Blog
Molecular Weight Of Potassium
Molecular Weight Of Potassium: Potassium is a chemical element found in the alkali metal group of the periodic table, denoted by the symbol K. In the field of chemistry, understanding the molecular weight of potassium is essential as it plays a significant role in various chemical reactions and scientific calculations.
This article will delve into the concept of molecular weight, explain how it relates to potassium, and discuss the significance of this knowledge in both theoretical and practical contexts.

Molecular Weight Of Potassium
Molecular Weight: An Overview
The mole weight, also known as molar mass, is a fundamental concept in chemistry. It represents the mass of one mole of a particular substance and is typically expressed in grams per mole (g/mol). A mole is a unit used to quantify the amount of a substance and contains approximately 6.022 x 10^23 entities, known as Avogadro’s number. Molecular weight is numerically equivalent to the atomic or mole weight of the substance.
The Molecular Weight of Potassium (K)
Potassium exists naturally in the form of various isotopes, but the most abundant and stable isotope is potassium-39 (^39K). To calculate the mole weight of potassium, we consider this dominant isotope.
The atomic mass of potassium-39 is approximately 38.963 g/mol. Therefore, the mole weight of potassium (K) is:
mole Weight of Potassium (K) = Atomic Mass of ^39K ≈ 38.963 g/mol
Significance of Molecular Weight of Potassium
Understanding the mole weight of potassium holds great significance in the field of chemistry and beyond:
1. Chemical Reactions:
The mole weight of potassium plays a pivotal role in chemical reactions that incorporate potassium compounds. It helps determine the stoichiometry, allowing chemists to balance equations and calculate reactant and product quantities accurately.
2. Analytical Chemistry:
In analytical chemistry, the knowledge of mole weight aids in the determination of concentrations, particularly in techniques like titration and gravimetric analysis.
3. Industrial Applications:
Potassium and its compounds have various industrial applications, such as in fertilizers, detergents, and glass production. Understanding mole weight is vital for precise formulation and quality control.
4. Medicine and Healthcare:
In the field of medicine, potassium is essential for numerous physiological processes in the human body. Knowing its molecular weight is essential for medication formulations and dosages.
5. Environmental Sciences:
mole weight plays a role in environmental studies, such as analyzing soil and water composition, where potassium content can impact ecosystem health.
6. Agriculture:
Potassium is a vital nutrient for plant growth. Understanding its mole weight is critical in formulating fertilizers and determining optimal application rates for crop production.
Conclusion
The mole weight of potassium (K) is a fundamental concept in chemistry with broad applications across various scientific disciplines and industries. It enables precise calculations, facilitates the formulation of chemicals and medications, and plays a vital role in understanding natural processes and environmental factors. By comprehending the mole weight of potassium, scientists, chemists, and professionals can make informed decisions and contributions to diverse fields, from agriculture to healthcare, ultimately benefiting society and advancing our understanding of the natural world.
Read More
- Molecular Weight Of O2
- Calcium Carbonate Molar Mass
- Molar Mass Of Ammonia
- Molecular Mass Of Acetic Acid
- Molecular Weight Of Calcium Carbonate
Frequently Asked Questions (FAQs) Molecular Weight Of Potassium
1. What is the molecular weight of potassium (K)?
The mole weight of potassium, denoted as K, is approximately 38.963 grams per mole (g/mol). This value is based on the most abundant and stable isotope of potassium, which is potassium-39 (^39K).
2. Why is the molecular weight of potassium important in chemistry?
The mole weight of potassium is crucial in chemistry because it helps in stoichiometry, which involves balancing chemical equations and determining the quantities of potassium compounds involved in chemical reactions.
3. How is the molecular weight of potassium calculated?
The mole weight of potassium is calculated by considering the atomic mass of its most abundant isotope, potassium-39 (^39K), which is approximately 38.963 g/mol.
4. What is the significance of knowing the molecular weight of potassium in analytical chemistry?
In analytical chemistry, the mole weight of potassium is important for accurately etermining concentrations of potassium compounds in various samples, such as in titration and gravimetric analysis.
4. Are there practical applications of the molecular weight of potassium in industries?
Yes, potassium and its compounds are used in various industries, including agriculture (fertilizers), detergents, glass production, and metallurgy. Knowing the molecular weight is vital for formulation and quality control in these applications.
AC Generator Class 12th
AC Generator Class 12th: In the realm of Class 12th Physics, students delve into various complex topics, and one of the intriguing subjects covered is the Alternating Current (AC) Generator.
AC generators, also known as alternators, are crucial devices that play a significant role in generating electrical energy in our everyday lives. In this article, we will explore the fundamentals of AC generators, their working principles, components, and practical applications relevant to Class 12th Physics.

AC Generator Class 12th
Understanding the AC Generator
1. Working Principle
An AC generator is a device that converts mechanical energy into electrical energy through the process of electromagnetic induction. It operates on the fundamental principle discovered by Michael Faraday, which states that when a magnetic field is moved relative to a conductor (or vice versa), it induces an electromotive force (EMF) or voltage in the conductor. This induced voltage creates an alternating current when the conductor forms a closed loop.
2. Components of an AC Generator
a. Rotor (Armature)
- The rotor is the part of the generator that rotates within a magnetic field. It typically consists of a coil of wire wound around an iron core.
b. Stator
- The stator is the stationary part of the generator that generates a magnetic field. It usually includes a set of magnets or electromagnetic coils.
c. Slip Rings and Brushes
- Slip rings and brushes are used to collect the generated electricity from the rotating rotor and transfer it to an external circuit.
3. Generation of Alternating Current
As the rotor rotates within the magnetic field generated by the stator, the relative motion between the two creates a constantly changing magnetic flux through the coil of wire on the rotor. This changing magnetic flux induces an EMF, causing an alternating current to flow in the coil. The resulting AC output is characterized by a continuously reversing direction of current flow, creating a sinusoidal waveform.
