
Q 1. What is political party?
A political party is an organized group of individuals with shared political beliefs and ideologies. Its primary goal is to participate in the electoral process to gain political power and influence government policies. Political parties play a vital role in democratic systems, representing the interests and concerns of their members and supporters. They develop policy platforms, nominate candidates for public offices, and engage in campaigns to attract voter support. Through their participation in elections, political parties seek to govern and enact their agenda, shaping the direction of a country’s governance and legislation.
Q 2. Importance of Class 10th political parties chapter in students life?
The importance of studying political parties in a student’s life is multifaceted and far-reaching. Firstly, it promotes civic awareness by helping students understand the functioning of the political system and the role of parties in representing people’s interests. This knowledge nurtures critical thinking as students learn to evaluate diverse ideologies and policies. It installs a sense of social responsibility, encouraging active participation in the democratic process and fostering leadership development through engagement in political discussions and student groups.
Moreover, studying political parties broadens students’ global awareness, as they explore different party systems worldwide and gain insights into international relations. It raises ethical considerations, promoting discussions on transparency, public interests, and corruption avoidance. This empowerment enables students to be proactive advocates for the causes they believe in and facilitates conflict resolution and negotiation skills development.
For those aspiring to careers in politics or public administration, this knowledge serves as a foundation. Additionally, it promotes diversity appreciation by exposing students to various perspectives. Overall, understanding political parties enriches students’ personal growth, cultivates active citizenship, and contributes to creating well-rounded, responsible, and informed global citizens.
Q 3. What are the main features of class 10th political parties?
Political parties in Class 10 are characterized by their organized structure, shared ideology, and participation in elections. They represent the interests of their members and formulate policy platforms. Winning parties or coalitions form the government, while others act as the opposition. Parties engage in election campaigns, use propaganda, and adapt to changing societal demands. Pluralism is evident in democratic societies, with multiple parties representing diverse interests and opinions. Understanding these features helps students comprehend the role and functioning of political parties in a democratic system.
Class 10 Development Notes of NCERT Economics Chapter 1
Class 10 development notes: The CBSE Notes for Class 10 Economics Chapter 1 introduce the concept of ‘Development’ and serve as a foundational understanding for higher classes where more development issues are explored. In this chapter, students will find answers to critical questions about a country’s ideal state, essential requirements, prospects for a better life for all, coexistence principles, and possibilities for increased equality. These answers aren’t confined to Economics alone; They are also intertwined with insights from History and Political Science. Understanding development necessitates a holistic approach as our present way of life is profoundly shaped by our past.

Topics Covered in the Class 10 development Notes
- Overview
- What Development Promises —Different People, Different Goals.
- Income and Other Goals.
- National Development.
- How to Compare Different Countries or States?
- Income and Other Criteria.
- Public Facilities.
- Sustainability of Development.
What Development Promises —Different People, Different Goals
People are unique in their perspectives, values, and priorities, which lead them to pursue different paths and set distinct goals in life. What may be important and meaningful to one person might not hold the same significance for another.
This phrase emphasizes the diversity and individuality of human beings, acknowledging that each person has their own set of dreams, desires, and aims. It also highlights the need for understanding and respecting these differences, as it is natural for people to have diverse goals and aspirations based on their backgrounds, experiences, and personal preferences. Recognizing and embracing these distinctions is essential for building a harmonious and inclusive society.
Income and Other Goals

Individuals desire increased income, as it enables the acquisition of material possessions and services, influencing our lives significantly. Nevertheless, the quality of life is also influenced by non-material factors like equitable treatment, freedom, security, and respect for others. Development encompasses a combination of goals, wherein people seek not only better financial prospects but also other essential aspects of life. These developmental objectives extend beyond mere income enhancement, encompassing broader aspects that contribute to a fulfilling and meaningful existence.
Class 10 Development Notes
National Development
People can have different and even opposite ideas about how a country should progress and develop.
How to Compare Different Countries or States?
To compare countries, we often look at their income, which is a crucial factor in determining how developed they are. Countries with higher incomes are generally more developed than those with lower incomes. Instead of comparing total income, we consider the average income of each country to understand what an average person earns.
Average income is the total income of a country divided by its total population, known as per capital income.
Per Capita Income = Total Income of Country / Total Population of Country
In World Development Reports, per capita income is used to classify countries.
Countries with a per capita income of US$ 12,056 or more in 2017 are called rich countries. Low-income Countries has income of US$ 955 or less. For example, India falls into this category.
Income and Other Criteria
When we consider a country or an area, we must not only focus on the average income but also pay attention to public facilities, which are equally important.
Public Facilities: These are the services that the government offers to its people. Some essential public facilities include infrastructure, sanitation, public transport, healthcare, water supply, and more.
Sustainability of Development
Sustainable development means progress that fulfills the current needs without harming the ability of future generations to meet their needs. Scientists have been cautioning that our current type and level of development are not sustainable. Some examples of this unsustainability include the overuse of groundwater and the depletion of natural resources.
Summery of Class 10 Development Notes
The CBSE Notes for Class 10 Economics Chapter 1 introduce the concept of ‘Development,’ exploring critical questions about a country’s progress and goals.
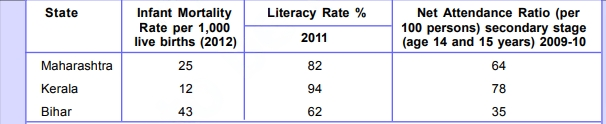
It emphasizes the individuality of people, recognizing diverse aspirations and priorities. Development is not solely about income; non-material factors like freedom, security, and respect also influence the quality of life. The chapter explains how countries are compared using per capital income and considers public facilities’ importance.
It presents data comparing Maharashtra, Kerala, and Bihar. Moreover, we talk about the cool concept of sustainable development! It’s all about finding a way to fulfill our current needs without messing up the ability of future generations to meet their own needs. We also look at some not-so-cool examples of unsustainability, like overusing groundwater and depleting natural resources.
Frequently Asked Question – FAQs: Class 10 Development Notes
Q 1. Importance of “Development” chapter for students?
- The “Development” chapter introduces students to the multidimensional nature of development, beyond economic growth.
- It emphasizes that development should cater to diverse individual goals and aspirations.
- Students gain a holistic perspective on development, considering material and non-material factors.
- The chapter fosters critical thinking as students evaluate the meaning of development in the context of a country or region.
- They learn methods to compare and analyze development levels among different countries or states.
- The concept of sustainable development raises awareness about responsible resource use and environmental preservation.
- Students develop a sense of social responsibility and active citizenship, understanding development challenges in their communities and beyond.
- The chapter is relevant for those interested in economics, international relations, sociology, and environmental studies careers.
- It provides a global perspective, showcasing how different countries face distinct developmental challenges.
- Overall, the chapter equips students with knowledge and values essential for becoming informed and responsible global citizens.
Q 2. What are the various key factor to determine a country’s development?
- Key factors to determine a country’s development include GDP, GNI/GNP, and per capital income, reflecting economic prosperity and living standards.
- The Human Development Index (HDI) considers life expectancy, education, and income to assess human development levels.
- Education, literacy rate, and healthcare indicators provide insights into a nation’s human capital and well-being.
- Adequate infrastructure and public facilities, like transportation and access to basic services, are vital for economic and social development.
- Low unemployment rates and stable governance contribute to economic growth and societal stability.
- Income inequality and poverty rates reflect the distribution of wealth and opportunities within a country.
