Category: Class 10
Median Formula Class 10th For Grouped and Ungrouped Data
Median Formula Class 10th: Statistics encompasses the gathering, examination, interpretation, and representation of extensive sets of numerical data. Captain John Graunt of London earned recognition as the pioneer of vital statistics due to his significant contributions in studying the statistics of births and deaths.
Among the key concepts in statistics, the median holds a vital position. Serving as a measure of central tendency, the median represents the middle-most value within a dataset. In this article, we will explore methods for calculating the median for both grouped and ungrouped data.
NCERT Math Median Formula Class 10th
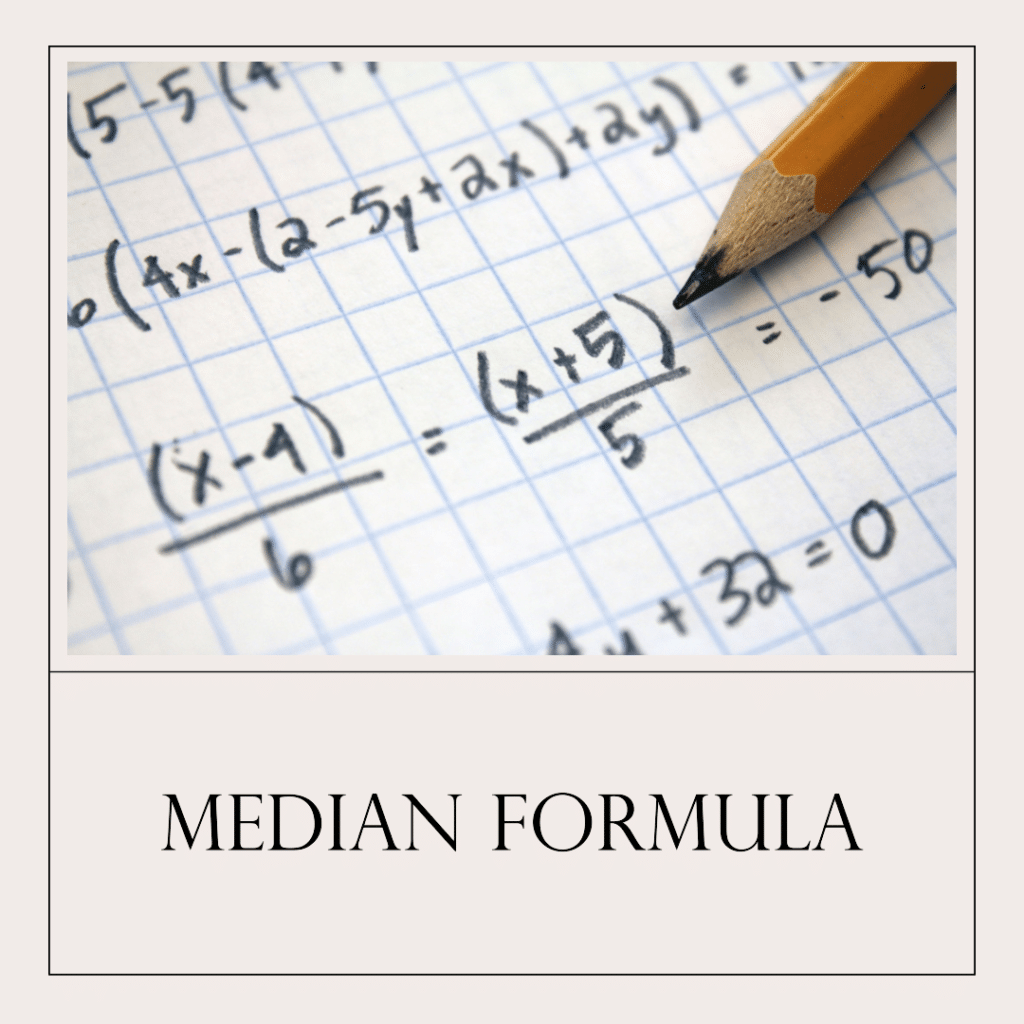
What is Median?
The median represents the middlemost value within a given set of data.
How to calculate Median Step by Step Process
Median Formula for ungrouped data:
1. Organize the provided values in ascending order.
2. Determine the total number of observations in the dataset, denoted as ‘n.’
3. If ‘n’ is an odd number, the median corresponds to the value of the [(n+1)/2]th observation.
4. If ‘n’ is an even number, calculate the median as the average of the (n/2)th and [(n/2)+1]th observations.
Median Formula for For grouped data:
- Step 1: Create a table with three columns – the first column is for the class interval, the second column for frequency (f), and the third column for cumulative frequency (cf).
- Step 2: Fill in the table by writing the class intervals and their corresponding frequencies.
- Step 3: Calculate the cumulative frequency by adding the frequency at each step in the third column (cf).
- Step 4: Find the sum of all frequencies (∑f); it should match the last number in the cumulative frequency column.
- Step 5: Calculate n/2 (where ‘n’ is the total number of observations). Identify the class whose cumulative frequency is greater than or closest to n/2; this class is known as the median class.
- Step 6: Now, apply the formula:
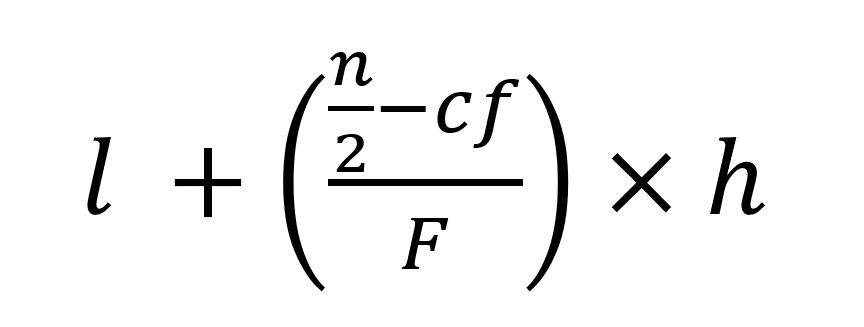
l = lower limit of the median class
n = number of observations
cf denotes the cumulative frequency of the class preceding the median class
f = frequency of the median class
h = class size (assuming classes are of equal size)
Median Formula Class 10th: Solved Example
Example 1: What is the median age of the students in a classroom with the following ages: 12, 14, 15, 16, 17, 18?
Solution:
To find the median age of the students in the classroom, follow these steps:
Step 1: Arrange the ages in ascending order:
12, 14, 15, 16, 17, 18
Step 2: Determine the total number of ages (n) in the dataset. In this case, n = 6.
Step 3: Since the number of ages (n) is even, we use the following formula to find the median:
Median = (n/2)th observation + [(n/2)+1]th observation / 2
Step 4: Substitute the values and calculate the median:
- Median = (6/2)th observation + [(6/2)+1]th observation / 2
- Median = 3rd observation + 4th observation / 2
- Median = (15 + 16) / 2
- Median = 31 / 2
- Median = 15.5
Therefore, the median age of the students in the classroom is 15.5 years.
Example 2: In a coding competition conducted at IIT, there were 50 participants. The median time taken by the participants to solve the coding problems was found to be 90 minutes. Now, you are given that 30 participants took more time than the median to solve the problems.
Can you calculate the maximum possible time (in minutes) taken by any participant to solve the problems in this coding competition?
Solution:
- Total number of participants (n) = 50
- Median time taken by participants = 90 minutes
- Number of participants taking more time than the median = 30
Since the median represents the middlemost value in the dataset, we can infer that there are 25 participants who took less time than the median (as there are 50 participants in total).
Now, we have the following information:
- Number of participants taking less time than the median = 25
- Number of participants taking more time than the median = 30
To find the maximum possible time taken by any participant, we can focus on the participants who took more time than the median. Since we don’t have the exact values of these 30 participants, we can only find the upper bound for the maximum time.
Since there are 30 participants who took more time than the median, the worst-case scenario for the maximum time taken by any participant would be if all these 30 participants took the exact same time, which is greater than the median.
Thus, the maximum possible time taken by any participant can be assumed to be the same as the median (90 minutes) or any value greater than 90 minutes.
In conclusion, the maximum possible time taken by any participant in the coding competition can be at least 90 minutes, but we cannot determine an exact value without more information about the individual times of those 30 participants.
Properties of Median in Statistics
The properties of the median in statistics can be summarized as follows:
- Independence: The median is not influenced by all the data values in a dataset. It is solely determined by its position within the sorted data.
- Positional Value: The median’s value is fixed by its position and does not directly represent any individual data point.
- Central Measure: The median’s distance to other values is smaller compared to any other point, making it a robust central measure.
- Uniqueness: Every dataset has a unique median, resulting in a single value that represents the middle observation.
- Non-Alterability: The median cannot be manipulated algebraically or combined through weighting; it retains its original position.
- Stability in Grouping: When data is grouped, the median remains stable and consistent.
- Limitation to Numeric Data: The median is not applicable to qualitative data and requires data to be grouped and ordered for calculation.
- Applicability to Scales: The median can be determined for datasets measured on ratio, interval, and ordinal scales.
- Robustness to Outliers: Outliers and skewed data have less impact on the median, making it a more resistant measure than the mean.
- Skewed Data Preference: In skewed distributions, the median is considered a better measure than the mean for representing the central tendency.
Read More
- Trigonometry Class 10 formulas List for NCERT Maths Students
- Sample Question Paper for Class 10 CBSE Maths With Solutions
- RD Sharma Class 10 Book Pdf Free Download Without Solutions
Frequently Asked Questions on Median Formula Class 10th
Q 1: How to find the median of ungrouped data?
To find the median of ungrouped data, follow these steps:
- Arrange the data in ascending order (from smallest to largest).
- Determine the total number of observations (n) in the dataset.
- If the number of observations (n) is odd, the median is the middlemost value. For example, if there are 9 data points, the 5th observation is the median.
- If the number of observations (n) is even, the median is the average of the two middlemost values. For example, if there are 10 data points, the 5th and 6th observations are the middlemost, and the median is the average of these two values.
Q 2: Which are the measures of central tendency?
The three main measures of central tendency are:
- Mean: The mean is the most commonly used measure of central tendency. It is calculated by summing up all the values in the dataset and then dividing by the total number of values (n).Mean = (Sum of all values) / n
- Median: The median is the middlemost value in an ordered dataset. It separates the higher half of the data from the lower half. To find the median, the data must be arranged in ascending or descending order, and if the number of observations (n) is odd, the median is the middle value; if n is even, it is the average of the two middle values.
- Mode: The mode is the value that appears most frequently in the dataset. It is possible for a dataset to have one mode (unimodal), two modes (bimodal), or more (multimodal), or it may have no mode if all values occur with the same frequency.
Q 3: What is the symbol of median?
The mean is commonly denoted by M, while the median is represented by Mdn. When referring to standard deviation, the symbol s (the Greek lower-case letter “sigma”) is typically used to represent the population standard deviation. On the other hand, s is employed to signify the standard deviation of a sample of scores.
Control and Coordination Notes Class 10: NCERT Science Ch. 6
Control and Coordination Notes Class 10: The intricately designed human body serves as a sophisticated mechanism, orchestrating a multitude of functions and processes crucial for sustaining life.
Within Class 10 Chapter 6 “Control and Coordination,” we delve into the fascinating exploration of how the body regulates its movements and harmonizes actions among different body parts and with the surrounding environment.
This study unveils the remarkable control systems at play that facilitate seamless coordination throughout the human organism.
NCERT Science Chapter 6 Control and Coordination Notes for Class 10
Control and Coordination Notes Class 10: The Nervous System
Movement in Organisms
Movement refers to the capability of organisms to manipulate specific body parts. When they relocate from one location to another, this process is known as locomotion. Furthermore, organisms demonstrate movements as a response to various stimuli.
Introduction to Control & Coordination
In response to diverse stimuli such as light, heat, nutrients/food, and more, organisms exhibit movement. The nervous and endocrine systems play a pivotal role in controlling and coordinating all activities in animals.
These two systems work hand in hand, with hormones serving as chemical messengers that aid the nervous system in executing various functions.
These hormones are secreted by endocrine glands. Additionally, in plants, hormones also serve as coordinators of movements, facilitating their responses to environmental cues.
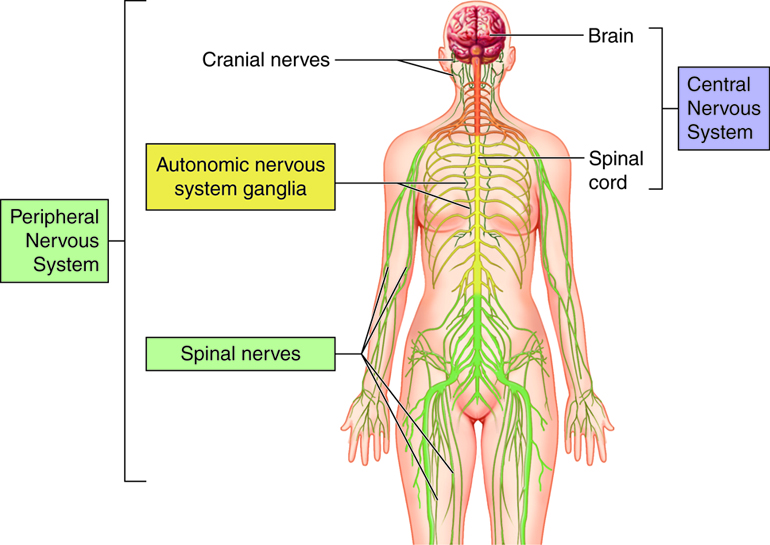
The Nervous System
Types of Nervous System
Neuron
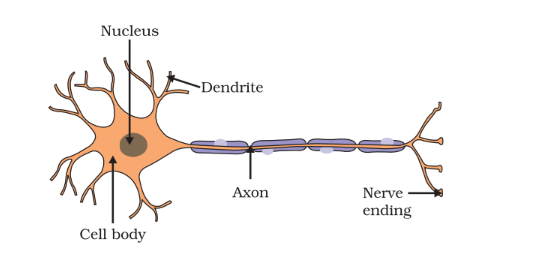
The neuron serves as the fundamental building block of the nervous system, encompassing both its structure and function.
Comprising three primary components, each neuron includes dendrites, cyton/soma/cell body, and an axon. Dendrites are responsible for receiving impulses from other neurons, while the cyton/soma processes these impulses. On the other hand, the axon is tasked with transmitting the impulse either to another neuron or to muscles, glands, and other target cells.
Neurons can exist in two forms: myelinated or non-myelinated. Myelinated neurons possess a myelin sheath, leading to faster impulse transmission compared to non-myelinated neurons. This intricate system of neurons facilitates swift and efficient communication within the nervous system.
Central Nervous System