Practical Applications
AC generators find wide-ranging applications in our daily lives and various industries:
1. Power Generation
- AC generators are the backbone of electrical power generation in power plants. They convert mechanical energy, often from turbines or engines, into electrical energy for distribution.
2. Electric Vehicles (EVs)
- Electric vehicles, including hybrid and fully electric cars, employ AC generators (alternators) to charge their batteries and power various electrical systems.
3. Portable Generators
- Portable AC generators are used in remote areas, construction sites, and during power outages to provide temporary electrical power.
4. Renewable Energy
- Wind turbines and hydroelectric generators often use AC generators to convert mechanical energy from wind or water into electricity.
5. Laboratories and Experiments
- In educational settings and scientific research, AC generators are used for experiments to study electrical phenomena and principles of electromagnetic induction.
Key Concepts to Remember
To excel in your Class 12th Physics studies and master the topic of AC generators, keep these key concepts in mind:
- Electromagnetic Induction: AC generators rely on Faraday’s law of electromagnetic induction, which states that a changing magnetic field induces an electromotive force (EMF) in a conductor.
- Rotor and Stator: Understand the roles of the rotor (armature) and stator in an AC generator. The rotor rotates within the magnetic field, inducing an EMF in the coils, while the stator generates a stationary magnetic field.
- Slip Rings and Brushes: These components are essential for transferring the generated electricity from the rotor to an external circuit. They allow continuous electrical contact without interrupting the rotor’s rotation.
- Alternating Current (AC): AC generators produce alternating current, characterized by its continuously changing direction. This alternating current is essential for transmitting electrical energy efficiently over long distances.
- Practical Applications: Recognize the diverse applications of AC generators, from electricity generation in power plants to charging electric vehicles and providing backup power during outages.
- Renewable Energy: Understand how AC generators are integral to renewable energy sources like wind turbines and hydroelectric generators, contributing to sustainable power generation.
- Scientific Experiments: Appreciate the role of AC generators in educational and laboratory settings, where they are used to conduct experiments and study electrical phenomena.
Beyond the Classroom
Beyond the classroom, students interested in AC generators and electrical engineering can explore various resources and opportunities:
- Online Simulations: Interactive simulations and virtual labs can provide a hands-on understanding of how AC generators work and allow students to experiment with different parameters.
- Engineering Competitions: Participating in engineering or science competitions can be an exciting way to apply knowledge and gain practical experience related to AC generators.
- Internships and Projects: Students with a strong interest in electrical engineering may consider internships or personal projects related to generator technology and electrical power systems.
- Further Education: Pursuing higher education in electrical engineering or a related field opens doors to in-depth study of AC generators and advanced topics in electrical power generation.
Conclusion
Understanding the principles and applications of AC generators is vital for Class 12th Physics students. These devices are not only integral to our electricity generation but also have widespread applications in modern technology and industry. By comprehending the working principles and components of AC generators, students gain valuable insights into the world of electrical engineering and energy production.
Read More
- Ethylene Glycol Molar Mass
- Organisms And Population Class 12
- Difference Between Capacitor And Inductor
- Van De Graaff Generator
- Dielectric Polarization In Polar And Nonpolar Material And Dielectric Constant
Frequently Asked Questions (FAQs) AC Generator Class 12th
1. What is an AC generator, and how does it work?
An AC generator, also known as an alternator, is a device that converts mechanical energy into electrical energy through the process of electromagnetic induction. It operates based on Faraday’s law, where a changing magnetic field induces an electromotive force (EMF) in a conductor, resulting in the generation of alternating current (AC).
2. What are the key components of an AC generator?
An AC generator typically consists of a rotor (armature), a stator, slip rings, and brushes. The rotor rotates within a magnetic field (stator), inducing an EMF in the coils. Slip rings and brushes are used to collect and transfer the generated electricity.
3. What is the role of slip rings and brushes in an AC generator?
Slip rings and brushes are crucial for maintaining continuous electrical contact between the rotating rotor and the external circuit. They enable the transfer of electrical energy without interrupting the rotor’s rotation.
4. What type of current does an AC generator produce?
An AC generator produces alternating current (AC). AC is characterized by its constantly changing direction of current flow, which is essential for efficient transmission of electrical energy over long distances.
5. How does an AC generator relate to the study of electromagnetic induction?
AC generators are practical examples of electromagnetic induction, a phenomenon discovered by Michael Faraday. The relative motion between a magnetic field and a conductor in an AC generator leads to the induction of an electromotive force (EMF), which is the basis of electrical energy generation.
Molecular Weight Of O2
Molecular Weight Of O2: Oxygen is a vital element for life on Earth, and it plays a crucial role in various chemical reactions and processes. In the realm of chemistry, understanding the mole weight of oxygen, specifically as O2 (diatomic oxygen), is fundamental.
In this article, we will explore what mole weight is, how it relates to O2, and its significance in both theoretical and practical contexts.

Molecular Weight Of O2
What Is Molecular Weight?
Mole weight, also known as molar mass, is a fundamental concept in chemistry. It represents the mass of one mole of a particular substance and is typically expressed in grams per mole (g/mol). A mole is a unit used to quantify the amount of a substance and contains approximately 6.022 x 10^23 entities, known as Avogadro’s number. Molecular weight is numerically equivalent to the atomic or molecular weight of the substance.
Understanding O2 (Diatomic Oxygen)
Oxygen, in its molecular form, exists as a diatomic molecule, O2. This means that each molecule of O2 consists of two oxygen (O) atoms bonded together. To calculate the molecular weight of O2, we need to sum the atomic masses of these two oxygen atoms.
Oxygen (O) has an atomic mass of approximately 16.00 g/mol. Therefore, to find the mole weight of O2 (diatomic oxygen), we simply add the atomic masses of the two oxygen atoms:
Mole Weight of O2 = 2 x Atomic Mass of O
Mole Weight of O2 = 2 x 16.00 g/mol = 32.00 g/mol
So, the mole weight of O2 (diatomic oxygen) is approximately 32.00 g/mol.