- Environmental sustainability and commitment to conservation impact a country’s long-term development.
- Access to clean water and sanitation facilities is essential for public health and human development.
- Gender equality and empowering women contribute to social progress and inclusive development.
- These factors collectively offer a comprehensive assessment of a country’s development status, guiding policymakers in prioritizing development strategies.
Q 3. When a country will known as “Developed Country”?
A “Developed Country” refers to a nation that has achieved a high level of economic prosperity, industrialization, technology, and social well-being.
The categorization varies among different organizations and indices. Common features of developed countries include high GDP per capita, advanced infrastructure, low poverty, access to quality healthcare and education, low infant mortality, high life expectancy, strong governance, and a high standard of living.
Examples of developed countries include the United States, Canada, Germany, Japan, Australia, and several European nations. Development is an ongoing process, and countries may transition from developing to developed status based on socio-economic improvements over time.
Manufacturing Industries Class 10th Notes of NCERT Geo. Ch. 6
Manufacturing industries class 10th notes: Manufacturing is the process of mass-producing goods through the transformation of raw materials. It encompasses various industries such as steel factories, automobile production, breweries, textile manufacturing, bakeries, and more. These industries are categorized as secondary activities. In CBSE Notes Class 10 Geography Chapter 6 – Manufacturing Industries, you will primarily focus on learning about manufacturing industries belonging to the secondary sector.

Topics Covered in the Article Manufacturing industries class 10th notes
- Importance of Manufacturing
- Agriculture and Industry
- Contribution of Industry to National Economy
- Industrial Location
- Classification of Industry
- Agro Based
- Textile Industry
- Cotton textiles
- Jute Textiles
- Sugar Industries
- Mineral Based Industries
- Iron and Steel Industry
- Aluminium Smelting
- Chemical Industries
- Fertiliser Industry
- Cement Industry
- Automobile Industry
- Information Technology and Electronics Industry
- Industrial Pollution and Environmental Degradation
- Control of Environmental Degradation
Geography Chapter 6 Manufacturing industries class 10th notes
Importance of Manufacturing
The manufacturing sector is widely regarded as the foundation of development, and this is attributed to several compelling reasons:
1. Modernizing Agriculture: Manufacturing industries play a crucial role in modernizing agriculture by creating job opportunities in the secondary and tertiary sectors. This helps to diversify the economy and provide alternative employment options for the workforce.
2. Combating Unemployment and Poverty: The presence of a thriving manufacturing sector aids in alleviating unemployment and poverty. By generating substantial employment opportunities, it enhances the standard of living for many individuals and families.
3. Boosting Trade and Commerce: Manufacturing industries contribute significantly to the expansion of trade and commerce, especially through the export of manufactured goods. This leads to a higher inflow of foreign exchange, which is essential for a well-functioning economy.
4. Fostering Economic Growth: The growth and success of the manufacturing sector have a cascading effect on the overall economy. It stimulates economic growth, encourages investments, and enhances the nation’s prosperity.
In summary, the manufacturing sector’s multifaceted contributions make it a vital pillar of development, impacting various aspects of a nation’s progress and prosperity.
Agriculture and Industry
Agriculture and industry share a mutually beneficial relationship, relying on each other for growth and sustainability.
→ Industries significantly enhance agriculture by elevating productivity through the provision of essential tools and products like fertilizers, among others.
→ Conversely, industries depend on agriculture as a valuable source of raw materials. They cater to farmers by offering products like irrigation pumps, fertilizers, insecticides, pesticides, plastic and PVC pipes, machinery, and tools, thereby strengthening the agricultural sector. This interdependence fosters a synergistic partnership between agriculture and industry, driving overall economic development.
Contribution of Industry to National Economy
The contribution of the industry to the national economy is paramount. Industries, particularly manufacturing, form the backbone of economic development, driving growth, and prosperity. They play a crucial role in generating employment opportunities, reducing unemployment, and improving livelihoods. Moreover, industries contribute significantly to the Gross Domestic Product (GDP) of a country, fueling economic expansion and enhancing the overall standard of living. Beyond economic benefits, industries promote innovation, technological advancements, and skill development. Additionally, they facilitate foreign trade, exporting goods and services, thereby boosting foreign exchange earnings. Overall, the industry’s contribution is indispensable, fostering a robust and vibrant national economy.
Industrial Location
The positioning of industries is influenced by the accessibility of the following factors:
1. Raw materials
2. Labor
3. Capital
4. Power
5. Market
6. Government policies
Manufacturing activities tend to gravitate towards locations where all these industrial factors are readily available or can be arranged at a lower cost. The figure below illustrates the crucial link between industries and markets in the context of industrial location.
Manufacturing industries class 10th notes
Classification of Industry
In terms of the source of raw materials used, industries can be classified as:
• Agro-based industries that rely on agricultural products as their primary input.
• Mineral-based industries that utilize minerals and natural resources for manufacturing processes.
Industries can also be categorized based on their main role:
• Basic or key industries, which supply products or raw materials essential for manufacturing other goods. Examples include iron and steel, and copper smelting industries.
• Consumer industries, which produce goods directly used by end consumers, like sugar and toothpaste manufacturing.
Another classification is based on the level of capital investment:
• Small-scale industries, which require investments up to rupees one crore and employ a smaller number of laborers.
• Large-scale industries, characterized by investments exceeding one crore, often employing a more extensive workforce.
Ownership also plays a role in classifying industries:
• Public sector industries, owned and operated by government agencies like BHEL and SAIL.
• Private sector industries, owned and operated by individuals or a group of individuals, such as TISCO, Bajaj Auto Ltd., and Dabur Industries.
Other ownership-based categories include:
• Joint sector industries, jointly run by the state and private individuals or groups. An example is Oil India Ltd. (OIL), which has both public and private ownership.
• Cooperative sector industries, owned and operated by producers or suppliers of raw materials, workers, or both.
Industries can also be distinguished based on the bulk and weight of raw materials and finished goods they handle:
• Heavy industries, like iron and steel, which deal with substantial raw materials and produce bulky goods.
• Light industries, such as electrical industries, which use lighter raw materials and produce less bulky goods.
Agro Based Industries
Industries based on agricultural raw materials include cotton, jute, silk, woollen textiles, sugar, edible oil, and more. Let’s explore each of them individually:
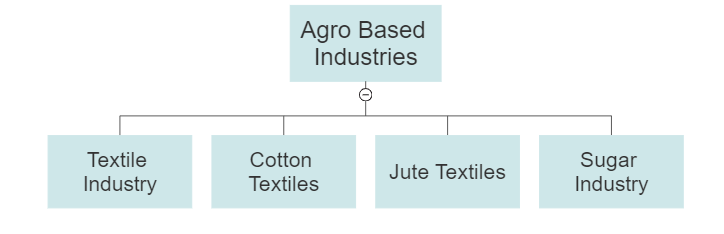
Textile Industry:
The Indian textile industry is unique for its self-reliance and complete value chain, covering everything from raw materials to the highest value-added products. It plays a significant role in industrial production, employment generation, and foreign exchange earnings.
Cotton Textiles:
This industry holds strong ties with agriculture, providing livelihoods to farmers, cotton boll pluckers, and workers involved in various stages like ginning, spinning, weaving, dyeing, designing, packaging, tailoring, and sewing. Additionally, it supports other related sectors, including chemicals and dyes, packaging materials, and engineering works.