The central nervous system (CNS) comprises the brain and the spinal cord. The distinct parts of the brain fulfill various functions:
1. The cerebrum governs reasoning, logic, emotions, speech, memory, visual processing, recognition of auditory and taste stimuli, and more.
2. The cerebellum regulates and coordinates body movements, posture, and balance.
3. The pons serves as a relay station, transmitting signals from the hindbrain to the forebrain.
4. The Medulla Oblongata controls involuntary movements, including functions like vomiting, sneezing, yawning, heartbeat, breathing, blood pressure, etc.
5. The Medulla oblongata continues as the spinal cord, coursing through the vertebral column, and overseeing reflex actions.
Each part of the brain plays a vital role in maintaining bodily functions and supporting complex cognitive processes.
Peripheral Nervous System
The peripheral nervous system (PNS) is composed of nerves that emanate from both the brain and the spinal cord. In humans, this system comprises 12 cranial nerves and 31 spinal nerves.
These nerves extend throughout the body, facilitating communication between the central nervous system and various organs, muscles, and sensory receptors.
Somatic Nervous System
The somatic nervous system constitutes a component of the peripheral nervous system (PNS). It comprises the nerves responsible for controlling voluntary actions within the body.
Through the somatic nervous system, individuals can consciously and deliberately perform movements, enabling interactions with the external environment.
Autonomic Nervous System
The autonomic nervous system encompasses all the nerves within the peripheral nervous system (PNS) that oversee involuntary actions in the body. Vital functions such as respiration, heart rate, blood pressure, digestion, and more are regulated by the autonomic nervous system. It operates through two divisions known as the sympathetic and parasympathetic nervous systems.
The sympathetic nervous system readies the body for intense physical activity, often referred to as the fight-or-flight response. On the other hand, the parasympathetic nervous system exerts an opposite effect, promoting relaxation and inhibiting or slowing down many high-energy functions. These two divisions work in harmony to maintain a delicate balance in the body’s physiological responses to various situations and stimuli.
Reflex Action
A reflex is a spontaneous and involuntary reaction to stimuli, playing a crucial role in our renowned survival instinct.
Many common reflexes are a result of our well-trained and accumulated knowledge of caution that we have internalized over time.
They can manifest in various ways, such as quickly pulling back our hand when it touches an extremely hot or cold object—an action known as a reflex action. These reflexes are intricately linked to our instinctual responses, enabling swift and automatic protective measures to safeguard our well-being.
Reflex Arc
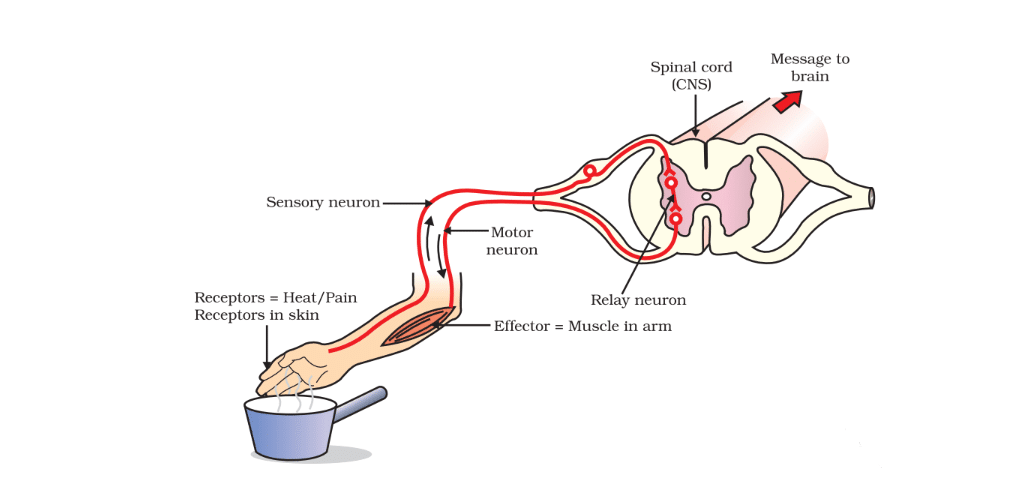
A reflex arc denotes the route taken by an electrical impulse during a reflex action.
The journey commences as the impulse travels from the receptor organ to either the spinal cord or the brain, where it undergoes processing. Once processed, the relevant information is relayed back to the appropriate muscle, prompting the action to be executed.
The components of a reflex arc encompass the receptor organ, the sensory/afferent neuron, the interneuron, the motor/efferent neuron, and the effector organ. These elements work in seamless coordination to ensure rapid and automatic responses to external stimuli, safeguarding the body from potential harm.
Protection of CNS
The brain is safeguarded by three primary layers of protection:
1. The bony skull, also known as the cranium, provides a sturdy and robust outer covering.
2. The cerebrospinal fluid acts as a cushioning and shock-absorbing layer that surrounds and supports the brain.
3. The meninges, comprising the Dura mater, Arachnoid, and Pia mater, form a protective membrane that encloses and shields the brain from potential harm.
Together, these layers form a formidable defense system, ensuring the brain’s well-being and functionality within the skull.
Plant Hormones and Movements
Plant Hormones
Hormones are responsible for orchestrating control and coordination in plants.
| Plant Hormone | Function |
|---|---|
| Auxin | It facilitates the growth of plant tissues. |
| Cytokinin | It stimulates cell division and retards cell aging. |
| Gibberellins | This hormone aids in stem growth, triggers seed germination, stimulates flowering, facilitates cell division, and supports seed development after germination. |
| Abscisic acid | It hinders growth, leading to leaf wilting, and encourages bud and seed dormancy. |
| Ethylene | This hormone is in gaseous form and induces the ripening of fruits. |
Growth Independent Movements
Movements that are unrelated to growth are referred to as nastic movements. These movements are triggered by environmental stimuli, but their direction of response is not determined by the direction of the stimulus.
The touch-me-not plant exhibits thigmonastic movement, which is a response to touch stimuli.
Growth-Related Movements in Plants
Movements that are related to growth are termed tropic movements. These movements arise in response to environmental stimuli, and the direction of their response is influenced by the direction of the stimulus.
Here are some examples of tropic movements:
1. Phototropic movement (light-dependent)
2. Geotropic movement (gravity-dependent)
3. Chemotropic movement (chemical-dependent)
4. Hydrotropic movement (water-dependent)
5. Thigmotropic movement (touch-dependent)
Geotropism
The response of plant parts to the Earth’s gravitational force is referred to as geotropism or gravitropism.
Positive geotropism is when plant parts grow towards gravity, while negative geotropism is when they grow away from gravity. For instance, roots exhibit positive geotropism as they grow towards the force of gravity, while shoots display negative geotropism by growing away from it.
Phototropism
Phototropism refers to the movement of plant parts in response to light.
Positive phototropism occurs when plant parts move towards light, while negative phototropism happens when they move away from light.
For example, stems exhibit positive phototropism as they bend towards the light source, while roots demonstrate negative phototropism by bending away from the light.
Hydrotropism
Hydrotropism refers to the movement of plant parts in response to water or moisture.
Positive hydrotropism occurs when plant parts move towards water, while negative hydrotropism happens when they move away from water.
For example, roots exhibit positive hydrotropism as they grow in search of water, such as moving towards areas with high humidity levels.
Chemotropism
Chemotropism refers to the movement of plant parts in response to chemical stimuli.
Positive chemotropism occurs when plant parts move towards the chemical source, while negative chemotropism happens when they move away from it. For example, the growth of the pollen tube towards the ovule is a demonstration of positive chemotropism, as it is attracted by chemical signals from the ovule.
Thigmotropism
Thigmotropism refers to the movement of plant parts in response to touch.
Positive thigmotropism occurs when plant parts move towards the touch stimulus, while negative thigmotropism happens when they move away from it.
For example, the movement of tendrils around a support is a demonstration of positive thigmotropism, as they respond to the touch of the support structure.
Importance of The Endocrine System for Control and Coordination Notes Class 10
Exocrine Glands
Exocrine glands are glands that release secretions through ducts, which open onto an epithelial surface.
Endocrine Glands
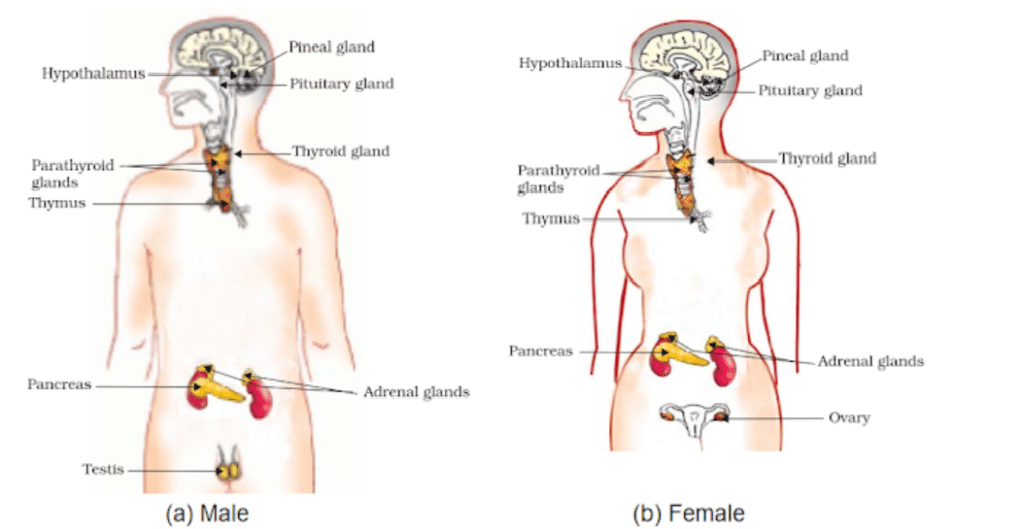
Endocrine glands in humans are ductless glands that release hormones directly into the bloodstream.
The human body houses various endocrine glands, including the pituitary, thyroid, adrenal, pineal, pancreas, ovary (female), testis (male), and more. Let’s delve deeper into the functions and roles of each of these glands below.
Pituitary Gland
Situated at the base of the brain, this gland is approximately the size of a pea.
Known as the master gland, it holds authority over the secretions of all other endocrine glands.
In addition, it releases the Growth Hormone (GH). If GH is under-secreted, it can lead to Dwarfism, while over-secretion results in Gigantism during childhood and Acromegaly in adulthood.
Thyroid Gland
Shaped like a butterfly, this gland resides in the throat region.
Its primary function involves releasing the hormone ‘Thyroxine,’ which plays a crucial role in regulating the body’s metabolism.
Iodine is an essential element required for synthesizing thyroxine within the body.
In situations of iodine deficiency, the gland under-secretes thyroxine, resulting in a condition known as goitre.
Pancreas
Located behind the stomach in the abdomen, this gland resembles a leaf in shape.
It functions as both an endocrine and exocrine gland.
As an endocrine gland, it produces two essential hormones – Insulin and glucagon. These hormones work in opposition to one another to regulate the blood sugar levels.
In its exocrine role, the gland secretes enzymes responsible for breaking down proteins, lipids, carbohydrates, and nucleic acids present in food.
Insufficient production of insulin by the pancreas can lead to diabetes, a condition characterized by abnormal blood sugar levels.
Adrenal Gland
Present in pairs above each kidney, these glands undergo a decrease in size as age advances.
They are responsible for secreting the hormone adrenaline, which plays a vital role in triggering the body’s flight or fight response during stressful situations.
Additionally, these glands also secrete noradrenaline, contributing to various physiological responses in the body.
Gonads
Gonads refer to the organs responsible for producing gametes – testes in males and ovaries in females.
In males, the testes produce the male hormone testosterone, while in females, the ovaries produce the female hormones estrogen and progesterone.
Testosterone and estrogen play crucial roles in gamete production and are responsible for the development of male and female sexual characteristics, respectively.
Progesterone is known as the pregnancy hormone, as it plays a vital role in supporting and maintaining pregnancy.
Other Endocrine Organs
In addition to the previously mentioned endocrine glands, the other endocrine organs encompass the hypothalamus, parathyroid, pineal, and thymus glands.
Read More
- Class 10 Notes for Science NCERT
- Chemical Reaction and Equation Notes Class 10 NCERT Sci. Ch.1
- Acids Bases and Salts Class 10 Notes of NCERT Science Ch. 2
- Life Process Notes Class 10 NCERT Science Chapter 5
- Class 10th How Do Organisms Reproduce Notes: Science Ch. 7
- Class 10th Heredity and Evolution Notes: NCERT Science Ch. 8
- Notes on Magnetic Effect of Electric Current Class 10 NCERT
- Human Eye and the Colourful World Notes Chapter 10 Science
- Metals and Non Metals Class 10 NCERT Science Chapter 3 Notes
- Class 10th Chapter 11 Science Notes for NCERT Students
- Notes of Our Environment Class 10: NCERT Science Chapter 13
Frequently Asked Questions on Control and Coordination Notes Class 10
What is the significance of the nervous system in control and coordination?
The nervous system plays a vital role in control and coordination as it enables the transmission of electrical impulses, facilitating communication among different body parts and the environment. It ensures swift responses to stimuli and aids in maintaining homeostasis, essential for the body’s proper functioning.
How do hormones contribute to control and coordination?
Hormones serve as chemical messengers in the endocrine system, responsible for control and coordination. They regulate various bodily functions, including growth, metabolism, and reproductive processes. By releasing hormones directly into the bloodstream, the endocrine system coordinates complex physiological responses throughout the body.
What are the key reflex actions in humans, and how do they work?
Reflex actions are involuntary responses to stimuli. Examples include the knee-jerk reflex and withdrawing a hand from a hot object. They occur through reflex arcs, involving sensory neurons, interneurons, and motor neurons. When a stimulus is detected, the impulse travels to the spinal cord or brain, which processes the information and sends a response back to the muscles, leading to the reflex action.
What are tropic movements in plants, and how do they work?
Tropic movements in plants are growth-related responses to environmental stimuli. Examples include phototropism (in response to light) and geotropism (in response to gravity). These movements are directed by the hormones auxin and gibberellin, which cause cells on the shaded side to elongate, leading to bending towards or away from the stimulus.
How does the endocrine system influence human growth and development?
The endocrine system significantly influences human growth and development. Hormones like growth hormone (GH), testosterone, and estrogen play pivotal roles in the growth of bones, muscles, and other tissues during puberty. Imbalances in these hormones can lead to growth disorders, affecting height and physical development. Additionally, thyroid hormones play a crucial role in metabolism, impacting energy levels and overall health throughout life.
Read Also:
- Chemical Reactions and Equations
- Acids, Bases and Salts
- Control and Coordination
- How Do Organisms Reproduce?
- Heredity and Evolution
- Magnetic Effects of Electric Current
- Our Environment
Class 10 Notes for Science NCERT
Providing a critical study resource for students, our chapter-wise NCERT Class 10 Notes for Science play a vital role in helping them excel in the CBSE Class 10 board exam. Effective studying and achieving good marks are essential for every student. To ensure their success, we offer comprehensive NCERT Class 10 Science Notes, complete with detailed explanations, illustrative examples, solved questions, and sample questions from NCERT books.
By meticulously referring to these notes during their studies, students can develop a proper study methodology. The notes are designed to give students a clear insight into all the important concepts and help them gain a strong grasp of the subject matter. The simplicity of the style and format used in these CBSE Notes makes it easier for students to retain and remember each concept effectively over an extended period.