Significance of Molecular Weight of O2
- Chemical Reactions: Understanding the molecular weight of O2 is essential in various chemical reactions where oxygen is involved as a reactant or product. It helps in stoichiometry, which is the study of the quantitative relationships between reactants and products in chemical reactions.
- Gas Laws: In the field of gas laws, the mole weight of O2 is crucial for determining the behavior of oxygen gas under different conditions of temperature and pressure. It contributes to calculations involving the ideal gas law and other gas-related concepts.
- Environmental Sciences: Mole weight plays a role in environmental sciences, especially in the measurement of air quality and the composition of Earth’s atmosphere, where O2 is a significant component.
- Industrial Applications: In industrial processes, the mole weight of O2 is important for precise calculations and control of reactions involving oxygen, such as combustion processes in power generation and metal smelting.
Conclusion
The mole weight of O2 (diatomic oxygen) is a fundamental concept in chemistry that holds significance in a wide range of scientific and practical applications. It aids in understanding chemical reactions, gas behaviors, environmental studies, and industrial processes. By grasping this concept, scientists and professionals can make informed decisions and contributions in various fields, furthering our understanding of oxygen’s role in the natural world and human endeavors.
Read More
- Calcium Carbonate Molar Mass
- Molar Mass Of Ammonia
- Molecular Mass Of Acetic Acid
- Molecular Weight Of Calcium Carbonate
- Molecular Weight Of Sodium Hydroxide
Frequently Asked Questions (FAQs) Molecular Weight Of O2
1. What is the molecular weight of O2 (diatomic oxygen)?
The mole weight of O2, which represents diatomic oxygen, is approximately 32.00 grams per mole (g/mol).
2. Why is the molecular weight of O2 important in chemistry?
The mole weight of O2 is essential in chemistry because it helps in stoichiometry, allowing chemists to determine the quantities of oxygen involved in chemical reactions. It also plays a role in gas laws and various practical applications.
3. How is the molecular weight of O2 calculated?
To calculate the mole weight of O2, you simply add the atomic masses of the two oxygen (O) atoms in the O2 molecule. Each oxygen atom has an atomic mass of approximately 16.00 g/mol, so the calculation is: Molecular Weight of O2 = 2 x Atomic Mass of O.
4. What is the significance of knowing the molecular weight of O2 in gas laws?
The mole weight of O2 is crucial in gas laws, such as the ideal gas law, because it helps determine the behavior of oxygen gas under different conditions of temperature and pressure. It contributes to calculations involving the behavior of gases.
5. In environmental sciences, why is the molecular weight of O2 important?
Understanding the mole weight of O2 is significant in environmental sciences when studying air quality and the composition of Earth’s atmosphere. O2 is a major component of the atmosphere, and its mole weight is relevant in atmospheric research.
Ethylene Glycol Molar Mass
Ethylene Glycol Molar Mass: Ethylene glycol is a common organic compound with a wide range of applications, from its use as a coolant in automobile radiators to its role as a key ingredient in the production of antifreeze.
Understanding the molar mass of ethylene glycol is essential in various chemical processes and industries. In this article, we will explore what molar mass is, how it relates to ethylene glycol, and its significance in practical applications.
Ethylene Glycol Molar Mass
What Is Molar Mass?
Molar mass, also known as molecular weight, is a fundamental concept in chemistry. It refers to the mass of one mole of a substance, expressed in grams per mole (g/mol). A mole is a unit that represents a specific number of atoms, molecules, or ions, approximately 6.022 x 10^23 entities, known as Avogadro’s number. The molar mass of a substance is numerically equal to its atomic or molecular weight.
Ethylene Glycol Composition
Ethylene glycol’s formula, C2H6O2, signifies two carbon (C) atoms, six hydrogen (H) atoms, and two oxygen (O) atoms per molecule. To determine ethylene glycol’s molar mass (C2H6O2), add the atomic masses of each element in its chemical formula together.
The atomic mass of carbon (C) is around 12.01 g/mol, hydrogen (H) is roughly 1.01 g/mol, and oxygen (O) is about 16.00 g/mol. Calculating the mol mass of ethylene glycol is as follows:
To calculate the molar mass of C2H6O2, sum the products of each element’s atomic mass by its respective count.
Molar Mass of C2H6O2 = (2 x 12.01 g/mol) + (6 x 1.01 g/mol) + (2 x 16.00 g/mol) ≈ 62.08 g/mol
Therefore, the mol mass of ethylene glycol is approximately 62.08 grams per mole (g/mol).
Significance of Ethylene Glycol Molar Mass
- Antifreeze Formulation: Ethylene glycol is a primary component of antifreeze, which prevents freezing and overheating in vehicle cooling systems. Accurate molar mass knowledge is essential for formulating effective and safe antifreeze products.
- Chemical Reactions: Mol mass plays a crucial role in chemical reactions involving ethylene glycol, such as its reaction with acids to produce esters or its use as a reactant in the synthesis of various organic compounds.
- Industrial Processes: Ethylene glycol is used in various industrial applications, including as a heat transfer fluid, deicing agent, and in the production of polyester fibers. Understanding its molar mass is vital for precise manufacturing processes.
- Environmental Considerations: Ethylene glycol’s molar mass is critical in evaluating its environmental impact, especially upon release, facilitating the assessment of ecological consequences.
Conclusion
The mol mass of ethylene glycol is a fundamental concept in chemistry, significant in various practical applications, including automotive maintenance and industrial processes. Understanding and applying this concept enables scientists and professionals to responsibly use ethylene glycol in various industries, promoting safety, efficiency, and environmental responsibility.
Read More
- Organisms And Population Class 12
- Difference Between Capacitor And Inductor
- Van De Graaff Generator
- Dielectric Polarization In Polar And Nonpolar Material And Dielectric Constant
- Chemistry In Everyday Life
Frequently Asked Questions (FAQs) On Ethylene Glycol Molar Mass
What is molar mass, and why is it important in chemistry?