Jute Textiles:
India ranks as the largest producer of raw jute and jute goods. Most of the jute mills are concentrated in West Bengal, primarily along the banks of the Hugli River.
Sugar Industry:
India holds the second position in global sugar production and is a leading producer of Gur and Khandsari. However, this industry is seasonal in nature, operating during specific periods.
Manufacturing industries class 10th notes
Mineral-Based Industries
Mineral-based industries are those that utilize minerals and metals as their primary raw materials. Let’s explore some of the industries falling under this category:
Iron and Steel Industry:
The iron and steel industry is considered the backbone of all other industries, including heavy, medium, and light, as they rely on it for their machinery. It is classified as a heavy industry due to the substantial weight of both raw materials and finished goods, leading to high transportation costs. India holds significant importance as an iron and steel producer globally, but certain challenges hinder its full potential, such as limited availability and high costs of coking coal, lower labor productivity, irregular energy supply, and inadequate infrastructure.
Aluminium Smelting:
Aluminium smelting ranks as the second most significant metallurgical industry in India. It is used to manufacture aircraft, utensils, and wires, with bauxite being the primary raw material used in the smelting process. Aluminium is increasingly favored as a substitute for steel, copper, zinc, and lead in various industries due to its lightweight, corrosion-resistant properties, good heat conductivity, malleability, and strength when mixed with other metals.
Chemical Industries:
The chemical industry comprises both large and small-scale manufacturing units and has witnessed rapid growth in both inorganic and organic sectors. Inorganic chemicals include sulphuric acid, nitric acid, alkalies, soda ash, and caustic soda, while organic chemicals encompass petrochemicals used in the production of synthetic fibers, synthetic rubber, plastics, dye-stuffs, drugs, and pharmaceuticals.
Fertilizer Industry:
Fertilizer industries are focused on the production of nitrogenous fertilizers, mainly urea, phosphatic fertilizers like ammonium phosphate (DAP), and complex fertilizers containing a combination of nitrogen, phosphate, and potash. States like Gujarat, Tamil Nadu, Uttar Pradesh, Punjab, and Kerala contribute significantly to fertilizer production in India.
Cement Industry
Essential for construction activities, the cement industry plays a vital role in building houses, factories, bridges, roads, airports, dams, and commercial establishments. The production of cement requires bulky and heavy raw materials such as limestone, silica, and gypsum.
Automobile Industry
The automobile industry involves the manufacturing of various vehicles, including trucks, buses, cars, motorcycles, scooters, three-wheelers, and multi-utility vehicles. This industry is typically concentrated around major cities like Delhi, Gurugram, Mumbai, Pune, Chennai, Kolkata, Lucknow, Indore, Hyderabad, Jamshedpur, and Bengaluru.
Information Technology and Electronics Industry
Encompassing a wide range of products, the electronics industry includes transistor sets, televisions, telephones, cellular telecom equipment, telephone exchanges, radars, computers, and other telecommunication devices. This industry has significantly contributed to employment generation in India, with Bengaluru being recognized as the electronic capital of the country.
Industrial Pollution and Environmental Degradation:
Industries are responsible for four types of pollution, namely air, water, land, and noise. Air pollution occurs due to undesirable gases emitted by various industries, impacting human health, animals, plants, and the atmosphere.
Water pollution results from industrial wastes and effluents discharged into rivers, leading to ecological and health hazards. Thermal pollution arises when hot water from factories and thermal plants is released into rivers and ponds before cooling.
Noise pollution, caused by industrial activities, affects human and animal life, leading to irritation, hearing impairment, and increased heart rate and blood pressure. Efforts to mitigate and manage industrial pollution are essential to protect the environment and ensure sustainable industrial development.
Control of Environmental Degradation
There are several effective ways to reduce industrial pollution:
1. Water Conservation: Minimize water usage by implementing measures for reusing and recycling water within industrial processes. Additionally, harvesting rainwater can be employed to meet water requirements.
2. Effluent Treatment: Treat hot water and industrial effluents before releasing them into rivers and ponds to minimize water pollution and protect aquatic ecosystems.
3. Air Pollution Control: Install smoke stacks equipped with electrostatic precipitators, fabric filters, scrubbers, and inertial separators to reduce particulate matter emissions from factories.
4. Cleaner Fuels: Switching from coal to cleaner fuels like oil or gas in industrial processes can significantly reduce smoke emissions and air pollution.
5. Energy Efficiency: Redesign machinery and processes to enhance energy efficiency, reducing overall energy consumption and environmental impact.
By adopting these practices, industries can play a pivotal role in curbing pollution and promoting sustainable development. For further knowledge on various subjects, access CBSE Class 10 Social Science Notes for History, Political Science, Geography, and Economics, conveniently compiled in one place. Stay curious and continue learning!
Summery of Manufacturing industries class 10th notes
The “Manufacturing Industries Class 10th Notes” emphasize the vital role of manufacturing in mass-producing goods from raw materials. It covers various industries in the secondary sector, such as steel factories, automobile production, and textile manufacturing.
The chapter highlights manufacturing’s significance in modernizing agriculture, reducing unemployment, and promoting economic growth. It explores the interdependence between agriculture and industry, the contribution of industries to the national economy, and factors affecting industrial location.
The classification of industries based on raw materials, role, capital investment, and ownership is explained. Additionally, the impact of industrial pollution on the environment and measures for its control are discussed.
Read Also:
Frequently Asked Question – FAQs: Manufacturing industries class 10th notes
Q 1. What is the best manufacturing industry?
- Reliance Industries Limited: A conglomerate with diverse interests, including petrochemicals, refining, telecommunications, retail, and more.
- Tata Group: A leading conglomerate with businesses in steel, automotive, information technology, consumer goods, and other sectors.
- Adani Group: Involved in various industries, including ports, logistics, power generation, renewable energy, and mining.
- Larsen & Toubro Limited (L&T): An engineering and construction company with significant contributions to infrastructure development.
- Mahindra & Mahindra Limited: A prominent player in the automotive industry, manufacturing cars, tractors, and other vehicles.
- Bajaj Auto Limited: A major two-wheeler and three-wheeler manufacturer, known for its motorcycles and scooters.
- ITC Limited: A diversified company with interests in cigarettes, consumer goods, hotels, and agribusiness.
- Hindustan Unilever Limited (HUL): A leading fast-moving consumer goods (FMCG) company with a wide range of products.
- Sun Pharmaceutical Industries Limited: One of the largest pharmaceutical companies in India, manufacturing generic drugs.
- Asian Paints Limited: A renowned paint manufacturer and market leader in India’s decorative paints segment.
Q 2. What are the type of manufacturing industry?
- Automobile Industry
- Pharmaceutical Industry
- Textile Industry
- Steel Industry
- Electronics Industry
- Food Processing Industry
- Chemical Industry
- Aerospace and Defense Industry
- Renewable Energy Industry
- Cement Industry and many more.
Q 3. Role of Industries in a country’s development?
Industries play a pivotal role in a country’s development by driving economic growth, creating jobs, fostering technological advancements, and generating foreign exchange earnings. They contribute to infrastructure development, skill enhancement, and regional growth, while also stimulating entrepreneurship and innovation. Industries are crucial for sustainable development and overall prosperity.
Acids Bases and Salts Class 10 Notes of NCERT Science Ch. 2
Acids Bases and Salts Class 10 Notes: A salt is created through the replacement of hydrogen ions by a metal or an ammonium ion in the precens of acid. A substance referred to as a base undergoes a reaction with an acid, resulting in the production of water and salt exclusively. Consequently, the combination of an acid and a base leads to the formation of a salt.