NCERT Class 10 Notes for Science
Class 10 Science primarily covers crucial chapters from the NCERT book, such as chemical reactions and equations, electricity, magnetic effects of electric current, acids, bases and salts, control and coordination, reflection and refraction of light, carbon and its compounds, and more.
For your convenience, we have compiled CBSE Class 10 Science Notes on each of these topics in the table below. Simply click on the links to access the chapter-wise notes for the Class 10 Science subject.
- Chapter 1 – Chemical Reactions and Equations
- Chapter 2 – Acids, Bases and Salts
- Chapter 3 – Metals and Non-metals
- Chapter 4 – Carbon and Its Compounds
- Chapter 5 – Life Processes
- Chapter 6 – Control and Coordination
- Chapter 7 – How Do Organisms Reproduce?
- Chapter 8 – Heredity and Evolution
- Chapter 9 – Light Reflection and Refraction
- Chapter 10 – Human Eye and Colourful World
- Chapter 11 – Electricity
- Chapter 12 – Magnetic Effects of Electric Current
- Chapter 13 – Our Environment
Benefits of Studying of Class 10 Notes for Science
The Science notes provide students with a concise overview of all the concepts.
Studying through these notes proves invaluable during revision.
It optimizes students’ time during exam preparation.
By referring to these notes, students can easily recall all the crucial topics from each chapter.
These notes significantly enhance students’ understanding of the chapters. Furthermore, they serve as the ultimate revision resource before board exams.
Read More
- RD Sharma Class 10 Book Pdf Free Download Without Solutions
- NCERT Books for Class 10 Science – Download pdf
- Class 10 Science Book Pdf in Hindi Download for NCERT Students
Frequently Asked Questions on Class 10 Notes for Science
Which is the easy chapter in science class 10?
In CBSE 10th Board Physics, students have the opportunity to secure full marks in two straightforward and significant chapters: ‘Human Eye’ and ‘Magnetic Effects of Current’. Similarly, in CBSE 10 Chemistry, the easiest and highest-scoring chapters for students to excel in are ‘Chemical Equation & Reaction’ and ‘Acid, Base & Salt’.
Which is the hardest subject in class 10?
Undoubtedly, mastering mathematics and science can be challenging in board examinations. However, with diligent studying and preparation, these two subjects can become the foundation for your success in the CBSE 2022 Term 2 Class 10 board examinations.
How to study science class 10 easily?
1. Familiarize Yourself With the Syllabus and Exam Pattern.
2. Craft a Well-Structured Study Schedule.
3. Create Concise and Effective Short Notes.
4. Utilize the Best Reference Books Available.
5. Enhance Preparedness Through Mock Tests and Previous Year’s Question Papers.
6. Emphasize a Clear Understanding of the Concepts.
How to score 80 percent in Class 10?
1. Create Comprehensive Notes: Ensure you take clear and organized notes for each lesson taught in class.
2. Challenge Yourself: Engage in self-assessment and practice to test your knowledge.
3. Prioritize Restful Sleep: Get sufficient sleep to rejuvenate your mind for effective studying.
4. Practice Writing: Enhance your understanding by writing and practicing the concepts learned.
5. Time Management: Maintain control over your study time and create a productive routine.
6. Work with Past Test Questions: Solve previous year’s test questions to gain confidence and familiarity.
7. Emphasize Revision: Regular revision is crucial for reinforcing what you’ve learned.
Notes on Magnetic Effect of Electric Current Class 10 NCERT
Notes on Magnetic Effect of Electric Current Class 10: In Chapter 12 of the Class 10 science curriculum, the main focus lies on magnetic fields and electromagnetic effects. The chapter thoroughly explores the application of the magnetic effect of electric current in both electromagnets and electric motors.
Notes on Magnetic Effect of Electric Current Class 10