Mol mass, often called molecular weight, represents the mass of one mole of a substance in grams per mole (g/mol). This concept is foundational in chemistry, assisting in stoichiometry—determining substance quantities in chemical reactions and various practical applications.
What is the chemical formula of ethylene glycol?
Ethylene glycol’s formula, C2H6O2, reveals two carbon (C), six hydrogen (H), and two oxygen (O) atoms per molecule.
How is the molar mass of ethylene glycol calculated?
To calculate the mol mass of ethylene glycol (C2H6O2), add the atomic masses of carbon (C), hydrogen (H), and oxygen (O) in its chemical formula: To find the molar mass of C2H6O2, add twice the atomic mass of carbon, six times the atomic mass of hydrogen, and twice the atomic mass of oxygen.
What is the molar mass of ethylene glycol?
The mol mass of ethylene glycol (C2H6O2) is approximately 62.08 grams per mole (g/mol).
Why is the molar mass of ethylene glycol important in antifreeze formulation?
Ethylene glycol is a key component of antifreeze used in vehicle cooling systems. Its molar mass is essential in formulating antifreeze to ensure the proper performance and safety of the product.
Calcium Carbonate Molar Mass
Calcium Carbonate Molar Mass: Calcium carbonate is a chemical compound that plays a crucial role in various aspects of our lives, from its presence in natural formations like limestone and marble to its use in industries such as agriculture, construction, and medicine.
One fundamental concept in chemistry that helps us understand and work with calcium carbonate is its molar mass. In this article, we will explore what molar mass is, how it relates to calcium carbonate, and its significance in various applications.

Calcium Carbonate Molar Mass
What Is Molar Mass?
Molar mass, also known as molecular weight, is a fundamental concept in chemistry that refers to the mass of one mole of a substance. Within the realm of chemistry, a mole stands as a foundational unit for quantifying substances, symbolizing approximately 6.022 x 10^23 particles—widely recognized as Avogadro’s number. The molar mass of a substance, expressed in grams per mole (g/mol), aligns numerically with the atomic or molecular weight of that particular substance.
Calcium Carbonate Composition
Calcium carbonate is represented by the chemical formula CaCO3, signifying that each calcium carbonate molecule comprises one calcium (Ca) atom, one carbon (C) atom, and three oxygen (O) atoms. Calculating its molar mass entails adding the atomic masses of these elements.
Calcium (Ca) has an atomic mass of approximately 40.08 g/mol, carbon (C) has an atomic mass of about 12.01 g/mol, and oxygen (O) has an atomic mass of roughly 16.00 g/mol. To calculate the molar mass of calcium carbonate (CaCO3), we add these values together:
Mol Mass of CaCO3 = (1 x Atomic Mass of Ca) + (1 x Atomic Mass of C) + (3 x Atomic Mass of O)
Mol Mass of CaCO3 = (1 x 40.08 g/mol) + (1 x 12.01 g/mol) + (3 x 16.00 g/mol) ≈ 100.09 g/mol
So, the mol mass of calcium carbonate is approximately 100.09 g/mol.
Significance of Calcium Carbonate Molar Mass
- Stoichiometry: Molar mass is vital in stoichiometry, the study of quantitative relationships in chemical reactions, aiding in precise substance quantities in synthesis and analysis.
- Medication Formulation and Dosage: Calcium carbonate is a commonly used antacid for heartburn and indigestion relief. Its molar mass is crucial in medication formulation and dosing.
- Agriculture: In agriculture, calcium carbonate acts as a soil conditioner, neutralizing acidity, and supplying vital calcium to plants. Precise molar mass measurements are vital for determining correct application rates.
- Construction and Building Materials: Within the construction sector, calcium carbonate plays a pivotal role in producing cement, mortar, and diverse concrete formulations. The molar mass helps engineers and construction workers ensure the correct proportions in these materials for structural integrity.
- Environmental Impact: The mol mass of calcium carbonate is significant in evaluating its environmental impact, including calculating the carbon footprint of related industries.
Conclusion
Understanding the mol mass of calcium carbonate is essential for a wide range of applications in chemistry, medicine, agriculture, and industry. It allows us to make precise calculations, formulate medications, improve soil quality, and create durable building materials. Comprehending mol mass and its relevance to calcium carbonate enables experts to make informed contributions, benefiting various fields and society.
Read More
- Molar Mass Of Ammonia
- Molecular Mass Of Acetic Acid
- Molecular Weight Of Calcium Carbonate
- Molecular Weight Of Sodium Hydroxide
- Molecular Weight Of Aluminium
Frequently Asked Questions (FAQs) On Calcium Carbonate Molar Mass
1. What is molar mass, and why is it important in chemistry?
Molar mass, also known as molecular weight, signifies the mass of one mole of a substance, expressed in g/mol. This concept plays a vital role in chemistry by helping determine the quantities of substances involved in chemical reactions. This determination is essential for stoichiometry, a fundamental aspect of chemistry, and is applicable in various areas within the discipline.
2. How is the molar mass of calcium carbonate calculated?
To find the molar mass of CaCO3, sum the atomic masses of its constituent elements in the formula. For CaCO3, you sum the atomic masses of calcium (Ca), carbon (C), and oxygen (O). Calculate the CaCO3 molar mass by adding the atomic masses of Ca, C, and O, as follows.
3. What is the molar mass of calcium carbonate?
The mol mass of calcium carbonate (CaCO3) is approximately 100.09 grams per mole (g/mol).
4. Why is knowing the molar mass of calcium carbonate important in medicine?
Calcium carbonate is a frequently employed antacid for alleviating symptoms of heartburn and indigestion. Knowledge of its molar mass is essential in formulating medications and determining the appropriate dosages to ensure effective treatment.
5. How is calcium carbonate used in agriculture, and why is its molar mass significant in this context?
In agriculture, calcium carbonate serves as a soil conditioner, addressing acidic soil conditions while supplying essential calcium to plants. Its molar mass is important for calculating the correct application rates to improve soil quality and promote healthy plant growth.