Household cleaning products are used to remove oil from windows and floors contain bases. Bases found in items like soaps, toothpaste, egg whites, dishwashing liquids, and household ammonia.
This article will give you a overview and quick short notes on class 10 chapter 2: Acid bases and salts.
CBSE Class 10 Science Notes Chapter 2 Acids Bases And Salts
Acids Bases and Salts Class 10 Notes
Introduction of Acid Base and Salts: Short Notes
An acid is defined as a substance that exhibits a sour taste when dissolved in water, turns blue litmus paper to red, and has the ability to neutralize bases. Conversely, a base is a substance whose aqueous solution tastes bitter, turns red litmus paper to blue, and can neutralize acids. On the other hand, salt is a neutral substance that does not have any impact on litmus paper when dissolved in an aqueous solution.
Acids Bases and Salts can be classified based on:
a) Composition: This includes elements, compounds, and mixtures.
b) State: Matter can exist in the form of solids, liquids, or gases.
c) Solubility: Matter can be categorized as suspensions, colloids, or solutions based on its ability to dissolve.
Mixtures can be further classified as homogeneous or heterogeneous, depending on the uniformity of their composition. Compounds, on the other hand, can be categorised as covalent or ionic compounds based on the types of chemical bonds present within them.
What Is an Acid, Base and Salts
An acid is characterised as any substance that contains hydrogen and has the ability to donate a proton, which is a hydrogen ion, to another substance. On the other hand, a base refers to a molecule or ion that can accept a hydrogen ion from an acid. Acidic substances are commonly recognised by their sour taste, which is a distinctive characteristic.
Ionisable and Non-Ionisable Compounds
As per chapter 2 of class 10 Ionizable compounds are substances that have the ability to undergo ionization, which is the process of forming ions by gaining or losing charged particles (usually electrons or protons) when dissolved in a solvent like water. In other words, ionizable compounds can dissociate into ions in solution.
On the other hand, non-ionizable compounds are substances that do not readily undergo ionization when dissolved in a solvent. These compounds remain predominantly in their molecular form and do not dissociate into ions.
Arrhenius’ Theory of Acids and Bases
According to the Arrhenius definition, an acid is a substance that, when dissolved in water, dissociates to produce H+ (aq) or H3O+ ions. Conversely, an Arrhenius base is a substance that, when dissolved in water, dissociates to yield OH− ions.
Examples:
Acids:
- Hydrochloric acid (HCl)
- Sulphuric acid (H2SO4)
- Nitric acid (HNO3)
Bases:
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Calcium hydroxide (Ca(OH)2)
Bronsted Lowry Theory
A Bronsted acid is defined as a substance that donates an H+ (aq) ion in a chemical reaction. Conversely, a Bronsted base is a substance that accepts an H+ (aq) ion.
Example:
In the reaction: HCl (aq) + NH3 (aq) → NH+4(aq) + Cl− (aq)
- HCl acts as a Bronsted acid because it donates an H+ (aq) ion.
- Cl− is the conjugate base of HCl because it remains after the acid donates its H+ (aq) ion.
- NH3 acts as a Bronsted base because it accepts the H+ (aq) ion from HCl.
- NH+4 is the conjugate acid of NH3 because it is formed after accepting the H+ (aq) ion.
Physical Test for Acid and Base

Two physical tests can be employed to identify an acid or a base.
a. Taste: An acid typically has a sour taste, while a base has a bitter taste. However, this method of taste is not recommended as it can be unsafe due to potential contamination or corrosiveness of acids and bases.
Example: Compounds like curd, lemon juice, orange juice, and vinegar possess a sour taste because they contain acids. Baking soda, on the other hand, has a bitter taste and serves as an example of a base.
b. Effect on Indicators by Acids and Bases: Indicators are chemical substances that undergo a change in their physical properties, such as color or odor, when in contact with an acid or a base. The following are commonly used indicators and the colors they exhibit:
a) Litmus:
- In a neutral solution: Purple
- In an acidic solution: Red
- In a basic solution: Blue
Litmus is available in the form of paper strips, with red litmus and blue litmus variants. An acid turns a moist blue litmus paper to red, while a base turns a moist red litmus paper to blue.
b) Methyl orange:
- In a neutral solution: Orange
- In an acidic solution: Red
- In a basic solution: Yellow
c) Phenolphthalein:
- In a neutral solution: Colorless
- In an acidic solution: Remains colorless
- In a basic solution: Pink
These indicators can be used to determine whether a substance is acidic or basic based on the observed color change.
Acid-Base Reactions for Class 10 Students
An acid-base neutralization reaction takes place when an acid reacts with a base. The outcome of this reaction is the formation of salt and water as the end products. In the standard approach, an acid-base neutralization reaction is represented as a double-replacement reaction.
Class 10 Reactions of Acids and Bases
a) Reaction of acids and bases with metals
Acids, in general, react with metals to produce salt and hydrogen gas. Bases, in general, do not react with metals and do not produce hydrogen gas.
Acid + active metal → salt + hydrogen + heat
2HCl + Mg → MgCl2 + H2 (↑)
Hydrochloric acid + Magnesium → Magnesium chloride + Hydrogen
Base + metal → salt + hydrogen + heat
2NaOH + Zn → Na2ZnO2 + H2 (↑)
Sodium hydroxide + Zinc → Sodium zincate + Hydrogen
A more reactive metal displaces the less reactive metal from its base.
2Na + Mg (OH) 2 → 2NaOH + Mg
Sodium + Magnesium hydroxide → Sodium hydroxide + Magnesium
b) Reaction of acids with metal carbonates and bicarbonates
Acids produce carbon dioxide, as well as metal salts and water, when they react with metal carbonates or metal bicarbonates. Sodium chloride, carbon dioxide, and water are formed when sodium carbonate interacts with hydrochloric acid. Allowing carbon dioxide gas to travel through lime water turns it milky.
Acid + metal carbonate or bicarbonate → salt + water + carbon dioxide.
2HCl + CaCO3 → CaCl2 + H2O + CO2
H2SO4 + Mg (HCO3)2 → MgSO4 + 2H2O + 2CO2
Effervescence indicates the liberation of CO2 gas.
c) Reaction of Acid with Base given in class 10
1. Reaction of metal oxides and hydroxides with acids
Metal oxides or metal hydroxides are basic in nature.
Acid + base → salt + water + heat
H2SO4 + MgO → MgSO4 + H2O
2HCl + Mg (OH) 2 → MgCl2 + 2H2O
2. Reaction of non-metal oxides with bases
Non-metal oxides are acidic in nature
Base + Nonmetal oxide → salt + water + heat
2NaOH + CO2→ Na2CO3 + H2O
3. Reaction of acids and base
A very common acid is hydrochloric acid. The reaction between strong acid, says hydrochloric acid and strong base say sodium hydroxide, forms salt and water. The complete chemical equation is shown below.
HCl (strong acid) + NaOH (strong base) → NaCl (salt) + H2O (water)
Water:
Water itself is a neutral substance and does not exhibit acidic or basic properties. However, it plays a crucial role in the dissociation of acids and bases when they are added to it. Acids and bases dissociate into their respective ions when dissolved in water, allowing them to conduct electricity.
Difference between a Base and an Alkali:
Base:
- Bases can undergo a neutralization reaction with acids.
- They are composed of metal oxides, metal hydroxides, metal carbonates, and metal bicarbonates.