Notes on Magnetic Effect of Electric Current Class 10
Magnet
A magnet refers to a substance capable of generating a magnetic field that can either attract or repel other materials possessing magnetic properties. A natural example of this is lodestone, which possesses magnetic properties and attracts materials like Iron, Nickel, Cobalt, and others.
Every magnet is inherently bipolar, featuring distinct north and south poles that are inseparable. These two poles coexist and cannot be isolated. When a magnet is freely suspended, its north pole is the side that aligns with Earth’s geographic north.
Just like electric charges, magnetic poles also demonstrate attractive and repulsive behavior. Similar poles repel each other, while unlike poles are drawn together.
Bar magnet
A bar magnet is a rectangular object made of iron, steel, or any ferromagnetic material with inherent permanent magnetic characteristics. It possesses distinct north and south poles. When suspended freely, the bar magnet aligns its north pole towards Earth’s geographic north pole.
Magnetic Field
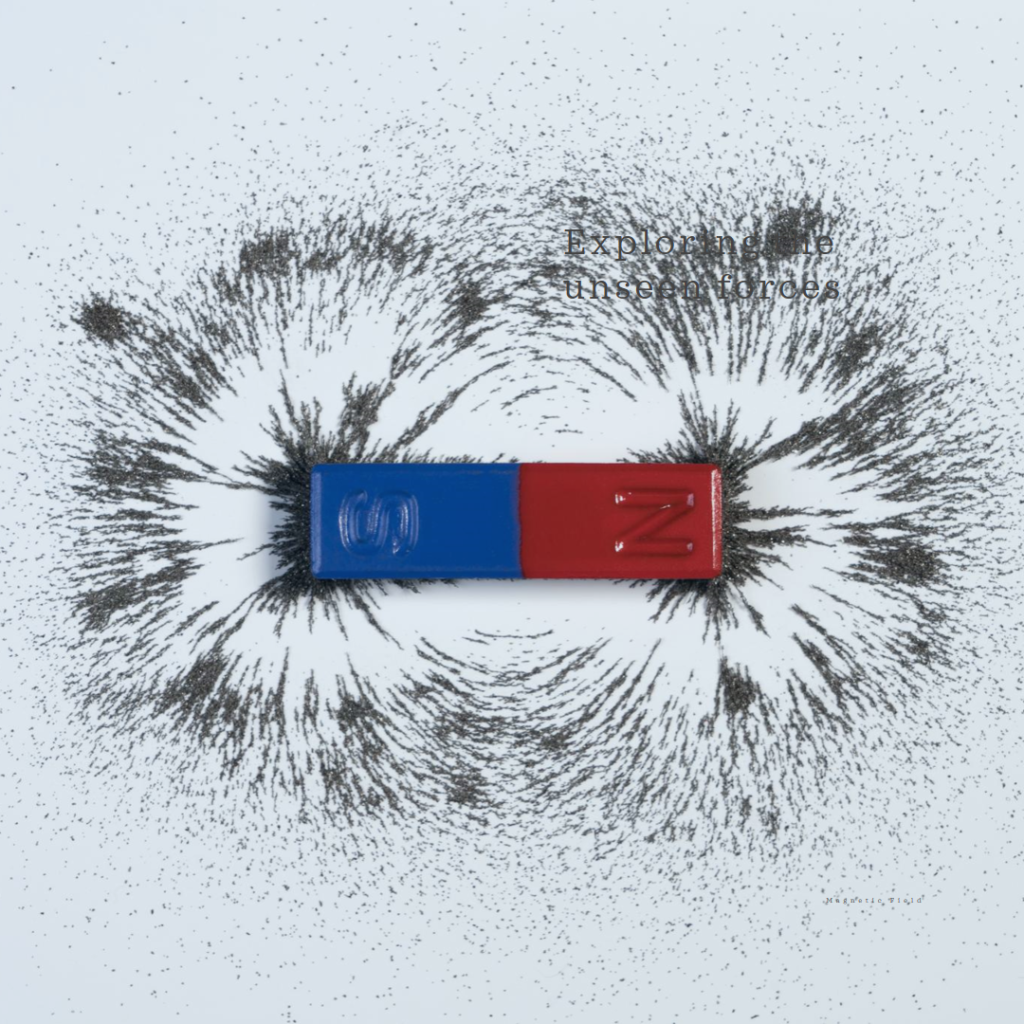
A magnetic field refers to the area surrounding a magnet where its magnetic influence can be observed. The direction and intensity of this magnetic field are depicted through lines of force known as magnetic lines.
Magnetic Field Lines
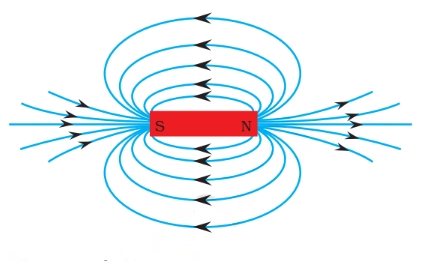
The magnetic field lines of a magnet form continuous and closed loops.
At any given point, the tangent to a field line indicates the direction of the total magnetic field.
The intensity and strength of the magnetic field increase with a greater number of field lines crossing per unit area.
The magnetic field lines do not intersect with each other.
Magnetic Field Lines for a Closed Loop
Due to the dipole nature of magnets, magnetic field lines must have a starting and ending point. As per convention, the field lines originate at the north pole and extend towards the south pole outside the bar magnet, while inside the magnet, they go from south to north.
This configuration results in the formation of closed loops. The strength of the magnetic field is greater where the field lines are closer or denser.
Iron Filings Test around a Bar Magnet
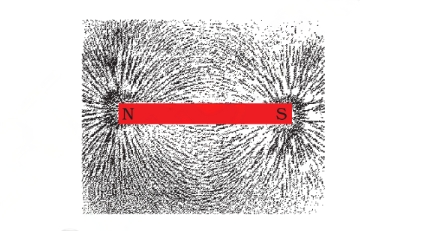
When iron filings are scattered around a bar magnet, they reveal the magnetic field lines that encircle the magnet. These magnetic field lines can be described as imaginary lines that visually depict the magnetic field encompassing any magnetic material.
Magnetic field lines do not intersect because it is not possible to have two different tangential magnetic field directions at the same point. If a compass needle were placed at such a point, it would indicate contradictory directions of the magnetic field, which is logically impossible.
Notes on Magnetic Effect of Electric Current Class 10
Oersted’s Experiment
Passing electric current through a conductor creates a magnetic field encircling it. This phenomenon becomes evident by observing the deflection of a magnetic needle near the conductor. The degree of deflection increases with a higher current flow. Reversing the direction of the current also leads to a reversal in the direction of the deflection shown by the magnetic needle.
Electromagnetism and Electromagnet
An electromagnet is a man-made magnet that generates a magnetic field when electric current flows through a conductor. This magnetic field vanishes when the current is switched off.
The process of creating or inducing a magnetic field through the flow of electric current is known as electromagnetism.
Magnetic Field Due to a Straight Current-Carrying Conductor
When electric current flows through a straight conductor, it generates a magnetic field surrounding it. This phenomenon becomes visible by using iron filings, which align themselves in concentric circles around the conductor.
Right-Hand Thumb Rule: Notes on Magnetic Effect of Electric Current Class 10
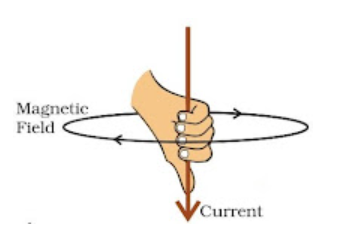
If you hold a straight conductor in your right hand with the thumb pointing in the direction of the current, the tips or curl of your fingers indicate the direction of the magnetic field encircling the conductor.
Magnetic Field Due to Current through a Circular Loop
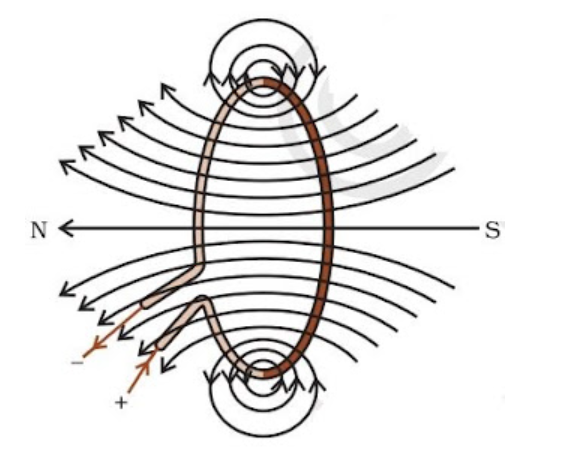
The right-hand thumb rule is applicable to a circular conducting wire as well, as it can be considered a collection of small straight segments. Each point along the wire carrying current generates a magnetic field that appears as straight lines at the center.
Magnetic Field Due to Current in a Solenoid
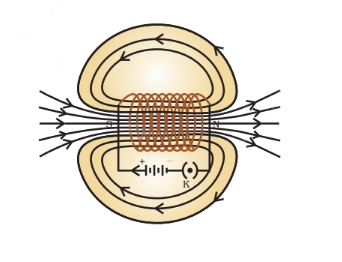
A solenoid is a cylindrical coil composed of numerous circular windings. When electric current passes through it, it exhibits magnetic properties akin to a bar magnet, producing a comparable field pattern. To enhance its magnetic strength, a soft iron core is often employed.
Notes on Magnetic Effect of Electric Current Class 10
Ampere’s Experiment
When an electric conductor is situated within a magnetic field, it encounters a force. The magnitude of this force is directly proportional to the current flowing through the conductor and is also perpendicular to both the length of the conductor and the magnetic field.
The force acting on a straight current-carrying conductor is mutually perpendicular to both the magnetic field and the direction of the current.
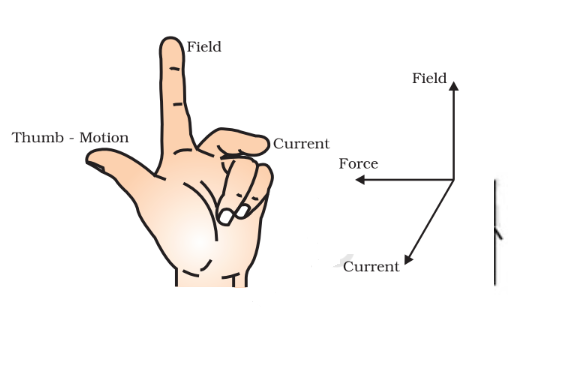
Fleming’s Left-Hand Rule
Fleming’s left-hand rule dictates that the direction of the force exerted on a current-carrying wire is perpendicular to both the direction of the current and the magnetic field.
Electric Motor
Through arm AB, current enters via brush X, and through brush Y, current flows from C to D. By applying Fleming’s Left-Hand Rule (LHR), we determine that the force pushes AB downwards and CD upwards.
In an electric motor, the split rings PQ function as a commutator, responsible for reversing the direction of the current periodically. This reversal occurs at each half-rotation, resulting in a continuous rotation of the coil.
Faraday’s Experiment: Notes on Magnetic Effect of Electric Current Class 10
Faraday made a significant discovery that a magnetic field can interact with an electric circuit and induce a voltage, which is referred to as EMF (electromotive force) through electromagnetic induction.
When a magnet is brought closer to a coil, it initiates a current in the coil circuit, which is evident by the deflection observed in the galvanometer needle.
Electromagnetic Induction
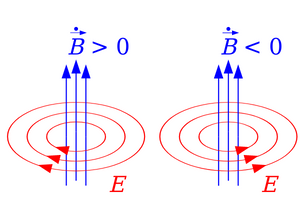
Electromagnetic induction is a phenomenon where an induced EMF and subsequent current are produced in a coil due to a changing magnetic field over time.
When a coil is positioned close to a current-carrying conductor, the magnetic field alters either due to a change in current (I) or due to the relative motion between the coil and conductor. The direction of the induced current is determined using Fleming’s right-hand rule.
Fleming’s Right-Hand Rule
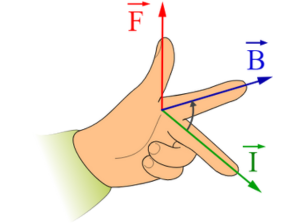
As per Fleming’s right-hand rule, you can use your right hand in the following manner: stretch your thumb, forefinger, and middle finger to be perpendicular to each other.
When the thumb represents the direction of the conductor’s movement, the forefinger points in the direction of the magnetic field, and the middle finger indicates the direction of the induced current.
Electric Generator
It transforms mechanical energy into electrical energy, functioning based on the principle of electromagnetic induction.
AC Generation: The setup comprises an axle connected to two rings, causing the arms AB and CD to move up and down within the generated magnetic field. Consequently, the induced current flows through the circuit ABCD.
After half a rotation, the current direction in both arms undergoes a change. By applying Fleming’s right-hand rule once more, the induced currents are established in these arms along the directions DC and BA. Therefore, the induced current flows through the circuit DCBA.
DC Generation: Similar to AC generation, the setup utilizes half rings to produce a current in one direction only, without any variations in magnitude.
Domestic Electric Circuits: Notes on Magnetic Effect of Electric Current Class 10
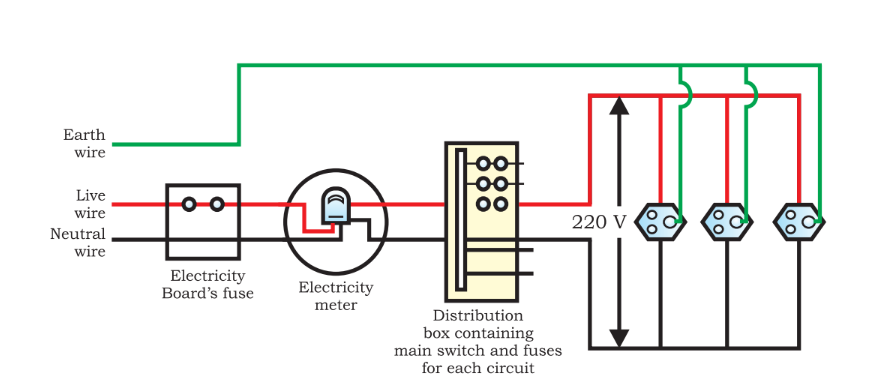
Fuse
A fuse serves as a protective device in electrical circuits during instances of overloading. Overloading occurs when the neutral and live wires come into contact due to insulation damage or a fault in the line.
During overloading, the current in the circuit increases, resulting in a short circuit that can be hazardous. The fuse device employs Joule’s heating (resistive or ohmic heating caused by the flow of current) to melt and break the circuit, thereby interrupting the current flow in the circuit.
Domestic Electric Circuits: Notes on Magnetic Effect of Electric Current Class 10
The live wire is coated with red insulation and carries a voltage of 220 V. The earth wire, covered with green insulation, maintains a voltage of 0 V, aligning with the Earth’s potential. The neutral wire, covered in black insulation, serves its purpose.
For domestic usage, our houses are supplied with alternating current (AC) electric power at 220 V with a frequency of 50 Hz.
Power Loss in Transmission
Power losses in transmission lines over long distances arise due to Joule’s heating. These losses are proportional to the square of the current (H ∝ I^2) and are caused by the line resistance (R).
Joule’s Law of Heating
Joule’s law provides a mathematical representation of how resistance in a circuit converts electric energy into heat energy. The first law of Joule describes the relationship between the heat produced by electric current flowing through a conductor, which can be expressed by the following formula:
Q = I^2 * R * T
Where Q represents the amount of heat generated, I is the electric current, R is the electric resistance in the circuit, and T denotes the time.
Read More
- Class 10 Notes for Science NCERT
- Metals and Non Metals Class 10 NCERT Science Chapter 3 Notes
- Chemical Reaction and Equation Notes Class 10 NCERT Sci. Ch.1
- Acids Bases and Salts Class 10 Notes of NCERT Science Ch. 2
- Life Process Notes Class 10 NCERT Science Chapter 5
- Control and Coordination Notes Class 10: NCERT Science Ch. 6
- Class 10th How Do Organisms Reproduce Notes: Science Ch. 7
- Class 10th Heredity and Evolution Notes: NCERT Science Ch. 8
- Class 10th Chapter 11 Science Notes for NCERT Students
- Notes of Our Environment Class 10: NCERT Science Chapter 13
Frequently Asked Questions on Notes on Magnetic Effect of Electric Current Class 10
Q 1. What is magnetic effect of electric current Class 10 concepts?
The magnetic effect of electric current is known as the electromagnetic effect. This implies that when a magnetic compass is brought close to an electrically charged conductor, the compass needle gets deflected due to the flow of electricity.
Q2. Who discovered the magnetic effect of electric current Class 10?
The discovery of the magnetic effect of current is credited to Hans Christian Oersted.
Q 3. What is the SI unit of magnetic flux?
The magnetic flux is measured in the SI unit called the Weber (Wb). When the flux density is one Weber per square meter (Wb/m^2), it is equivalent to one Tesla (T).
Q 4. What are two examples of magnetic effect of electricity?
Cranes utilize robust electromagnets for moving metallic objects. Electric bells employ electromagnets to generate sound. Additionally, induction motors harness the magnetic effect of current in their operation.
Q 5. How do magnetic effects relate to electromagnetic induction?
Electromagnetic induction is the process by which a changing magnetic field induces an electric current in a conductor. This phenomenon is the basis for many electrical devices and technologies, including generators and transformers.
Q 6. Can magnetic effects of electric currents be harmful?
While magnetic effects of electric currents are essential for many applications, strong magnetic fields can be harmful to certain electronic devices and even living organisms. It is crucial to exercise caution and use appropriate shielding when dealing with high-power magnets or magnetic fields.
Q 7. What is the significance of magnetic fields in transformers?
Transformers use magnetic fields to transfer electrical energy between two or more coils of wire. By inducing a magnetic field in one coil, it creates a varying magnetic flux that, in turn, induces a voltage in another coil, allowing for efficient electrical energy transfer.
Q 8. How do magnetic effects impact electric power transmission?
Magnetic effects play a crucial role in electric power transmission systems. They can cause power losses due to heat generation in transmission lines, requiring efficient designs to minimize energy wastage during long-distance electricity transmission.
Q 9. What is the application of magnetic effects of electric currents in class 10?
Magnetic effects of electric currents find applications in various devices and technologies, including electromagnets, electric motors, generators, transformers, and many other electrical appliances.
Q 10. How does an electric motor work?
An electric motor operates based on the principle of electromagnetic induction. When an electric current flows through a coil placed within a magnetic field, it experiences a force that causes the coil to rotate, leading to the movement of the motor’s shaft.
Our Environment Class 10 Chapter 13 Solution for NCERT
Our Environment Class 10: The NCERT Solutions for Class 10 Science Chapter 15, titled “Our Environment,” offer significant benefits to students in thoroughly grasping the concepts. These solutions are meticulously crafted by subject experts in accordance with the latest CBSE syllabus.
The comprehensive questions and detailed answers provided in the NCERT Solutions enable students to comprehend the core ideas and principles covered in the CBSE Class 10 Science examination. By studying from these solutions, students can enhance their chances of achieving good marks in their exams.
This chapter delves into various aspects of the environment, such as the composition of air, the significance of air in soil, the role of oxygen in supporting living organisms, and the importance of the atmosphere for both plants and our survival. By exploring these topics, students can gain valuable knowledge about their surroundings and their impact on life.
In conclusion, utilizing the NCERT Solutions for Class 10 Science Chapter 15 equips students with a deeper understanding of the subject matter, enabling them to excel in their academic endeavors.
Our Environment Class 10 Chapter 13 Solution for NCERT