Molar Mass Of Zinc
Molar Mass Of Zinc: In the world of chemistry, understanding the molar mass of elements is fundamental. It’s a concept that plays a crucial role in various chemical calculations, from determining the amount of a substance in a reaction to understanding its physical properties. In this article, we explore the molar mass of zinc (Zn), a vital element with a wide range of applications.

Molar Mass Of Zinc
What is Molar Mass?
Molar mass, also known as molecular weight, is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). In the realm of chemistry, a mole serves as a foundational unit for quantifying entities like atoms, molecules, or ions. This number is approximately 6.022 x 10^23, known as Avogadro’s number.
Molar mass is significant because it allows chemists to relate the weight of a substance to the number of moles present, which is essential for performing stoichiometric calculations and understanding the behavior of substances in chemical reactions.
Calculating the Molar Mass of Zinc (Zn)
To determine the molar mass of zinc (Zn), we follow these steps:
- Find the atomic mass: The atomic mass of an element is the average mass of its atoms, taking into account the relative abundance of its isotopes. For zinc, the atomic mass is approximately 65.38 atomic mass units (amu).
- Multiply by the molar mass constant: The molar mass constant, which relates atomic mass units to grams per mole, is 1 g/mol. So, to find the molar mass of zinc, we multiply the atomic mass by this constant.
Mol Mass of Zn = Atomic Mass of Zn x Molar Mass Constant Molar Mass of Zn ≈ 65.38 g/mol
Hence, the mol mass of zinc (Zn) is approximately 65.38 g/mol.
Significance of Zinc’s Molar Mass
Understanding the mol mass of zinc holds significant importance across various applications:
- Chemical Reactions: In chemical reactions involving zinc, its molar mass is vital for accurately calculating the amount of zinc needed or produced, ensuring the reaction’s efficiency.
- Galvanization: In industry, zinc is commonly used to coat steel in a process known as galvanization. Knowledge of its molar mass is essential for quality control and determining the appropriate amount of zinc coating.
- Pharmaceuticals: Zinc is an essential mineral for human health and is used in various pharmaceutical formulations. Understanding its molar mass aids in dosage calculations.
- Alloys: Zinc alloys, such as brass and bronze, have numerous engineering applications. Molar mass information is crucial for alloy composition and properties.
- Corrosion Protection: Zinc is employed in sacrificial anodes to protect metal structures from corrosion in marine and industrial environments. Its molar mass is vital for designing effective corrosion protection systems.
In conclusion, the mol mass of zinc (Zn) is a fundamental concept in chemistry that has wide-ranging implications in various fields, including industry, medicine, and materials science. It enables precise measurements and calculations, making it an indispensable tool for understanding and utilizing this essential element in our daily lives.
Read More
- Differences Between Enthalpy And Entropy
- Periodic Table Class 11
- Molecular Weight Of Sodium Carbonate
- Difference Between Isothermal And Adiabatic Process
- Simple Harmonic Motion Examples
Frequently Asked Questions (FAQs) On Molar Mass Of Zinc
1. What is the molar mass of zinc, and why is it important?
The mol mass of zinc (Zn) is approximately 65.38 grams per mole (g/mol). It’s crucial because it allows chemists to relate the weight of zinc to the number of moles, aiding in various chemical calculations.
2. How is the molar mass of zinc calculated?
The mol mass of zinc is calculated by multiplying its atomic mass by the mol mass constant, which is approximately 1 g/mol.
- Mol Mass of Zn = Atomic Mass of Zn x Molar Mass Constant
3. What is the atomic mass of zinc, and how is it determined?
The atomic mass of zinc is approximately 65.38 atomic mass units (amu). The determination of zinc’s atomic mass involves considering the isotopes’ relative abundances and their corresponding atomic masses.
4. Why is the molar mass of zinc important in chemical reactions?
In chemical reactions involving zinc, its mol mass is crucial for calculating the precise amount of zinc needed or produced. This accuracy is essential for reaction efficiency.
5. What are some common applications of zinc that rely on its molar mass?
Zinc finds application in diverse fields, including galvanization for steel corrosion protection, pharmaceuticals for health benefits, and various alloy formulations. Its molar mass is essential for quality control, dosage calculations, and alloy composition.
Sodium Carbonate Molar Mass
Sodium Carbonate Molar Mass: Unveiling the Molecular Weight of Na2CO3 Molar mass, also known as molecular weight, is a fundamental concept in chemistry that plays a pivotal role in understanding and quantifying chemical reactions. In this article, we delve into the world of sodium carbonate (Na2CO3), a compound of significant importance in various industrial, scientific, and everyday applications, to explore its molar mass and its relevance in the realm of chemistry.

Sodium Carbonate Molar Mass
What is Molar Mass?
Molar mass is the mass of a substance expressed in grams per mole (g/mol). It represents the weight of one mole of that substance, where a mole is a unit that contains approximately 6.022 x 10^23 particles, be they atoms, molecules, ions, or any other entity. Molar mass is crucial in chemistry because it helps determine the quantity of a substance involved in a chemical reaction, making it an essential tool for stoichiometry and analytical chemistry.
Calculating the Molar Mass of Sodium Carbonate (Na2CO3)
To determine the molar mass of sodium carbonate (Na2CO3), we follow these steps:
1. Identify the elements: Sodium carbonate consists of three elements: sodium (Na), carbon (C), and oxygen (O).
2. Find the atomic masses: Consult the periodic table to find the atomic masses of each element.
- Atomic mass of Na ≈ 22.99 g/mol
- Atomic mass of C ≈ 12.01 g/mol
- Atomic mass of O ≈ 16.00 g/mol
3. Determine the number of atoms: In sodium carbonate, there are two sodium (Na) atoms, one carbon (C) atom, and three oxygen (O) atoms.
4. Multiply and sum: Calculate the molar mass by multiplying the atomic mass of each element by the number of atoms of that element in the compound and then summing these values.