- Most bases are insoluble or only slightly soluble in water.
Alkali:
- An alkali refers specifically to an aqueous solution of a base, primarily consisting of metallic hydroxides.
- Alkalis readily dissolve in water and dissociate to produce OH− ions.
- It’s important to note that while all alkalis are bases, not all bases are alkalis. The term “alkali” specifically applies to bases that are soluble in water and form alkaline solutions.
Hydronium Ion
Acids Bases and Salts Class 10 Notes
A hydronium ion is created when a hydrogen ion (H+) accepts a lone pair of electrons from the oxygen atom of a water molecule. This interaction leads to the formation of a coordinate covalent bond between the hydrogen ion and the water molecule.
Dilution: Dilution refers to the process of decreasing the concentration of a solution by adding more solvent, usually water. This addition of solvent is typically an exothermic process, releasing heat. When diluting an acid, it is important to add the acid to water gradually, and not the other way around, to prevent potential splattering or hazardous reactions.
Strength of Acids and Bases: Strong acid or base: A strong acid or base is characterized by complete dissociation in water, where all the molecules of a given amount fully separate into their respective ions, resulting in the presence of H+(aq) ions for acids and OH−(aq) ions for bases.
Weak acid or base: In contrast, a weak acid or base only partially dissociates in water, with only a few of the molecules from a given amount breaking apart to form their respective ions. This results in the presence of H+(aq) ions for acids and OH−(aq) ions for bases, but in smaller quantities compared to strong acids or bases.
pH
A universal indicator is a type of pH indicator that provides a pH range from 0 to 14, allowing it to indicate the acidity or alkalinity of a solution. It changes color depending on the pH value of the solution, providing a visual representation of its acid-base nature.
PH Scale for Acids Bases and Salts
A neutral solution, which is neither acidic nor alkaline, has a pH value of 7.
The pH of a solution can be calculated using the equation pH = -log10[H+]. In pure water, the concentrations of both hydrogen ions ([H+]) and hydroxide ions ([OH-]) are equal to 10^-7 mol/L. Therefore, the pH of pure water is 7.
The pH scale spans from 0 to 14, where values below 7 indicate an acidic solution, values above 7 indicate a basic (alkaline) solution, and a pH of 7 represents a neutral solution.
Importance of pH in Everyday Life: Class 10 Notes
1. pH sensitivity of plants and animals:
Plants and animals exhibit sensitivity to pH levels. Vital biological processes such as food digestion, enzyme functions, and hormonal activities rely on specific pH values for proper functioning.
2. pH of soil:
For optimal plant growth and cultivation, the pH of soil is ideally maintained between 6.5 and 7.0. This range provides favorable conditions for nutrient availability and absorption by plants.
3. pH in the digestive system:
The digestive process within our stomach occurs at a specific pH range of 1.5 to 4. The acidic environment, influenced by hydrochloric acid (HCl), aids in the breakdown of food. Enzymatic activities during digestion are also dependent on the pH conditions within the stomach.
4. pH in tooth decay:
Tooth decay occurs when teeth are exposed to an acidic environment with a pH of 5.5 and below. Acidic conditions promote the erosion of tooth enamel, leading to decay and cavities.
5. pH in self-defence mechanisms of animals and plants:
Both animals and plants employ acidic substances as a means of self-defense. For instance, bees and plants like nettles secrete highly acidic substances to deter threats. These secreted acidic substances possess specific pH levels that contribute to their defensive properties.
Manufacture of Acids and Bases

Acids Bases and Salts Class 10 Notes
a) Nonmetal oxide + water → acid
SO2(g) + H2O(l) → H2SO3(aq)
SO3(g) + H2O(l) → H2SO4(aq)
4NO2(g) + 2H2O(l) + O2(g) → 4HNO3(aq)
Non-metal oxides are thus referred to as acid anhydrides.
b) Hydrogen + halogen → acid
H2(g) + Cl2(g) → 2HCl(g)
HCl(g) + H2O(l) → HCl(aq)
c) Metallic salt + conc. sulphuric acid → salt + more volatile acid
2NaCl(aq) + H2SO4(aq) → Na2SO4(aq) + 2HCl(aq)
2KNO3(aq) + H2SO4(aq) → K2SO4(aq) + 2HNO3(aq)
d) Metal + oxygen → metallic oxide (base)
4Na(s) + O2(g) → 2Na2O(s)
2Mg(s) + O2(g) → 2MgO(s)
e) Metal + water → base or alkali + hydrogen
Zn(s) + H2O(steam) → ZnO(s)+ H2(g)
f) Few metallic oxides + water → alkali
Na2O(s) + H2O(l) → 2NaOH(aq)
g) Ammonia + water → ammonium hydroxide
NH3(g) + H2O(l) → NH4OH(aq)
Salts
Salt is a general term used to refer to a class of compounds composed of ions held together by ionic bonds. In chemistry, salt specifically refers to a compound formed when the hydrogen ions of an acid are replaced by metal ions or ammonium ions. This process is known as neutralization. Salts are typically solid crystals with high melting points and can be either soluble or insoluble in water.
Common examples of salts include sodium chloride (table salt), calcium carbonate, potassium nitrate, and magnesium sulfate. Salts play essential roles in various biological, chemical, and industrial processes. They are widely used in cooking, food preservation, manufacturing, and as nutrients for plants and animals.
Types of Salts
Common Salt:
Sodium Chloride (NaCl) is commonly known as common salt because it is extensively used worldwide for culinary purposes.
Family of Salts:
Salts that share the same cation or anion belong to the same family. For instance, NaCl, KCl, and LiCl are members of the same salt family.
pH of Salts:
A salt resulting from the combination of a strong acid and a strong base will be neutral in nature, indicated by a pH value of around 7.
A salt formed from a weak acid and a strong base will be basic, with a pH value greater than 7.
Conversely, a salt produced from a strong acid and a weak base will be acidic, with a pH value below 7.
The pH of a salt derived from a weak acid and a weak base can be determined by conducting a pH test.
Chemicals from Common Salt
Sodium chloride is a widely recognized common salt, represented by its molecular formula NaCl. It is an essential component of our meals, serving as both a flavor enhancer and a preservative in our food. From common salt, we can derive the following four compounds:
1. Sodium hydroxide, also known as lye or caustic soda.
2. Baking soda, also referred to as sodium hydrogen carbonate or sodium bicarbonate.
3. Washing soda, scientifically known as sodium carbonate decahydrate.
4. Bleaching powder, which is calcium hypochlorite.
Preparation of Sodium Hydroxide:
Sodium hydroxide (NaOH) is a strong base with wide applicability. Caution must be exercised when preparing a sodium hydroxide solution by dissolving it in water, as the reaction is highly exothermic and can generate significant heat. It is crucial to ensure safety during this process to prevent splattering or boiling. The following steps outline a safe procedure for manufacturing a sodium hydroxide solution, along with recipes for different NaOH strengths:
Chemical formula: NaOH
Also known as: Caustic soda
Bleaching Powder:
Bleaching powder, which is soluble in water, is widely employed as a bleaching agent in textile industries. It serves as an oxidising agent and a disinfectant in various industrial applications. Bleaching powder is synthesised by reacting chlorine gas with dry slaked lime, represented as Ca(OH)2.
Chemical formula: Ca(OCl)Cl or CaOCl2
Preparation: Ca(OH)2(aq) + Cl2(g) → CaOCl2(aq) + H2O(l)
Upon interaction with water, bleaching powder releases chlorine, which is responsible for its bleaching action.