Our Environment Class 10 Chapter 13 Solution for NCERT
Q 1. What are the trophic levels? Give an example of a food chain and state the different trophic levels in it.
The food chain operates through the transfer of food or energy at different levels, which are referred to as trophic levels.
For instance:
Grass → Goat → Man
In this food chain:
– Grass occupies the first trophic level.
– The goat occupies the second trophic level.
– Man occupies the third trophic level.
At each trophic level, energy is passed from one organism to another, demonstrating the flow of nutrients and energy through the ecosystem.
Q 2. What is the role of decomposers in the ecosystem?
Decomposers play crucial roles within the ecosystem:
1. Acting as environmental cleansers, they decompose deceased plants and animals, aiding in the breakdown and removal of organic matter.
2. They contribute to nutrient recycling, breaking down organic material and releasing essential nutrients back into the ecosystem for reuse.
3. By decomposing the deceased, they create room for new life forms to thrive in the biosphere, ensuring the continuous cycle of life.
4. Decomposers play a vital role in returning various elements to the water, soil, and air, making them available again for producers like crop plants to utilize. This process sustains the ecosystem’s balance and productivity.
Q 3. Why are some substances biodegradable and some non-biodegradable?
The distinction between biodegradable and non-biodegradable substances stems from the specific roles of microorganisms, such as bacteria, and decomposers, like saprophytes. These natural agents are equipped to break down certain materials in the environment, like paper and wood, which are considered biodegradable. However, human-made products like plastics cannot be broken down by these organisms. As a result, some substances fall into the category of biodegradable, while others are classified as non-biodegradable.
Q 4. Give any two ways in which biodegradable substances would affect the environment.
Biodegradable substances have several positive impacts on the environment:
1. Environmental cleanliness is maintained, as biodegradable materials readily undergo decomposition.
2. Biodegradable substances efficiently participate in the geochemical cycle with the assistance of decomposers, ensuring a smooth and natural recycling process.
Q 5. Give any two ways in which non-biodegradable substances would affect the environment.
Non-biodegradable substances can have adverse effects on the environment:
1. They contribute to pollution of the air, soil, and water, posing a significant threat to ecosystems and living organisms.
2. Non-biodegradable substances may lead to bio-magnification within the food chain, potentially impacting humans at the top of the chain, posing serious risks to their health and well-being.
Q 6. What is ozone, and how does it affect the ecosystem?
Ozone is a unique molecule consisting of three oxygen atoms, and it is considered an isotope of oxygen. The ozone layer’s primary role is to shield the Earth’s surface from the detrimental effects of the sun’s harmful UV rays. These rays pose a significant risk to living organisms and have the potential to cause skin cancer. Thus, the ozone layer acts as a crucial protective barrier safeguarding life on our planet from the harmful impacts of excessive ultraviolet radiation.
Q 7. How can you help in reducing the problem of waste disposal? Give any two methods.
To address the issue of waste disposal effectively, consider the following approaches:
1. Embrace the 3 Rs: To minimize waste disposal problems, adopt the principles of the 3 Rs: reduce, recycle, and reuse. Reduce your consumption and waste generation, recycle materials whenever possible, and find ways to reuse items to extend their lifespan. Additionally, opting for public transport over private vehicles not only reduces waste but also helps in lowering air pollution.
2. Composting: Dispose of biodegradable waste, such as kitchen scraps, through composting. By composting organic materials, you can divert them from landfills and create nutrient-rich compost, benefiting soil health and reducing overall waste.
By implementing these waste reduction strategies, we can make a significant positive impact on waste management and promote a cleaner and more sustainable environment.
Q 8. Which of the following groups contain only biodegradable items?
a. Grass, flowers and leather
b. Grass, wood and plastic
c. Fruit peels, cake and lime juice
d. Cake, wood and grass
a) Grass, flowers, and leather
c) Fruit peels, cake, and lime juice
d) Cake, wood, and grass
The groups listed above consist exclusively of biodegradable items. However, since plastic is not biodegradable, any group that includes plastic cannot be considered biodegradable.
Q 9. Which of the following constitutes a food chain?
a. Grass, wheat and mango
b. Grass, goat and human
c. Goat, cow and elephant
d. Grass, fish and goat
In the group comprising of grass, goat, and human:
The grass functions as the producer, as it generates its own food through photosynthesis.
The goat takes on the role of the primary consumer, as it directly consumes the grass for sustenance.
The human serves as the secondary consumer, as they consume the goat, which in turn relies on the grass for its nourishment.
Q 10. What will happen if we kill all the organisms at one trophic level?
Eliminating all the organisms within a specific trophic level will disrupt the flow of food to the next level, leading to an ecological imbalance. Consequently, the higher-level animals will suffer, potentially causing a surge in the population of lower trophic level animals. This uncontrolled growth can severely impact the overall equilibrium within the ecosystem. Maintaining the delicate balance between trophic levels is crucial for the stability and sustainability of the entire ecosystem.
Q 11.Will the impact of removing all the organisms in the trophic level be different for different trophic levels? Can the organisms of any trophic level be removed without causing any damage to the ecosystem?
Indeed, the consequences of eliminating all organisms within a trophic level will vary across different trophic levels. For instance, if all the producers are removed, it could lead to the death or migration of primary consumers, thereby disrupting the balance of trophic levels. This principle applies uniformly to all trophic levels.
Hence, the removal of organisms at any level would result in an upset of the entire ecosystem, as the disruption of the food chain would reverberate throughout. The survival of higher-level animals is entirely reliant on the presence and stability of animals at the lower levels within the ecosystem.
Q 12. What is biological magnification? Will the levels of this magnification be different at different levels of the ecosystem?
Biological magnification refers to the gradual rise in the concentration of non-biodegradable pollutants within the food chain.
With each successive trophic level in the ecosystem, the magnification intensifies, impacting all the other levels and leading to varying concentrations compared to the initial level. This phenomenon highlights the potential risks and consequences of accumulating harmful substances as they move up the food chain, posing a threat to organisms at higher levels.
Q 13. What are the problems caused by the non-biodegradable wastes that we generate?
Non-biodegradable wastes give rise to several issues:
1. Microorganisms cannot decompose these substances, leading to their persistence in the environment.
2. As the quantity of non-biodegradable wastes increases, disposal becomes a challenging problem.
3. Hazardous non-biodegradable wastes, such as heavy metals, may enter the food chain, affecting organisms at higher trophic levels.
4. These wastes have the potential to contaminate groundwater, resulting in soil infertility and disruption in soil pH levels.
Q 14. If all the waste we generate is biodegradable, will this have no impact on the environment?
Biodegradable wastes undergo decomposition by microorganisms, producing simpler substances that can be utilized as raw materials by producers. However, excessive biodegradable waste can lead to the following effects:
1. Slow decomposition of biodegradable wastes results in the emission of unpleasant odors, posing potential harm to humans when inhaled.
2. Dumping areas of excessive biodegradable wastes can become breeding grounds for harmful organisms, posing risks to humans, plants, and animals alike.
3. A surge in the population of aquatic organisms due to biodegradable waste can lead to oxygen depletion in water bodies, potentially harming aquatic ecosystems.
Q 15. Why is damage to the ozone layer a cause for concern? What steps are being taken to limit this damage?
The ozone layer serves as a protective shield for the Earth, safeguarding it from harmful UV rays that can lead to skin cancer. However, the ozone layer’s depletion is primarily caused by air pollutants like chlorofluorocarbons (CFCs). Excessive UV rays can have detrimental effects on plants, affecting photosynthesis, and harming vital organisms such as plankton and decomposers. This concern arises from the potential consequences of ozone layer damage.
To address this issue, both developing and developed countries have taken measures to combat the problem. Many nations have signed agreements with and are adhering to the guidelines set forth by the United Nations Environment Programme (UNEP). These guidelines aim to freeze or limit the production and usage of CFCs, serving as a crucial step to mitigate the depletion of the ozone layer.
Key Features of NCERT Solutions for Science Chapter 13 – Our Environment Class 10
Comprehensive answers to the chapter questions have been compiled below using lucid language that can be understood by all.
These answers are genuine and appropriate, making them suitable for CBSE exams, Olympiads, and other competitive exams. The provided responses are concise to aid students in better understanding the concepts.
Please feel free to refer to these solutions to enhance your preparation for various examinations. If you have any further queries, do not hesitate to ask for more detailed explanations. Happy learning!
Read More
- NCERT Solutions Science Class 10 All Chapter
- Chemical Equations and Reactions Class 10: Solution of Sci. Ch.1
Frequently Asked Questions on Our Environment Class 10 NCERT Solutions for Science Chapter 13
Q 1. What is the importance of our environment Chapter for class 10 student?
The environment chapter holds significant importance for Class 10 students as it offers essential insights into the delicate balance of nature and its impact on our lives.
Understanding the environment helps students comprehend the interdependence between living organisms and their surroundings, fostering ecological awareness and responsible behavior.
Knowledge about air, water, soil, and ecosystem components empowers them to become environmentally conscious citizens, contributing to sustainable practices and conservation efforts. Moreover, learning about biodegradable and non-biodegradable substances, pollution, and waste management equips students with practical solutions to address pressing environmental challenges.
This knowledge enables them to make informed decisions, promoting a healthier and harmonious coexistence with the natural world.
Q 2. What is the summary of our environment?
The environment can be divided into three main components:
1. Natural components, which include air, water, land, and all living organisms present in the ecosystem.
2. Human components, encompassing individuals, families, and communities that interact and influence the environment.
3. Human-made components, which consist of artificial structures such as roads, monuments, and industries.
Our environment is a dynamic blend of both natural and human-made elements, creating a complex interplay of phenomena that shape the world around us.
Q 3. What are the things that make our environment?
The environment comprises both a physical and a biological component. The physical part includes non-living elements and conditions such as mountains, valleys, rivers, streams, rocks, soils, sunlight, heat, rain, and snow.
On the other hand, the biological part encompasses living organisms like plants, animals, fungi, and bacteria. Together, these components interact to shape the ecosystem, influencing the life and activities of all living beings in the environment.
Q 4. How can we protect the environment?
Adopt sustainable practices to protect our environment:
1. Reduce, reuse, and recycle to conserve resources and reduce landfill waste.
2. Volunteer for community cleanups and watershed protection to contribute to a cleaner environment.
3. Educate yourself and others about the value of natural resources.
4. Conserve water to minimize ocean pollution from runoff and wastewater.
5. Make sustainable seafood choices to support responsible fishing practices.
6. Opt for eco-friendly shopping by reducing plastic use and using reusable bags.
7. Use energy-efficient light bulbs to reduce greenhouse gas emissions, and remember to turn off lights when not in use.
8. Plant trees to promote oxygen, energy savings, and combat climate change.
9. Use non-toxic chemicals at home and work to avoid contaminating waterways.
10. Opt for biking over driving to reduce emissions and promote a greener environment.
Notes of Our Environment Class 10: NCERT Science Chapter 13
Notes of Our Environment Class 10: The environment encompasses the habitat in which an organism flourishes, encompassing living and non-living elements such as physical, chemical, and biotic factors. In this chapter, we delve into the diverse components of the environment, explore their intricate interconnections, and gain insights into how human activities impact this delicate ecosystem.
Notes of Our Environment Class 10: NCERT Science Chapter 13