- Molar Mass of Na2CO3 = (2 x Atomic Mass of Na) + (1 x Atomic Mass of C) + (3 x Atomic Mass of O)
- Molar Mass of Na2CO3 = (2 x 22.99 g/mol) + (1 x 12.01 g/mol) + (3 x 16.00 g/mol)
- Molar Mass of Na2CO3 ≈ 105.99 g/mol
Hence, the molar mass of sodium carbonate (Na2CO3) is approximately 105.99 grams per mole (g/mol).
Significance of Sodium Carbonate’s Molar Mass
Understanding the mol mass of sodium carbonate holds practical significance in several areas:
- Chemical Reactions: It is vital for determining the amount of sodium carbonate required or produced in chemical reactions, making it essential for proper dosing and yield estimation.
- Stoichiometry: Sodium carbonate’s molar mass plays a critical role in stoichiometric calculations, facilitating the precise relationship between reactants and products in chemical reactions.
- Industry Applications: Industries, including the detergent, glass, and chemical manufacturing sectors, rely on accurate molar mass values for process optimization and quality control.
- Analytical Chemistry: Mol mass information is invaluable in analytical techniques such as mass spectrometry, aiding in the identification and quantification of substances in samples.
- Household Uses: Sodium carbonate, commonly known as soda ash or washing soda, is used in various household applications, including cleaning, pH adjustment, and water softening.
To sum up, the mol mass of sodium carbonate is a fundamental factor in chemistry, allowing precise measurements and applications. In industry, research, or daily life, a solid grasp of molar mass calculations is crucial for accurate chemical analysis.
Read More
- Layers Of The Earth
- Environmental Pollution And Recycle
- Batteries In Series Parallel
- Difference Between Work And Energy
- Difference Between Concave Convex Lens
Frequently Asked Questions (FAQs) Sodium Carbonate Molar Mass
1. What is sodium carbonate, and why is its molar mass important?
Sodium carbonate, also known as soda ash or washing soda, is a chemical compound with the formula Na2CO3. Its molar mass is important because it allows us to calculate the amount of sodium carbonate in chemical reactions and solutions accurately.
2. How is the molar mass of sodium carbonate calculated?
- To find the mol mass of sodium carbonate (Na2CO3), sum the atomic masses of its elements: sodium (Na), carbon (C), and oxygen (O). The formula for calculating it is:
- Calculate the molar mass of Na2CO3 by adding: 2(Atomic Mass of Na) + Atomic Mass of C + 3(Atomic Mass of O).
3. What are the atomic masses of the elements in sodium carbonate?
- Atomic mass of Na (sodium) ≈ 22.99 grams per mole (g/mol)
- Atomic mass of C (carbon) ≈ 12.01 g/mol
- Atomic mass of O (oxygen) ≈ 16.00 g/mol
4. What is the molar mass of sodium carbonate (Na2CO3)?
The mol mass of sodium carbonate is approximately 105.99 g/mol.
5. Why is molar mass important in chemical reactions?
Molar mass is crucial for stoichiometry, a branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It allows chemists to calculate the precise amounts of substances involved in reactions.
Molar Mass Of Ammonia
Molar Mass Of Ammonia: Ammonia, represented chemically as NH3, is a compound that plays a significant role in various industries, from agriculture to manufacturing. To understand its properties and behavior in chemical reactions, it’s essential to calculate its molar mass.

In this article, we will explore what molar mass is, how to calculate the molar mass of ammonia, and its practical sign
Molar Mass Of Ammonia
What is Molar Mass?
Molar mass, often referred to as molecular weight, is a fundamental concept in chemistry. It represents the mass of a given substance (usually in grams) per mole of that substance. The mole is a unit used to express the amount of a chemical substance, and it is defined as approximately 6.022 x 10^23 entities, which could be atoms, molecules, ions, or any other particle.
Molar mass is crucial in chemical calculations because it allows chemists to relate the mass of a substance to the number of moles, making it possible to determine the amount of a substance in a chemical reaction accurately.
Calculating the Molar Mass of Ammonia (NH3)
To determine ammonia’s molar mass (NH3), we add the atomic weights of its elements, nitrogen (N) and hydrogen (H). Here’s how you can calculate it:
1. Find the atomic masses: Look up the atomic masses of nitrogen (N) and hydrogen (H) on the periodic table. The atomic mass of nitrogen is approximately 14.01 atomic mass units (amu), and the atomic mass of hydrogen is approximately 1.01 amu.
2. Determine the number of atoms: In ammonia (NH3), there is one nitrogen atom and three hydrogen atoms.
3. Multiply and sum: Multiply the atomic mass of each element by the number of atoms of that element in the compound and then sum these values.
Molar Mass of NH3 = (1 x Atomic Mass of N) + (3 x Atomic Mass of H)
Molar Mass of NH3 = (1 x 14.01 amu) + (3 x 1.01 amu)
The Molar Mass of NH3 = 14.01 amu + 3.03 amu
Molar Mass of NH3 ≈ 17.04 amu
Therefore, the molar mass of ammonia (NH3) is approximately 17.04 grams per mole (g/mol).
Practical Significance of Molar Mass of Ammonia
The molar mass of ammonia is a crucial factor in various applications, including:
1. Chemical Reactions: It is used to determine the amount of ammonia needed or produced in chemical reactions. For example, in the Haber-Bosch process, which synthesizes ammonia for fertilizer production, molar mass calculations are essential for optimizing reactant quantities.
2. Stoichiometry: Molar mass is essential for stoichiometric calculations, allowing chemists to relate the quantities of reactants and products in a chemical reaction.
3. Industrial Processes: Industries that use ammonia, such as the production of cleaning products, refrigerants, and explosives, rely on accurate molar mass values for process control and efficiency.
4. Environmental Monitoring: In environmental science, understanding the molar mass of ammonia is vital for studying its emissions and impacts on air quality and ecosystems.
5. Analytical Chemistry: Molar mass information is valuable in analytical techniques like mass spectrometry, where it helps identify and quantify ammonia and other compounds in samples.