Uses of Bleaching Powder:
1. It is used for bleaching soiled clothes in laundry and as a bleaching agent for cotton and linen in the textile industry.
2. As a strong oxidizing agent, it finds applications in various industries.
3. It is used as a disinfectant for purifying water, making it potable.
Baking Soda:
Sodium bicarbonate, commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It consists of a sodium cation (Na+) and a bicarbonate anion (HCO3). Sodium bicarbonate exists as a white, crystalline substance, often found in the form of a fine powder. It has a slightly salty and alkaline taste, similar to washing soda (sodium carbonate).
Chemical name: Sodium hydrogen carbonate
Chemical formula: NaHCO3
Preparation (Solvay process):
a. Limestone is heated: CaCO3 → CaO + CO2
b. CO2 is passed through a concentrated solution of sodium chloride and ammonia:
NaCl(aq) + NH3(g) + CO2(g) + H2O(l) → NaHCO3(aq) + NH4Cl(aq)
Uses:
1. It reduces acidity in the stomach and acts as an antacid for treating stomach upset and indigestion.
2. It is utilized as a water softener in the process of washing.
3. Sodium bicarbonate finds applications in various household cleaning products.
Washing Soda:
Washing soda is another name for sodium carbonate decahydrate, which is a chemical compound with the formula Na2CO3·10H2O. It is commonly used in the glass, soap, and paper industries. Washing soda also functions as a water softener and is utilized as a domestic cleaner.
Chemical name: Sodium carbonate decahydrate
Chemical formula: Na2CO3·10H2O
Preparation (Solvay process):
a. Limestone is heated: CaCO3 → CaO + CO2
b. CO2 is passed through a concentrated solution of sodium chloride and ammonia:
NaCl(aq) + NH3(g) + CO2(g) + H2O(l) → NaHCO3(aq) + NH4Cl(aq)
Uses:
1. It finds applications in the glass, soap, and paper industries.
2. Washing soda is utilized as a water softener to counteract hard water.
3. It serves as a domestic cleaner for various cleaning purposes.
Crystals of Salts:
Certain salts have the ability to form crystals by combining with a specific proportion of water. The water molecules that join with the salt in a crystal are referred to as water of crystallization. Crystallization is the process by which a solid substance arranges its atoms or molecules into a highly organized structure called a crystal. This process can occur through precipitation from a solution, freezing, or, in rare cases, direct deposition from a gas.
Examples:
Table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes are common examples of materials that can form crystals. Many gemstones, such as quartz and diamond, also exhibit a crystal structure.
Plaster of Paris
Plaster of Paris is a widely used chemical compound that finds extensive application in sculpting materials and gauze bandages. It is a white powdery substance composed of hydrated calcium sulfate, which is typically obtained by calcining gypsum. In other words, Plaster of Paris is manufactured by heating gypsum at a high temperature.
Plaster of Paris is chemically expressed as CaSO4·1/2H2O, indicating that it contains one molecule of water of crystallization.
Uses:
Plaster of Paris, also known as gypsum plaster, is employed for creating casts to aid in the healing of fractures.
Summery: Acids Bases and Salts Class 10 Notes
The “Acids Bases and Salts Class 10 Notes” provide comprehensive information on the properties and reactions of acids, bases, and salts.
The notes of Class 10’s chapter 2 “Acid bases and salts” cover various aspects, including the definition of acids and bases, their characteristics, pH scale, neutralization reactions, and the preparation of chemicals from common salt.
Additionally, the summary highlights the importance of pH in everyday life, the differences between bases and alkalis, and the types of salts.
The notes also elaborate on the formation of crystals in certain salts and the uses of Plaster of Paris. This detailed summary serves as a valuable resource for students studying these topics in their class 10 curriculum.
Read Also:
- Class 10 Notes for Science NCERT
- Life Process Notes Class 10 NCERT Science Chapter 5
- Control and Coordination Notes Class 10: NCERT Science Ch. 6
- Notes on Magnetic Effect of Electric Current Class 10 NCERT
- Notes of Our Environment Class 10: NCERT Science Chapter 13
- Class 10th How Do Organisms Reproduce Notes: Science Ch. 7
- Class 10th Heredity and Evolution Notes: NCERT Science Ch. 8
- Chemical Reaction and Equation Notes Class 10 NCERT Sci. Ch.1
- Acids Bases and Salts Class 10 Notes of NCERT Science Ch. 2
- Class 10th Chapter 11 Science Notes for NCERT Students
- Human Eye and the Colourful World Notes Chapter 10 Science
Frequently Asked Question – FAQ on Acids Bases and Salts Class 10 Notes
Q 1. In the absence of water, acids do not exhibit their characteristic acidic behaviour.
Ans: Acids require water to display their typical acidic behavior due to a process known as ionization or dissociation. Acids release positively charged hydrogen ions (H+) into the water. These hydrogen ions are responsible for the acidic properties of the solution, as they can donate protons to other substances, leading to various acid-base reactions.
In the absence of water, there is no medium for the acids to dissociate into ions. Therefore, without water, acids cannot release hydrogen ions, and as a result, they do not exhibit their usual acidic characteristics such as the ability to lower pH, react with bases, or corrode certain materials.
Q 2. What are the three properties of Acids bases and salts?
Ans: The three main properties of acids are:
1. Sour taste: Many acids have a sour taste. Tasting acids is not recommended due to their potential for high corrosiveness and harm to health.
2. Acidic pH: Acids typically have a pH value lower than 7. The pH scale ranges from 0 to 14, considering 7 as neutral, and classifying values below 7 as acidic and values above 7 as alkaline (basic). The lower the pH value, the stronger the acid.
3. Acid-base reactions: Acids have the ability to react with bases to form salts and water in a process known as neutralization. In these reactions, acids donate hydrogen ions (H+) to bases, which then accept the ions to form water. This neutralization reaction results in the reduction of acidic or basic properties, respectively.
It’s important to handle acids with care, as some can be highly corrosive and pose significant risks to health and safety. When handling acids, it’s essential to prioritize safety and follow proper laboratory protocols.
Q 3. Write the pH range for acids and alkalis?
Ans: The pH scale ranges from 0 to 14, where:
- pH values below 7 indicate acidity
- pH 7 is neutral
- pH values above 7 indicate alkalinity (also known as basic).
Q 4. What is the chemical name of gypsum?
Ans: The chemical name of gypsum is “calcium sulfate dihydrate.” The gypsum (CaSO4·2H2O) made up of calcium ions (Ca2+), sulfate ions (SO42-), and two water molecules (H2O) in each unit.
Q 5. What is the common name of the compound CaOCl2?
Ans: The common name of the compound CaOCl2 is “bleaching powder” or “chlorinated lime.” It is a white or pale grayish powder with a strong smell of chlorine.
Nationalism in India Class 10 NCERT History Chapter 2 Notes
Nationalism in India Class 10: The article delves into Congress’ efforts to foster the national movement. It also include the diverse participation of social groups, and the profound impact of nationalism on people’s minds. The outbreak of the First World War in 1919 had global ramifications, fueling India’s struggle for independence. This event ignited the spirit of nationalism, and the Non-Cooperation Movement, pivotal elements in India’s fight for freedom.
Nationalism stands as the largest mass movement of modern times, uniting millions across classes and ideologies in political action. This result in ultimately leading to the downfall of the colonial empire. If you’re seeking Nationalism in India class 10 notes, we’ve covered all the significant aspects here. Keep reading to explore further!