Notes of Our Environment Class 10: NCERT Science Chapter 13
Ecosystem
An ecosystem encompasses the dynamic interplay of both biotic and abiotic factors within a specific area. Biotic elements encompass all living organisms like plants, animals, microorganisms, and humans. On the other hand, abiotic components include sunlight, temperature, air, wind, rainfall, soil, minerals, and more. Examples of ecosystems include pond ecosystems, grassland ecosystems, and various others where these components interact harmoniously.
Mode of Nutrition in Animals and Plants
Living organisms exhibit two primary modes of nutrition: Autotrophic and Heterotrophic. Autotrophic organisms, such as plants and certain bacteria, can produce their own food through photosynthesis. On the other hand, Heterotrophic organisms, including animals, fungi, and some bacteria, rely on consuming other organisms for their nutritional needs.
Saprophytes and Decomposers
These factors play vital roles in the ecosystem’s nutrient cycling. Saprophytes, like fungi and microorganisms, feed on dead and decaying materials, absorbing nutrients from decomposing plant and animal matter. Decomposers, such as bacteria, worms, slugs, and snails, break down organic matter and waste, releasing nutrients back into the soil. Their work is crucial for soil biology as they transform complex organic substances into simpler compounds that plants can utilize for various metabolic activities.
Biotic Components
The living entities within the environment encompass a diverse range of organisms, including plants, animals, microbes, and fungi.
Abiotic Components
The Abiotic Components of the environment comprise non-living chemical and physical elements, including soil, air, water, temperature, and others. These abiotic factors play a significant role in shaping the ecosystem and influencing the distribution and behavior of living organisms within it.
Trophic Levels
The term “trophic levels” pertains to the different stages in a food web, delineated by the flow of energy. These trophic levels are as follows:
1. Producers (T1): Organisms capable of synthesizing their own food, usually through photosynthesis, forming the foundation of the food chain.
2. Primary consumers (herbivores – T2): The organisms that directly feed on the producers, consuming plant material as their source of energy.
3. Secondary consumers (primary carnivores – T2): These are the carnivores that feed on the primary consumers, acquiring energy by consuming herbivorous animals.
4. Tertiary consumers (secondary carnivores – T3): Carnivores that occupy the third trophic level, preying on other carnivores to obtain their energy.
5. Quaternary consumers (tertiary carnivores – T4): Carnivores situated at the fourth trophic level, consuming other tertiary carnivores.
6. Decomposers: Organisms that break down organic matter and detritus, returning nutrients to the environment, and playing a crucial role in nutrient recycling.
Pyramid of Trophic Levels of Our Environment in Class 10
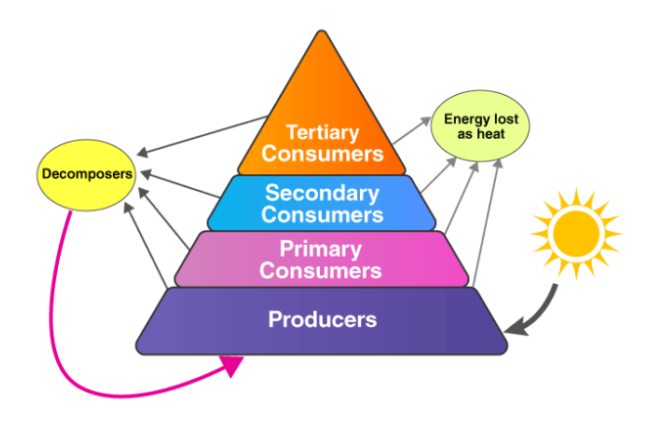
A graphical representation in ecology portrays the organization of trophic levels and can take the form of three types:
1. Pyramid of numbers: This depicts the number of organisms present at each trophic level, starting with producers. It can appear either upright or inverted, depending on the population distribution across trophic levels.
2. Pyramid of biomass: Here, the biomass of each trophic level is illustrated, with producers at the base. Similar to the pyramid of numbers, it can be either upright or inverted, reflecting variations in biomass distribution.
3. Pyramid of energy: This representation is always upright as it showcases the flow of energy from one trophic level to the next. Producers form the foundation, and the energy decreases as it moves up the pyramid to higher trophic levels.
Each type of pyramid provides valuable insights into the ecosystem’s structure and energy dynamics, contributing to a comprehensive understanding of its functioning.
Law of Conservation of Energy
The principle of energy conservation states that energy cannot be created nor destroyed; instead, it undergoes transformation from one form to another.
In biological systems, this energy flows from one organism to another, transferring across trophic levels in the food chain.
Energy Flow
The transfer of energy from one trophic level to another is depicted by the pyramid of energy, illustrating both its direction and amount.
In any given food chain, only approximately 10% of the energy is passed on from one trophic level to the next.
Food Chain
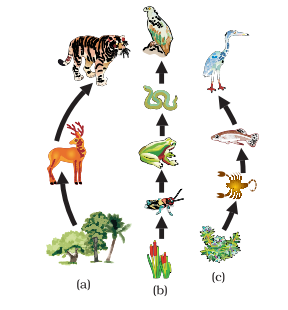
A food chain is the sequence of events within an ecosystem where one living organism consumes another, and subsequently, that organism becomes prey to a larger organism. This process of nutrient and energy transfer from one organism to another at various trophic levels creates the food chain.
Furthermore, the food chain illuminates the feeding patterns and relationships among living organisms. Trophic levels represent the distinct stages in a food chain, beginning with producers at the base, followed by primary, secondary, and tertiary consumers. Each level in the food chain is referred to as a trophic level.
Food Web
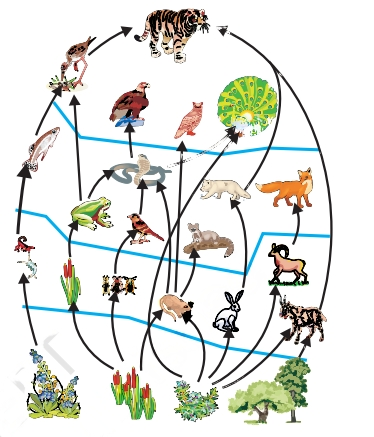
A food web is a complex network formed by several interconnected food chains. While it shares similarities with a food chain, a food web is notably larger in scope. In a food web, some organisms are either consumed by multiple predators or consume several other organisms themselves, leading to a multitude of interconnections between trophic levels.
This interconnectedness is where the food chain falls short in accurately showcasing the flow of energy. In contrast, the food web excels in representing the proper flow of energy by illustrating the intricate interactions between various organisms within the ecosystem. The food web provides a more comprehensive and realistic portrayal of the complex relationships and energy transfers that occur within a given ecosystem.
What is Environment? – Notes for Class 10 Students
The environment encompasses everything surrounding us, comprising both living and non-living elements, such as soil, water, animals, and plants, all of which adapt harmoniously to their surroundings. It is a precious gift from nature that sustains life on Earth.
The environment plays a pivotal role in supporting life on our planet. The term “Environment” originates from the French word “Environ,” signifying “surrounding.” An ecosystem encompasses all living and non-living entities within the environment, forming the foundational basis of the Biosphere, which profoundly influences the overall health of Earth.
Within the realm of life sciences, Ecology and Environmental science are significant branches that primarily focus on studying organisms, their interactions with one another, and their surroundings. These disciplines provide invaluable insights into the complex dynamics of life and its interconnectedness with the environment.
Ecosystem
An ecosystem constitutes a fundamental building block of ecology, encompassing both the structural and functional aspects of living organisms and their interactions with the surrounding environment. Simply put, it represents a chain of intricate relationships between organisms and their ecological surroundings. The term “Ecosystem” was initially coined by A.G. Tansley, an English botanist, in 1935.
Continue reading to delve into comprehensive notes that explore the ecosystem’s structure, components, various types, and the essential functions it fulfills within the natural world.
Pollution
Numerous types of pollution predominantly arise due to human activities, known as anthropogenic causes. Additionally, globalisation has played a part in pollution by driving humanity’s persistent demand for natural resources, leading to significant changes in the Earth’s landscape.
While advancements have improved the quality of life, they have also brought about new challenges that increasingly affect human health and the environment. This article aims to delve into the concept of pollution, its underlying causes, and the various types it manifests. Furthermore, we will examine the far-reaching impacts of pollution on both human health and the environment.
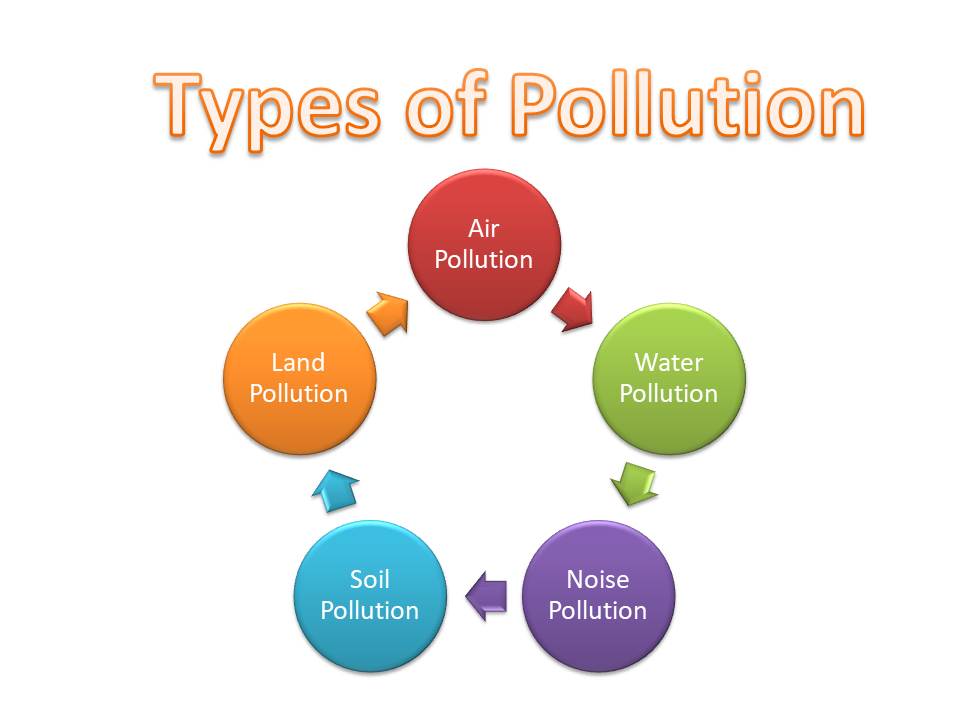
Types of Pollution
As mentioned earlier, pollution can be of various types, stemming from either natural occurrences like forest fires or human-made activities such as emissions from cars, industrial operations, and nuclear waste disposal. The primary classifications of pollution include:
1. Air Pollution
2. Water Pollution
3. Soil Pollution
4. Noise Pollution
In addition to these four main types, there are other forms of pollution, including light pollution, thermal pollution, and radioactive pollution. While radioactive pollution is less common than other types, it is considered the most hazardous.
Air Pollution
Air pollution pertains to the emission of harmful substances (such as chemicals, toxic gases, particulates, biological molecules, etc.) into the Earth’s atmosphere. These pollutants can have severe adverse effects, including significant health concerns.
Water Pollution
Water pollution arises when toxic pollutants and particulate matter are introduced into water bodies, such as lakes, rivers, and seas.
These contaminants are often a result of human activities, such as improper sewage treatment and oil spills. However, even natural processes like eutrophication can contribute to water pollution.
Soil Pollution
Soil pollution, also known as soil contamination, signifies the deterioration of land caused by the presence of chemicals or other man-made substances in the soil. These xenobiotic substances disrupt the natural soil composition and have adverse effects on it. Consequently, this pollution can significantly impact life both directly and indirectly.
For instance, any toxic chemicals present in the soil can be absorbed by plants, which, being primary producers in the environment, transfer these contaminants up the food chain. Although the effects of soil pollution may be less apparent compared to other types of pollution, their implications are highly noticeable and can have far-reaching consequences.
Noise Pollution
Noise pollution denotes the presence of an excessive amount of disruptive noise in the environment that disturbs its natural balance. Typically, it is caused by human activities, but certain natural events like volcanic eruptions can also contribute to noise pollution.
Sounds exceeding 85 decibels are generally considered harmful. Moreover, the duration of exposure to such noise can also have an impact on an individual’s health. To put it into perspective, a normal conversation registers around 60 decibels, while a jet taking off can reach approximately 150 decibels. As a result, noise pollution is more conspicuous than other types of pollution.
Ozone Layer Depletion
The ozone layer acts as a shield, safeguarding the Earth from the sun’s harmful ultraviolet (UV) radiation. However, when CFCs (chlorofluorocarbons) are released into the atmosphere, they undergo chemical reactions with ozone molecules, leading to the depletion of this protective layer.
Garbage Management