In summary, ammonia’s molar mass (NH3) is a fundamental concept in chemistry, enabling us to comprehend and control its behavior in diverse applications. For students or industry scientists, understanding molar mass is crucial for accurate chemical analysis and experimentation.
Read More
- Molecular Mass Of Acetic Acid
- Molecular Weight Of Calcium Carbonate
- Molecular Weight Of Sodium Hydroxide
- Molecular Weight Of Aluminium
- Molecular Weight Of CaCl2
Frequently Asked Questions (FAQs) Molar Mass Of Ammonia
1. What is molar mass, and why is it important?
Molar mass is the mass of a substance (usually in grams) per mole of that substance. It’s essential because it allows chemists to relate the mass of a substance to the number of moles, aiding in precise chemical calculations.
2. How is the molar mass of ammonia (NH3) calculated?
The molar mass of ammonia (NH3) is calculated by summing the atomic masses of its constituent elements, nitrogen (N) and hydrogen (H).
Atomic mass of N = approximately 14.01 atomic mass units (amu)
Atomic mass of H = approximately 1.01 amu
Molar Mass of NH3 = (1 x Atomic Mass of N) + (3 x Atomic Mass of H)
The Molar Mass of NH3 = (1 x 14.01 amu) + (3 x 1.01 amu)
Molar Mass of NH3 ≈ 17.04 grams per mole (g/mol)
3. Why is ammonia’s molar mass important in the context of agriculture?
Ammonia is a key component of fertilizers, and its mol mass is essential for calculating the correct amount of ammonia needed to provide essential nitrogen to crops, promoting healthy growth and increased yield in agriculture.
4. How is the molar mass of ammonia used in the chemical industry?
In the chemical industry, the mol mass of ammonia is crucial for determining the precise amount of ammonia required in various chemical reactions, such as in the production of cleaning products, explosives, and refrigerants.
5. What is the significance of ammonia’s molar mass in environmental science?
In environmental science, understanding ammonia’s mol mass is essential for monitoring and regulating ammonia emissions, which can impact air quality and ecosystems. It is used to calculate the amount of ammonia released into the environment and its potential effects.
Layers Of The Earth
Layers of the Earth:The Earth, our home and a planet of immense beauty, harbors secrets beneath its surface that are as intriguing as the landscapes we see above ground. As we embark on a journey to explore the layers of the Earth, we’ll uncover the mysteries hidden beneath our feet and gain a deeper understanding of the geological forces that shape our world.

Layers Of The Earth:A Journey to the Center of Our Planet
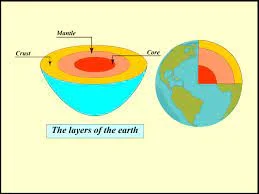
1. The Crust: Earth’s Outer Shell
The Earth’s outermost layer is known as the crust. It’s the thinnest of all the Earth’s layers, making up just a tiny fraction of the planet’s total volume. Beneath our oceans, the crust is relatively thin, about 3 to 5 miles (5 to 8 kilometers) thick, while beneath the continents, it’s significantly thicker, ranging from 20 to 30 miles (30 to 50 kilometers). The crust is where we find the diverse landscapes, continents, and the ocean floors.
2. The Mantle: A Region of Heat and Movement
Beneath the crust lies the mantle, which extends to a depth of about 1,800 miles (2,900 kilometers). The mantle is primarily composed of solid rock, but it behaves like a slow-moving, viscous fluid over geological timescales. Convection currents in the mantle are responsible for the movement of tectonic plates on the Earth’s surface, which leads to the formation of mountains, earthquakes, and volcanoes.
3. The Outer Core: A Molten Sea of Iron and Nickel
Beneath the mantle, we encounter the outer core, a region composed of liquid iron and nickel. This molten layer is responsible for generating the Earth’s magnetic field through a process called the geodynamo effect. It extends from a depth of about 1,800 miles (2,900 kilometers) to 3,700 miles (5,950 kilometers).
4. The Inner Core: Earth’s Solid, Superheated Heart
At the Earth’s center lies the inner core, which extends from a depth of 3,700 miles (5,950 kilometers) to the very center of the planet, approximately 4,000 miles (6,400 kilometers) down. Despite the extreme pressure, the inner core is solid, primarily composed of iron and nickel, with temperatures reaching up to 9,000 degrees Fahrenheit (5,000 degrees Celsius) — hotter than the surface of the sun!
Why Do These Layers Matter?
Understanding the layers of the Earth is fundamental to comprehending the planet’s geological processes and phenomena. Here are some key reasons why these layers matter:
- Tectonic Plate Movement: The movement of tectonic plates, which float on the semi-fluid asthenosphere of the upper mantle, is responsible for earthquakes, mountain formation, and the shifting of continents.
- Magnetic Field Generation: The molten iron in the outer core generates the Earth’s magnetic field, which protects our planet from harmful solar radiation and guides migratory animals.
- Earth’s History: The study of Earth’s layers helps scientists unravel the planet’s geological history, including the formation of continents, oceans, and the evolution of life.
- Natural Resources: Knowledge of Earth’s interior aids in the exploration and extraction of valuable resources such as minerals, oil, and natural gas.
- Volcanoes and Earthquakes: The understanding of the mantle and core’s composition and behavior helps predict volcanic eruptions and seismic activity, contributing to public safety.
In conclusion, the Earth’s layers are a testament to the complexity and wonder of our planet. As we explore the depths of our world, we gain insight into the forces that have shaped its past and continue to mold its future. These layers remind us that there is still much to discover and learn about the remarkable planet we call home.
Read More
- Environmental Pollution And Recycle
- Batteries In Series Parallel
- Difference Between Work And Energy
- Difference Between Concave Convex Lens
- Difference Between Electric Field And Magnetic Field
Frequently Asked Questions (FAQs) Layers Of The Earth
1. How many layers does the Earth have, and what are they called?
The Earth has four main layers:
- Crust: The outermost layer.
- Mantle: Located beneath the crust.
- Outer Core: Lies beneath the mantle.