Visit history of class 10th for all chapter’s solution.
Nationalism in India Class 10 Notes

Class 10’s “Nationalism in India” chapter explores how a sense of common belonging fosters nationalism. It explores how their struggles bind them together, fostering a shared identity. The chapter also examines nationalism in various parts of the world, including Germany, France, Britain, Vietnam, India, and more. Below are the comprehensive notes on Nationalism in India Class 10 for your reference:
Nationalism in India Class 10 Notes
World War 1 (1914 -1918)
World War 1, had a profound impact on the world, and India was no exception. While India was a colony under British rule during this period, its involvement in the war was significant. The war brought forth numerous changes and challenges for the Indian subcontinent, shaping its political, social, and economic landscape.
As a part of the British Empire, India was automatically drawn into World War 1. The British government utilized Indian resources, manpower, and finances to support the war effort. Around 1.5 million Indian soldiers were recruited to serve on various battlefronts across the globe, making it the largest voluntary army of that time.
![]()
Indian soldiers exhibited tremendous bravery and dedication, playing a vital role in several major battles, including the Battle of Neuve Chapelle, Battle of Ypres, and the Mesopotamian campaign. Their sacrifices were significant, with over 74,000 Indian soldiers losing their lives and many more injured.
The war also had a significant impact on the home front in India. The British government imposed heavy taxes and requisitioned resources, leading to economic hardships and food shortages. Additionally, the war heightened political consciousness among Indians, and demands for self-rule and independence intensified.
India’s involvement in World War 1 became a turning point in the Indian nationalist movement. The war’s promise of democracy and self-determination for all nations highlighted the hypocrisy of British colonial rule. This led to the emergence of more assertive demands for independence and galvanized leaders like Mahatma Gandhi, who initiated nonviolent movements to demand self-rule.
The aftermath of World War 1 had a lasting impact on India’s political landscape. The disillusionment with British promises of self-determination fueled the demand for complete independence, leading to the Non-Cooperation Movement and the Civil Disobedience Movement in the subsequent years.
Satyagraha
As per the NCERT 10th class history book, Satyagraha is derived from the words “Satya” (truth) and “Agraha” (holding on to truth). Mahatma Gandhi launched three regional Satyagrahas, as follows:

1. Champaran: In 1916, Gandhi initiated the first Satyagraha in Champaran, Bihar, encouraging people to protest against the oppressive teenkathiya system (plantation system).
2. Kheda: In 1917, Gandhi organized a Satyagraha in the Kheda district of Gujarat, supporting poor peasants demanding relief from high revenue collection.
3. Ahmedabad: In 1918, a Satyagraha was organized for cotton mill workers in Ahmedabad.
Gandhi Ji based these movements on two principles: truth and non-violence. He believed that pure intentions could render force unnecessary in Satyagraha. Being a practitioner of non-violence, he emphasized that truth and non-violence (Dharma) were potent tools to win any battle. Throughout India’s nationalist struggle, Gandhi upheld this Dharma, as detailed in the 10th class history books.
Rowlatt Act of 1919
Following the regional Satyagrahas, Gandhi decided to launch a nationwide movement. However, in response, the Imperial legislative council passed the Rowlatt Act in 1919, granting the British significant power to suppress political activities and detain political prisoners without trial for two years.

In retaliation, Gandhi initiated a hartal (strike) on 6th April, marking the beginning of India’s workers’ national movement. This led to widespread disruptions, with closed shops, disrupted railways, and telegraph lines. In certain areas, martial law was imposed, and local leaders were arrested, while Gandhi himself was barred from entering Delhi.
On 13th April, during the Baisakhi festival in Amritsar, unaware of martial law, a gathering of people witnessed a tragic incident. General Dyer opened fire upon them, resulting in a large number of casualties to instill fear and awe. This dark episode is etched in the history of India’s nationalist movement.
As mentioned in the chapter “Nationalism in India Class 10,” Gandhi staunchly upheld non-violence. In response to the Jallianwala Bagh massacre, he immediately called off the hartal, adhering to his principles of peaceful resistance.
Nationalism in India Class 10 Notes
Non Cooperation and Khilafat
According to the Nationalism in India Class 10 Notes, The Non-Cooperation Movement was launched by Mahatma Gandhi in 1920. It was a nationwide campaign aimed at resisting British rule through nonviolent means.
The movement called for Indians to boycott British institutions, schools, colleges, courts, and civil services, and to withdraw support from the British government. People were urged to adopt swadeshi (buying Indian-made goods) and spin their own cloth using the charkha (spinning wheel) to promote self-reliance.
The Non-Cooperation Movement saw widespread participation from people across different sections of society. It marked a turning point in India’s freedom struggle and brought millions of Indians together in a collective effort for independence.
However, the movement was called off in 1922 after a violent incident at Chauri Chaura, where protestors clashed with police, leading to casualties. Mahatma Gandhi believed that nonviolence was the essence of the movement, and he called it off to avoid further escalation of violence.
Non-Cooperation Movement in Stages
The Non-Cooperation Movement, launched by Mahatma Gandhi in 1920, unfolded in several stages, each marked by distinct activities and events. Here are the key stages of the Non-Cooperation Movement:

1. Launch and Mass Participation (1920):
The Non-Cooperation Movement was officially launched on August 1, 1920, during the special session of the Indian National Congress in Calcutta (now Kolkata). Mahatma Gandhi called for nonviolent non-cooperation with the British government as a means to resist colonial rule. The movement aimed to unite people from all sections of society in a nonviolent struggle for independence.
2. Boycott of Institutions (1920-1921):
During the initial phase, Indians were urged to boycott British institutions, including schools, colleges, and law courts. Lawyers and students withdrew from these institutions, leading to a significant impact on the British administration.
3. Surrender of Titles and Resignation from Government Posts (1920-1922):
Many Indians, especially leaders and government officials, resigned from their posts and returned their titles to the British government. This act symbolized their rejection of British authority and demonstrated their commitment to the cause of independence.
4. Boycott of Foreign Goods and Promotion of Swadeshi (1920-1922):
The Non-Cooperation Movement encouraged Indians to boycott foreign goods and promote the use of swadeshi (Indian-made) products. People began spinning their own cloth using the charkha and boycotted British-manufactured textiles, leading to a surge in the use of Indian textiles.
5. Mass Demonstrations and Protests (1920-1922):
Throughout the movement, mass demonstrations, processions, and public meetings were held across India. These gatherings became platforms for expressing discontent against British rule and demanding self-governance.
6. Suspension of Civil Disobedience (1922):
In 1922, the Non-Cooperation Movement took an unfortunate turn when violent clashes occurred in Chauri Chaura, Uttar Pradesh. A mob attacked a police station, resulting in the death of several police officers. Mahatma Gandhi, who always emphasized nonviolence, was deeply disappointed by the incident. In response, he suspended the movement, believing that nonviolence was essential for its success.
The Non-Cooperation Movement was a pivotal phase in India’s freedom struggle, bringing millions of people together in a nonviolent resistance against British rule. While it was suspended in 1922, its impact continued to inspire future movements, leading India closer to achieving independence in 1947.
Civil Disobedience Movement
In the Nationalism in India Class 10, a significant milestone highlighted is the Civil Disobedience Movement, characterized by the following key features:
1. Advancement from Non-Cooperation: The movement went beyond Non-Cooperation, urging people not only to abstain from cooperation but also to defy colonial laws.
2. Boycott of Foreign Cloth and Picketing of Liquor Shops: People actively boycotted foreign cloth and engaged in picketing liquor shops as acts of protest.
3. Peasants’ Refusal to Pay Revenue and Chowkidar Taxes: Peasants were encouraged to resist paying revenue and chowkidar taxes as a form of civil disobedience.
4. Refusal to Attend English Medium Schools, Colleges, Offices, and Courts: Students, village officials, and lawyers were urged not to attend English medium institutions and colonial offices and courts.
The Civil Disobedience Movement marked a bold step in the struggle for India’s independence, as people engaged in nonviolent resistance and non-cooperation with oppressive colonial laws and institutions.
Beginning of Civil Disobedience
In 1930, Gandhiji initiated the Dandi march to protest against the monopoly rights on salt manufacturing. Together with his supporters, he openly defied the salt law by making salt themselves. This marked the beginning of a civil disobedience movement, aiming to mobilize people from all backgrounds and classes to unite against the oppressive rule of the British Government. The Dandi march became a significant event among other national movements in India.
However, in the present, we observe threats to our society’s framework from negative elements seeking to divide our nation along lines of caste, creed, religion, and class. As responsible citizens, it becomes our duty to safeguard the hard-fought freedom and unity of our nation.
Participants in the Civil Disobedience Movement
The civil disobedience movement witnessed the active involvement of various social groups expressing their discontent with colonial policies. Rich peasant communities joined to combat high revenue policies, while the Indian business classes reacted against restrictive colonial regulations on their businesses. However, the poor peasant and industrial working classes did not participate in significant numbers. One notable aspect of the movement was the remarkable participation of women in picketing and protests, highlighting their crucial role in the struggle for independence.
Limits of the Civil Disobedience Movement
The impact of the civil disobedience movement was relatively limited. Dalits showed minimal participation in the movement, as they focused on seeking political empowerment through increased representation and demanded separate electorates. B.R. Ambedkar led this movement, culminating in the Poona Pact of 1932, despite opposition from Gandhi.
Muslim communities also felt somewhat disconnected from the movement, particularly after the Khilafat movement lost its significance. The apprehension of potential Hindu dominance played a significant role in the minority Muslim communities’ reluctance to respond actively to the movement.
Salt March
The Salt March, also known as the Dandi March, was a significant event during India’s struggle for independence from British colonial rule. It was a nonviolent protest led by Mahatma Gandhi in 1930 to oppose the British-imposed salt tax, which heavily burdened the Indian population and symbolized British economic exploitation.
The Salt March began on March 12, 1930, when Mahatma Gandhi, along with 78 volunteers, embarked on a 240-mile journey from his ashram in Sabarmati, Gujarat, to the coastal town of Dandi. The march lasted for 24 days, covering about 10 miles per day. Thousands of people joined Gandhi along the way, making it a powerful mass movement.
During the march, the participants followed strict principles of nonviolence and civil disobedience. They picked up salt along the seashore, openly defying the British salt laws, which granted the British a monopoly on salt production and taxed the production and sale of salt.
The Salt March garnered widespread attention and support from Indians across the country. It inspired people to boycott British salt and produce their own salt, marking a symbol of defiance against British authority and economic exploitation. This act of civil disobedience demonstrated the power of nonviolent resistance and the unity of the Indian masses in their quest for independence.
The Salt March, along with other civil disobedience movements, put immense pressure on the British government and led to negotiations with Mahatma Gandhi. Ultimately, it played a crucial role in shaping India’s freedom struggle and strengthening the nationalist movement. The Salt March remains a remarkable example of the effectiveness of nonviolent resistance and Gandhi’s leadership in India’s journey towards independence.
Gandhi Irwin Pact
To address the escalating tensions and widespread unrest, Mahatma Gandhi and Lord Irwin engaged in negotiations. The talks culminated in the Gandhi-Irwin Pact, which had the following key provisions:
- Suspension of Civil Disobedience: Gandhi agreed to suspend the Civil Disobedience Movement, calling off the mass protests and non-cooperation activities.
- Participation in Round Table Conference: As part of the pact, the British government agreed to invite Indian representatives to attend the Second Round Table Conference in London. The conference aimed to discuss constitutional reforms and the future of India’s political structure.
- Release of Political Prisoners: In return for suspending the movement, the British government agreed to release political prisoners who were arrested during the Civil Disobedience Movement.
The signing of the Gandhi-Irwin Pact marked a temporary truce between the Indian National Congress, led by Gandhi, and the British government. It provided an opportunity for negotiations and discussions on India’s future political setup. However, the Round Table Conferences did not lead to substantial outcomes, as there were disagreements between the Indian leaders and the British authorities.
Despite the temporary setback in achieving complete independence, the Gandhi-Irwin Pact played a crucial role in providing a platform for discussions and raising international awareness about India’s struggle for freedom. It highlighted Gandhi’s commitment to nonviolence and peaceful negotiations in the pursuit of India’s independence. The pact also paved the way for future negotiations and agreements that ultimately led to India’s independence in 1947.
Development of a Sense of Collective Belonging
The development of a sense of collective belonging played a crucial role in the growth of nationalism. Various cultural processes, including the use of symbols, folklore, and Indian history, contributed to spreading ideas of nationalism. However, some aspects, such as the glorification of a Hindu past, alienated people from other communities.
Read Also:
- The Making of A Global World Notes: NCERT Hist. Class 10 Ch. 3
- Print Culture and The Modern World
- Notes of The Rise of Nationalism in Europe
- The Age of Industrialisation
Frequently Asked Questions – FAQ
Q 1. What is nationalism in India explain in detail?
Nationalism in India evolved as a powerful force during the struggle for independence against British colonial rule. Factors such as historical context, socio-religious reforms, education, print media, and visionary leaders contributed to its development.
The Indian National Congress advocated for representation, while the Swadeshi Movement promoted Indian goods. Mahatma Gandhi led the Non-Cooperation and Civil Disobedience Movements. Post-independence, challenges included national consolidation and development.
India’s nationalism upholds principles of unity in diversity, promoting social and economic progress. Rooted in peace, tolerance, and global cooperation, Indian nationalism reflects a collective journey toward freedom, dignity, and a pluralistic future.
Q 2. What was the reason for nationalism in India?
Nationalism in India emerged as a response to British colonial rule, fueled by oppressive policies and economic exploitation. India’s rich cultural heritage and historical unity fostered a sense of common identity and pride.
Socio-religious reform movements, access to education, and print media further strengthened nationalist sentiments. Political organizations like the Indian National Congress provided platforms for demanding self-rule.
Influenced by global nationalist movements, India’s visionary leaders, including Mahatma Gandhi, Jawaharlal Nehru, and Subhash Chandra Bose, united people in their pursuit of independence, ultimately leading to India’s liberation in 1947.
Q 3. What is the main cause of nationalism?
Nationalism stems from a shared identity and pride within a distinct community or nation. Causes include historical unity, cultural revival, oppression, and common struggles.
External domination or colonization can trigger a desire for sovereignty. Efforts to preserve cultural traditions strengthen national identity. Political representation demands and economic disparities may drive nationalist movements.
Modern media spread nationalist ideas. Defined geographical boundaries contribute to territorial identity. Visionary leaders play a key role in inspiring nationalist goals. Nationalism arises from historical, cultural, socio-economic, and political factors that strengthen a sense of shared identity and aspiration for a unified nation.