- Inner Core: At the Earth’s center.
2. What is the Earth’s crust made of?
The Earth’s crust is primarily composed of solid rock. It consists of a variety of minerals, including silicates, oxides, and carbonates.
3. How thick is the Earth’s crust?
The thickness of the Earth’s crust varies depending on whether it’s under the oceans or continents. Oceanic crust is relatively thin, about 3 to 5 miles (5 to 8 kilometers), while continental crust is thicker, ranging from 20 to 30 miles (30 to 50 kilometers).
4. What is the main component of the Earth’s mantle?
The Earth’s mantle is primarily composed of solid rock, mainly silicate minerals like olivine and pyroxene. It behaves like a slow-moving, viscous fluid over geological timescales.
5. How does the mantle contribute to plate tectonics?
The mantle’s convective currents are responsible for the movement of tectonic plates on the Earth’s surface. This movement leads to the formation of mountains, earthquakes, and volcanoes.
Environmental Pollution And Recycle
Environmental Pollution And Recycle: Environmental pollution poses a significant threat to the planet’s health and well-being. From air and water pollution to the accumulation of non-biodegradable waste, our activities have far-reaching consequences for the environment.
However, recycling offers a ray of hope by mitigating the impact of pollution and conserving resources. In this article, we will delve into the various forms of environmental pollution, the importance of recycling, and how recycling can help us achieve a more sustainable future.

Environmental Pollution And Recycle
Understanding Environmental Pollution
- Air Pollution: This occurs when harmful substances, such as particulate matter, nitrogen oxides, and volatile organic compounds, are released into the atmosphere. Sources include industrial processes, vehicular emissions, and deforestation. Air pollution leads to respiratory diseases, climate change, and damage to ecosystems.
- Water Pollution: Contamination of water bodies, such as rivers, lakes, and oceans, by chemicals, pathogens, and heavy metals is a major concern. Agricultural runoff, industrial discharges, and improper waste disposal contribute to water pollution, harming aquatic life and affecting drinking water quality.
- Land Pollution: Land pollution results from the disposal of hazardous waste, including plastics, electronic waste (e-waste), and chemicals, on the ground. It contaminates soil and groundwater, making land unsuitable for agriculture or other productive uses.
- Noise Pollution: Excessive noise from transportation, industrial activities, and urbanization can lead to stress, hearing loss, and other health issues. It also disrupts wildlife habitats and communication among species.
The Role of Recycling in Mitigating Pollution
Recycling is a powerful tool for addressing environmental pollution and fostering sustainability:
- Reduction in Waste: Recycling diverts materials from landfills and incinerators, reducing the burden of land and air pollution. This is particularly important for items like plastics, which can persist in the environment for centuries.
- Conservation of Resources: Recycling conserves natural resources like metals, paper, and plastics. For example, recycling aluminum uses significantly less energy than producing it from raw materials, reducing greenhouse gas emissions.
- Energy Savings: The recycling process typically requires less energy than manufacturing products from raw materials. This reduces the emissions of pollutants associated with energy production, contributing to cleaner air.
- Reducing Water Pollution: Recycling reduces the need for extracting and processing virgin materials, which can lead to water pollution through industrial runoff and mining activities.
- Mitigating Climate Change: Recycling helps lower greenhouse gas emissions by decreasing the energy demand associated with resource extraction and manufacturing.
The Importance of Responsible Recycling Practices
While recycling is a vital part of environmental conservation, it’s essential to practice responsible recycling:
- Proper Disposal: Ensure that recyclables are disposed of in the correct recycling bins or centers. Contaminated recycling streams can lead to waste being sent to landfills.
- Education and Awareness: Public awareness campaigns and education about recycling practices help ensure that individuals know what can and cannot be recycled.
- Support for Recycling Programs: Advocate for and support local recycling programs and policies that promote recycling initiatives within your community.
- Reduce and Reuse: Prioritize reducing and reusing materials before recycling them. Reducing consumption is often the most effective way to minimize environmental impact.
Conclusion
Environmental pollution remains a pressing global challenge, but recycling offers a practical and effective solution. By reducing waste, conserving resources, and mitigating pollution, recycling contributes to a more sustainable future.
Individuals, communities, and governments must work together to promote responsible recycling practices, reduce waste generation, and minimize our collective impact on the environment. It is through these efforts that we can pave the way for a cleaner, healthier planet for generations to come.
Read More
- Batteries In Series Parallel
- Difference Between Work And Energy
- Difference Between Concave Convex Lens
- Difference Between Electric Field And Magnetic Field
- Difference Between Transducer And Sensor
Frequently Asked Questions (FAQs) Environmental Pollution And Recycle
1. What is environmental pollution, and why is it a concern?
Environmental pollution refers to the introduction of harmful substances or contaminants into the environment, leading to adverse effects on ecosystems, human health, and the quality of air, water, and soil. It is a concern because it can harm natural habitats, endanger species, and negatively impact human well-being.
2. How can I reduce air pollution in my daily life?
- Reduce vehicle emissions by carpooling, using public transportation, or driving fuel-efficient vehicles.
- Support clean energy sources like wind and solar power.
- Minimize the use of products that release volatile organic compounds (VOCs).
- Plant trees and promote green spaces to help filter the air.
3. What are some common sources of water pollution, and how can we prevent it?
- Common sources include industrial discharges, agricultural runoff, sewage, and improper waste disposal.
- Prevention measures include proper waste disposal, using eco-friendly products, and supporting regulations to limit industrial pollution.
4. How can individuals reduce land pollution in their daily lives?
- Practice responsible waste disposal by recycling, composting, and disposing of hazardous materials properly.
- Reduce single-use plastics and opt for reusable products.
- Support initiatives to clean up litter and polluted areas.
5. What are the benefits of recycling?
- Recycling reduces waste sent to landfills and incinerators.
- It conserves natural resources and energy.
- Recycling lowers greenhouse gas emissions.
- It contributes to a circular economy where materials are reused, reducing the need for raw material extraction.