Waste management encompasses a series of activities and measures aimed at handling waste from its creation to its ultimate disposal.
It ensures adherence to environmental best practices through rigorous monitoring and regulation.
The steps involved in waste management are as follows:
1. Waste Segregation
2. Collection
3. Transportation
4. Treatment
5. Processing & Recycling
6. Disposal
Biodegradable Waste
Waste originates from plants or animals and can naturally decompose in the soil due to various natural agents such as weather, water, air, heat, microorganisms, and more.
Biodegradation
The process of decomposing garbage or waste materials through the activities of living organisms or biological processes.
Read More
- Class 10 Notes for Science NCERT
- Chemical Reaction and Equation Notes Class 10 NCERT Sci. Ch.1
- Acids Bases and Salts Class 10 Notes of NCERT Science Ch. 2
- Life Process Notes Class 10 NCERT Science Chapter 5
- Control and Coordination Notes Class 10: NCERT Science Ch. 6
- Class 10th How Do Organisms Reproduce Notes: Science Ch. 7
- Class 10th Heredity and Evolution Notes: NCERT Science Ch. 8
- Human Eye and the Colourful World Notes Chapter 10 Science
- Class 10th Chapter 11 Science Notes for NCERT Students
- Notes on Magnetic Effect of Electric Current Class 10 NCERT
- Metals and Non Metals Class 10 NCERT Science Chapter 3 Notes
Frequently Asked Questions on Notes of Our Environment Class 10
Q 1: What are the four types of environment?
The four types of environment are the natural environment (consisting of all living and non-living elements in nature), the built environment (human-made structures and infrastructures), the social environment (interactions and relationships between individuals and groups), and the cultural environment (beliefs, values, customs, and traditions).
Q 2: How can we reduce biomagnification?
Biomagnification can be reduced by controlling the use and release of harmful substances into the environment, particularly those that are persistent and non-biodegradable. Implementing proper waste management, recycling, and using eco-friendly products can help minimize the accumulation of pollutants in the food chain, thereby reducing the potential for biomagnification.
Q 3: What is biological magnification Class 10 notes?
In Class 10 notes, biological magnification, also known as biomagnification, is described as a process in which certain harmful substances like pesticides or heavy metals become increasingly concentrated in living organisms as they move up the food chain. The notes emphasize the importance of understanding this phenomenon and its consequences on the environment and human health, highlighting the need for pollution control measures.
Q 4: What are the main points of our environment Class 10?
The main points covered in Class 10 regarding our environment include the significance of preserving natural resources, understanding ecological balance, identifying various types of pollution. The concept of biodiversity, and the need for sustainable practices. Students also learn about the impact of human activities on the environment. Also, how individual actions can contribute to conservation efforts for a healthier planet.
Electricity of Class 10 NCERT Science Chapter 11 Solutions
Discover comprehensive NCERT Science Electricity of Class 10 Chapter 12 Solutions containing well-explained answers to all the exercise questions found in your textbook. These solutions cover a wide range of topics, including electric cells, electric bulbs, electric circuits, switches, conductors, insulators, as well as examples of each.
You’ll encounter diverse question formats such as ‘Fill in the Blanks’, ‘True or False’, circuit diagrams, and descriptive answering questions, which will facilitate a deeper understanding of the concepts.
In this article, we will explore the very essence of electricity, from its historical roots and foundational principles to practical applications in our daily lives and cutting-edge technologies. Whether you’re just beginning your journey into the realm of electrical knowledge or seeking to reinforce your existing understanding, we have tailored this article to cater to all learning levels.
Electricity of Class 10 NCERT Science Chapter 11 Solutions
Electricity of Class 10
NCERT Electricity of Class 10 Science Chapter 11 Solutions
1. What does an electric circuit mean?
Answer:
An electric circuit refers to a seamless, unbroken loop comprising electric components, allowing the flow of an electric current. The fundamental elements of a basic circuit include:
(a) Conductors – These materials facilitate the easy movement of electric charges, enabling the flow of current within the circuit.
(b) Cell – The cell, acting as a power source, provides the necessary electric potential that propels the electrons to move through the circuit.
(c) Switch – A switch serves as a control device within the circuit, allowing us to open or close the path for the electric current, thus controlling its flow.
(d) Load – The load represents any device or component in the circuit that consumes electrical energy, converting it into various forms of useful output, such as light, heat, or motion.
2. Define the unit of current.
Answer:
The unit of current is known as the ampere. An ampere is defined as the rate of flow of one coulomb of electric charge per second.
3. Calculate the number of electrons constituting one coulomb of charge.
Answer:
The charge of an electron is determined to be 1.6 × 10^-19 C.
Based on the principle of charge quantization, the charge (Q) can be expressed as Q = n * qe, where ‘n’ represents the number of electrons, and ‘qe’ is the charge of an electron.
By substituting the given values into the above equation, we can calculate the number of electrons in one coulomb of charge as follows:
n = 1 C/1.6 * 10^-19 = 6.25 * 10^18
Consequently, the number of electrons comprising one coulomb of charge is found to be 6.25 × 10^18.
4. Name a device that helps to maintain a potential difference across a conductor.
Answer:
One of the devices responsible for sustaining a potential difference across a conductor is a battery, which comprises one or more electric cells.
5. What is meant by saying that the potential difference between two points is 1 V?
Answer:
The potential difference between two points is defined as 1 volt (V) when 1 joule (J) of work is expended to move a charge of 1 coulomb (C) from one point to another.
6. How much energy is given to each coulomb of charge passing through a 6 V battery?
Answer:
As per the potential difference equation:
V = W / Q, where:
V represents the potential difference between two points,
W is the work done in moving the charge from one point to another,
Q denotes the charge.
Using the above equation, we can determine the energy imparted to each coulomb:
W = V × Q
Upon substituting the given values into the equation, we find:
W = 6V × 1C = 6 J
Therefore, when passing through a 6 V battery, each coulomb of charge receives 6 joules of energy.
7. On what factors does the resistance of a conductor depend?
Answer:
The resistance of a conductor is influenced by the following factors:
a. Temperature of the conductor: The resistance tends to change with variations in the conductor’s temperature.
b. Cross-sectional area of the conductor: A larger cross-sectional area typically results in reduced resistance.
c. Length of the conductor: Longer conductors generally have higher resistance compared to shorter ones.
d. Nature of the material of the conductor: The resistance is determined by the specific material properties of the conductor. Different materials have different inherent resistances.
8. Will current flow more easily through a thick wire or a thin wire of the same material, when connected to the same source? Why?
Answer:
The resistance of a wire can be calculated using the formula:
R = ρ * l / A
where:
ρ represents the resistivity of the wire’s material,
l is the length of the wire, and
A denotes the cross-sectional area of the wire.
As we observe from the equation, the cross-sectional area of the wire is inversely proportional to its resistance. Consequently, a thinner wire exhibits higher resistance, while a thicker wire offers lower resistance. Therefore, current flows more effortlessly through a thick wire as compared to a thin wire.
9. Let the resistance of an electrical component remain constant while the potential difference across the two ends of the component decreases to half of its former value. What change will occur in the current through it?
Answer:
Ohm’s Law provides a means to calculate the change in the current flowing through an electrical component. According to Ohm’s Law, the current (I) can be determined by the equation:
I = V / R
Now, if we reduce the potential difference by half while keeping the resistance constant, we get:
New voltage: V’ = V / 2
New resistance: R’ = R
Let the new amount of current be denoted as I’.
We can determine the change in the current using Ohm’s Law as shown below:
NCERT Solutions for Class 10 Chapter 12 Image 2
Thus, the current flowing through the electrical component is reduced by half when the potential difference is halved while keeping the resistance constant.
10. Why are coils of electric toasters and electric irons made of an alloy rather than a pure metal?
Answer:
Alloys possess significantly higher melting points compared to pure metals due to their elevated resistivity. This inherent property allows alloys to resist melting easily at high temperatures.
As a result, alloys find extensive application in heating appliances, such as electric toasters and electric irons, where they can endure and maintain their structural integrity even when subjected to elevated temperatures.
11. Use the data in the table given below and answer the following questions.
a. Which among iron and mercury is a better conductor?
b. Which material is the best conductor?
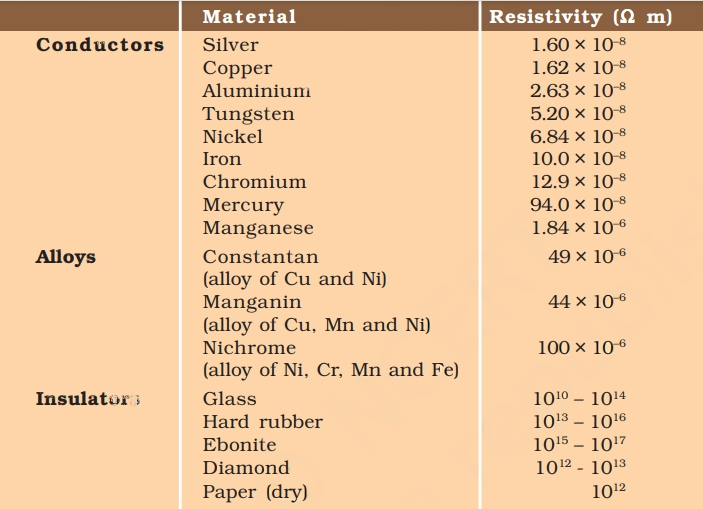
Answer
a. Iron exhibits better conductivity than mercury due to the lower resistivity of iron when compared to mercury.
b. Among all the materials listed in the table, silver stands out as the best conductor as it possesses the lowest resistivity of all, measuring at 1.60 × 10^-8.
12. Draw a schematic diagram of a circuit consisting of a battery of three cells of 2 V each, a 5 Ω resistor, an 8 Ω resistor, and a 12 Ω resistor, and a plug key, all connected in series.
“A set of three cells, each having a voltage of 2 V, combines to form a battery with a total potential of 6 V. The circuit illustration provided depicts three resistors with resistances of 12 Ω, 8 Ω, and 5 Ω, connected in a series arrangement, along with the 6 V potential battery.”
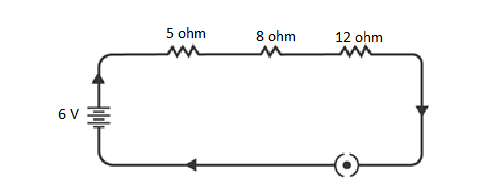
Q 13. Redraw the circuit of Question 1, putting in an ammeter to measure the current through the resistors and a voltmeter to measure the potential difference across the 12 Ω resistor. What would be the readings in the ammeter and the voltmeter?
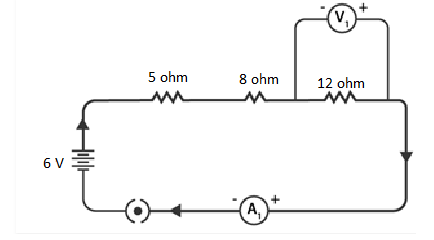
To measure current and potential difference in a circuit, it is important to connect the ammeter in series with the resistors and the voltmeter in parallel to the resistor, as depicted in the figure below.
NCERT Solutions for Class 10 Chapter 12 Image 4
By utilizing Ohm’s Law, the readings of the ammeter and the voltmeter can be determined.
The total resistance of the circuit is obtained by adding the individual resistances: 5 Ω + 8 Ω + 12 Ω = 25 Ω.
Given that the potential difference of the circuit is 6 V, the current flowing through the circuit (or the resistors) can be calculated as follows:
I = V / R = 6 V / 25 Ω = 0.24 A
Let the potential difference across the 12 Ω resistor be denoted as V1.
Using the obtained current, V1 can be calculated as follows:
V1 = I × 12 Ω = 0.24 A × 12 Ω = 2.88 V
Hence, the ammeter reading will be 0.24 A, and the voltmeter reading will be 2.88 V.
Q 14. Judge the equivalent resistance when the following are connected in parallel – (a) 1 Ω and 106 Ω, (b) 1 Ω, 103 Ω, and 106 Ω.
When 1 Ω and 106 are connected in parallel, the equivalent resistance is given by

Therefore, the equivalent resistance is 1 Ω.
(b) When 1 Ω, 103 Ω, and 106 Ω are connected in parallel, the equivalent resistance is given by

Therefore, the equivalent resistance is 0.999 Ω.
Q 15. An electric lamp of 100 Ω, a toaster of resistance 50 Ω, and a water filter of resistance 500 Ω are connected in parallel to a 220 V source. What is the resistance of an electric iron connected to the same source that takes as much current as all three appliances, and what is the current through it?
The circuit diagram illustrates the electric lamp, toaster, and water filter connected in parallel to a 220 V power source, as shown below:
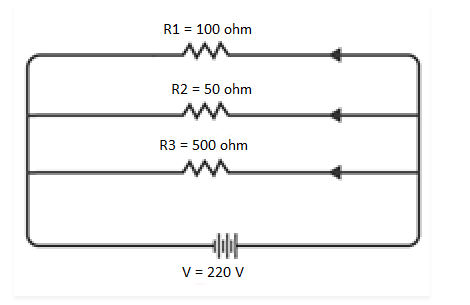
NCERT Solutions for Class 10 Chapter 12 Image 7
To determine the equivalent resistance of the resistors in the circuit, follow these calculations:

NCERT Solutions for Class 10 Chapter 12 Image 8
Furthermore, the resistance of the electric iron box is measured to be 31.25 Ω.
Q 16. What are the advantages of connecting electrical devices in parallel with the battery instead of connecting them in series?
When electrical devices are connected in parallel, the voltage across each appliance remains the same and is equal to the supply voltage. In this configuration, there is no division of voltage among the appliances. Each device receives the full potential difference of the source.
Additionally, connecting devices in parallel reduces the effective resistance of the circuit. The equivalent resistance of the parallel configuration is lower than the individual resistances of the devices. This leads to an increase in the total current flowing through the circuit, as the path for current is effectively widened by the parallel arrangement of appliances.
Q 17. How can three resistors of resistances 2 Ω, 3 Ω, and 6 Ω be connected to give a total resistance of (a) 4 Ω, (b) 1 Ω?
In the first circuit diagram shown below, three resistors are connected:

It can be observed that the resistors of 3 Ω and 6 Ω are connected in parallel. Their equivalent resistance can be calculated as follows:


The equivalent resistor of 2 Ω is then connected in series with another 2 Ω resistor. Consequently, the equivalent resistance of this combination can be calculated as follows:
Req = 2 Ω + 2 Ω = 4 Ω
Thus, the total resistance of the circuit is 4 Ω.
(b) In the second circuit diagram displayed below, three resistors are connected:
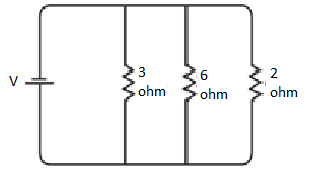
It is evident that all the resistors are connected in parallel. Therefore, their equivalent resistance can be calculated as follows:

The total resistance of the circuit is 1 Ω.
Q 18. What is (a) the highest, (b) the lowest total resistance that can be secured by combinations of four coils of resistance 4 Ω, 8 Ω, 12 Ω, 24 Ω?
(a) When the four resistors are connected in series, their total resistance will be the sum of their individual resistances, and it will be the highest. The total equivalent resistance of the resistors connected in series will be 4 Ω + 8 Ω + 12 Ω + 24 Ω = 48 Ω.
(b) If the resistors are connected in parallel, their equivalent resistance will be the lowest. The total equivalent resistance connected in parallel is:

Therefore, the lowest total resistance is 2 Ω.
Q 19. Why does the cord of an electric heater not glow while the heating element does?
The heating element in an electric heater is constructed using an alloy with high resistance. When electricity passes through the heating element, it generates significant heat, causing the element to glow red.
In contrast, the cord connecting the heater is typically made of materials like copper or aluminum, which have low resistance. As a result, the cord does not heat up enough to glow since the low resistance allows the electricity to flow through it smoothly without generating excessive heat.
Q 20. Compute the heat generated while transferring 96000 coulomb of charge in one hour through a potential difference of 50 V.
Given the following values:
Charge, Q = 96000 C
Time, t = 1 hr = 60 x 60 = 3600 s
Potential difference, V = 50 volts
Now, to calculate the current (I), we can use the formula:
I = Q / t
where Q is the charge, and t is the time.
Therefore, I = 96000 C / 3600 s = 80/3 A
Now, we can find the amount of heat generated (H) using Joule’s law:
H = V * I * t
H = 50 V * (80/3 A) * 3600 s = 4.8 x 10^6 J
Thus, the heat generated is approximately 4.8 x 10^6 joules.
Q 21. An electric iron of resistance 20 Ω takes a current of 5 A. Calculate the heat developed in 30 s.
The quantity of heat generated can be determined using Joule’s law of heating, given by the equation:
H = VIt
By substituting the given values into the equation, we find:
H = 100 × 5 × 30 = 1.5 × 10^4 J
Thus, the amount of heat developed by the electric iron in 30 seconds is 1.5 × 10^4 joules.
Q 22. What determines the rate at which energy is delivered by a current?
Electric power refers to the rate at which electrical energy is consumed by electric appliances. Consequently, it represents the rate at which energy is delivered by a current to the appliance.
Q 23. An electric motor takes 5 A from a 220 V line. Determine the power of the motor and the energy consumed in 2 h.
The power of the motor can be determined using the equation:
P = VI
By substituting the given values into the equation, we find:
P = 220 V × 5 A = 1100 W
Thus, the power of the motor is 1100 watts.
The energy consumed by the motor can be calculated using the equation:
E = P × T
By substituting the given values into the equation, we find:
E = 1100 W × 7200 s = 7.92 × 10^6 J
Therefore, the energy consumed by the motor in 2 hours is 7.92 × 10^6 joules.
Q 24. A piece of wire of resistance R is cut into five equal parts. These parts are then connected in parallel. If the equivalent resistance of this combination is R′, then the ratio R/R′ is _____.
(a) 1/25
(b) 1/5
(c) 5
(d) 25
Answer:
The original resistance is divided into five equal parts, which means each part has a resistance of R/5.
Since each part is connected to one another in parallel, the equivalent resistance (R’) can be calculated as shown below:

The ratio of R to R’ is 25.
Q 26. Which of the following does not represent electrical power in a circuit?
(a) I2R
(b) IR2
(c) VI
(d) V2/R
Answer:
Electrical power is expressed by the equation P = VI. (1)
According to Ohm’s law, V = IR.
By substituting the value of V in equation (1), we obtain:
P = (IR) × I
P = I^2R
Similarly, from Ohm’s law, I = V/R.
Substituting the value of I in equation (1), we get:
P = V × V/R = V^2/R
From this, it is evident that the expression IR^2 does not represent electrical power in a circuit. Instead, the correct representation is V^2/R.
Q 27. An electric bulb is rated 220 V and 100 W. When it is operated on 110 V, the power consumed will be _____.
(a) 100 W
(b) 75 W
(c) 50 W
(d) 25 W
The energy consumed by the appliance can be determined by the expression:
P = VI = V^2/R
The resistance of the light bulb can be calculated as follows:
R = V^2/P
By substituting the values, we find:
R = (220)^2 / 100 = 484 Ω
Even if the supply voltage is reduced, the resistance remains the same. Hence, the power consumed can be calculated as follows:
P = V^2 / R
Substituting the value, we get:
P = (110)^2 V / 484 Ω = 25 W
Thus, the power consumed when the electric bulb operates at 110 V is 25 W.
Q 28. Two conducting wires of the same material and of equal lengths and equal diameters are first connected in series and then parallel in a circuit across the same potential difference. The ratio of heat produced in series and parallel combinations would be _____.
(a) 1:2
(b) 2:1
(c) 1:4
(d) 4:1
Answer:
(c) 1:4
Q 29. How is a voltmeter connected in the circuit to measure the potential difference between two points?
To measure the voltage between any two points, the voltmeter should be connected in parallel across the two points.
Q 30. A copper wire has diameter 0.5 mm and resistivity of 1.6 × 10–8 Ω m. What will be the length of this wire to make its resistance 10 Ω? How much does the resistance change if the diameter is doubled?

Q 31. The values of current I flowing in a given resistor for the corresponding values of potential difference V across the resistor are given below –
| I (Ampere) | 0.5 | 1.0 | 2.0 | 3.0 | 4.0 |
| V (Volts) | 1.6 | 3.4 | 6.7 | 10.2 | 13.2 |
Plot a graph between V and I and calculate the resistance of that resistor.
The plot depicting the relationship between voltage and current is known as the IV characteristic. The current is represented on the y-axis, while the voltage is shown on the x-axis. The table provides various current values corresponding to different voltage values. The IV characteristic for the given resistor is illustrated below.
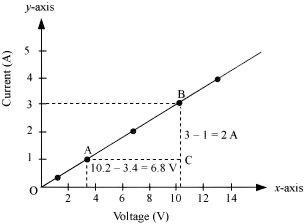
The value of resistance can be obtained from the slope of the line.
The slope is calculated as follows:
Slope = 1/R = BC/AC = 2/6.8
To calculate R:
R = 6.8/2 = 3.4 Ω
Thus, the resistance of the resistor is 3.4 Ω.
Q 32. When a 12 V battery is connected across an unknown resistor, there is a current of 2.5 mA in the circuit. Find the value of the resistance of the resistor.
The resistance (R) of a resistor is determined by Ohm’s law, which states V = IR.
To find R, we use the formula R = V/I, where:
Potential difference, V = 12 V
Current in the circuit, I = 2.5 mA = 2.5 x 10^-3 A
Therefore, the resistance of the resistor is 4.8 kΩ.
Q 33. A battery of 9 V is connected in series with resistors of 0.2 Ω, 0.3 Ω, 0.4 Ω, 0.5 Ω and 12 Ω, respectively. How much current would flow through the 12 Ω resistor?
In a series connection, there is no division of current, and the same current flows across all the resistors.
To calculate the amount of current flowing through the resistors, we can use Ohm’s law.
First, let’s determine the equivalent resistance of the series connection:
R = 0.2 Ω + 0.3 Ω + 0.4 Ω + 0.5 Ω + 12 Ω = 13.4 Ω
Now, applying Ohm’s law:

The current flowing through the 12 Ω resistor is 0.671 A.
Q 34. How many 176 Ω resistors (in parallel) are required to carry 5 A on a 220 V line?
Let’s assume that “n” 176 Ω resistors are connected in parallel.
The formula for calculating the equivalent resistance (Req) of “n” resistors connected in parallel is:
1/Req = 1/R₁ + 1/R₂ + 1/R₃ + … + 1/Rₙ
where R₁, R₂, R₃, …, Rₙ are the individual resistances.
Given that the current (I) is 5 A and the voltage (V) is 220 V, and we want to find the number of 176 Ω resistors (n).
First, let’s find the equivalent resistance (Req) using Ohm’s law:
V = I * Req
Req = V / I Req = 220 V / 5 A Req = 44 Ω
Now, we can calculate the number of resistors (n) using the equivalent resistance:
1/Req = 1/176 Ω + 1/176 Ω + … + 1/176 Ω (n times)
1/44 Ω = n/176 Ω
n = 4
Q 35. Show how you would connect three resistors, each of resistance 6 Ω, so that the combination has a resistance of (i) 9 Ω, (ii) 4 Ω.
If we connect the resistors in series, the equivalent resistance will be the sum of the resistors, i.e., 6 Ω + 6 Ω + 6 Ω = 18 Ω, which is not the desired outcome. If we connect them in parallel, the equivalent resistance will be 6/2 = 3 Ω, which is also not desired. Thus, we need to explore other combinations to achieve the desired total resistance.
(a) Two resistors in parallel:
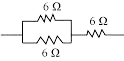
Two 6 Ω resistors are connected in parallel. Their equivalent resistance will be:
1/Req = 1/6 Ω + 1/6 Ω
1/Req = 2/6 Ω
Req = 6/2 = 3 Ω
The third 6 Ω resistor is in series with 3 Ω. Hence, the equivalent resistance of the circuit is 6 Ω + 3 Ω = 9 Ω.
(b) Two resistors in series:
![]()
Two 6 Ω resistors are in series. Their equivalent resistance will be the sum: 6 Ω + 6 Ω = 12 Ω.
The third 6 Ω resistor is in parallel with 12 Ω. Hence, the equivalent resistance will be:
1/Req = 1/6 Ω + 1/12 Ω
1/Req = 3/12 Ω
Req = 12/3 = 4 Ω
Therefore, the total resistance of the circuit is 4 Ω.
Q 36. Several electric bulbs designed to be used on a 220 V electric supply line, are rated 10 W. How many lamps can be connected in parallel with each other across the two wires of 220 V line if the maximum allowable current is 5 A?
The resistance (R1) of the bulb is given by the expression:
R1 = V^2 / P
Where:
Supply voltage, V = 220 V
Rating of the electric bulb, P = 10 watts
Since R = V^2 / P, we can substitute the given values to find R1.

Q 37. A hot plate of an electric oven connected to a 220 V line has two resistance coils A and B, each of 24 Ω resistance, which may be used separately, in series, or in parallel. What are the currents in the three cases?
Case (i) When coils are used separately:
Using Ohm’s law, we can calculate the current flowing through each coil as follows:

When used separately, 9.166 A of current flows through each resistor.
Case (ii) When coils are connected in series:
The total resistance in the series circuit is 24 Ω + 24 Ω = 48 Ω
The current flowing through the series circuit is calculated as follows:

Therefore, a current of 4.58 A flows through the series circuit.
Case (iii) When coils are connected in parallel:
When the coils are connected in parallel, the equivalent resistance is calculated as follows:

The current in the parallel circuit is 18.33 A.
Q 38. Compare the power used in the 2 Ω resistor in each of the following circuits: (i) a 6 V battery in series with 1 Ω and 2 Ω resistors, and (ii) a 4 V battery in parallel with 12 Ω and 2 Ω resistors.
(i) When the potential difference is 6 V and the resistors 1 Ω and 2 Ω are connected in series, their equivalent resistance is given by 1 Ω + 2 Ω = 3 Ω. The current in the circuit can be calculated using Ohm’s law as follows:

Therefore, the power consumed by the 2 Ω resistor is 8 W.
(ii) When 12 Ω and 2 Ω resistors are connected in parallel, the voltage across the resistors remains the same. Knowing that the voltage across the 2 Ω resistor is 4 V, we can calculate the power consumed by the resistor as follows:

The power consumed by the 2 Ω resistor is 8 W.
Q 39. Two lamps, one rated 100 W at 220 V, and the other 60 W at 220 V, are connected in parallel to electric mains supply. What current is drawn from the line if the supply voltage is 220 V?
As both bulbs are connected in parallel, the voltage across each of them will be the same.
The current drawn by the bulb with a rating of 100 W can be calculated using the formula:
P = V × I
I = P / V
Substituting the given values, we get:
I = 100 W / 220 V = 100/220 A
Similarly, the current drawn by the bulb with a rating of 60 W can be calculated as follows:
I = 60 W / 220 V = 60/220 A
Therefore, the total current drawn from the line is:

Q 40. Which uses more energy, a 250 W TV set in 1 hr, or a 1200 W toaster in 10 minutes?
The energy consumed by electrical appliances is determined by the equation:
H = Pt, where P is the power of the appliance, and t is the time.
Using this formula, we can calculate the energy consumed by a TV with a power rating of 250 W as follows:
H = 250 W × 3600 seconds = 9 × 10^5 J
Similarly, the energy consumed by a toaster with a power rating of 1200 W is:
H = 1200 W × 600 s = 7.2 × 10^5 J
From the calculations, it can be observed that the energy consumed by the TV is greater than the toaster.
Q 41. An electric heater of resistance 8 Ω draws 15 A from the service mains 2 hours. Calculate the rate at which heat is developed in the heater.
The rate at which heat develops in the heater can be calculated using the following formula:
P = I^2 * R
By substituting the given values into the equation, we find:
P = (15A)^2 * 8 Ω = 1800 watts
Therefore, the electric heater produces heat at the rate of 1800 watts.
Q 42. Explain the following.
a. Why is the tungsten used almost exclusively for filament of electric lamps?
b. Why are the conductors of electric heating devices, such as bread-toasters and electric irons, made of an alloy rather than a pure metal?
c. Why is the series arrangement not used for domestic circuits?
d. How does the resistance of a wire vary with its area of cross-section?
e. Why copper and aluminium wires are usually employed for electricity transmission?
a. Tungsten is an ideal choice for the filament of electric lamps due to its high resistivity and melting point. These properties prevent it from burning readily when heated, making it suitable for operating at high temperatures in electric lamps.
b. Alloys are preferred as conductors for electric heating devices because of their high resistivity. Compared to pure metals, alloys have higher resistivity, leading to the generation of a substantial amount of heat when current passes through them, which is crucial for heating applications.
c. The series arrangement is not commonly used for domestic circuits due to the following reasons:
– The overall voltage gets divided in a series circuit, which may cause electric appliances not to receive their rated power for proper operation.
– All connected appliances in a series circuit cannot be operated independently. If one device is defective, the entire circuit will be affected.
– The total resistance increases in a series circuit, leading to reduced current flow, which may cause appliances to operate inefficiently.
d. Resistance is inversely proportional to the area of cross-section. When the area of the cross-section increases, the resistance decreases, and vice versa. This relationship between resistance and the cross-sectional area is an important factor to consider in electrical circuits and material design.
e. Copper and aluminum are widely used for electricity transmission due to their low resistivity and excellent conductivity. Their low resistivity results in significantly less power losses in the form of heat during the transmission of electricity, making them efficient choices for power transmission applications.
Electricity of Class 10 NCERT Science Chapter 11 Solutions
Chapter 11 – Electricity in Class 10 Science is an essential topic carrying at least 8 marks as per previous examination trends. However, the 2018 Class 10 Science exam had questions totalling up to 7 marks for this chapter. To strengthen your understanding of the key concepts in this chapter, make use of NCERT Solutions for Class 10.
The topics covered in the NCERT Solutions for Class 10 Science, Chapter 11 are as follows:
1. Ohm’s law
2. Resistivity and Resistance
3. Factors affecting the Resistance of a Conductor
4. Parallel and Series Combination of Resistors and their applications
5. Heating Effect of Electric Current and its Applications
6. Electric Power
7. The interrelation between P, V, I, and R
Electricity is a vital aspect of our society, shaping our civilization since the industrial revolution. It powers entire industries and businesses, and life without electricity would result in chaos, given its importance as a source of energy.
Through NCERT Solutions for Class 10 Science, you can explore how electricity works at the molecular level, understand crucial concepts, and discover its practical applications. The learning resources are designed to facilitate efficient learning.
Key Features of NCERT Solutions for Class 10 Science, Chapter 11 – Electricity:
– Content presented in an easy-to-understand language
– Solutions crafted by highly qualified teachers and industry experts
– Additional questions based on the latest prescribed syllabus
– Detailed explanations of challenging exam questions
– Access to additional learning resources like sample papers and previous year question papers
Read Also:
- NCERT Solutions Science Class 10 All Chapter
- Our Environment Class 10 Chapter 13 Solution for NCERT
- Chemical Equations and Reactions Class 10: Solution of Sci. Ch.1
Frequently Asked Question – FAQs on Electricity of Class 10 NCERT Science Chapter 11 Solutions
Q 1. What are the 4 types of electricity?
The four types of electricity are static electricity (buildup of charge due to friction), current electricity (flow of charge through conductors), direct current (DC, flows in one direction), and alternating current (AC, changes direction periodically, commonly used in household electricity).
Q 2. What is electricity in class 10?
In Class 10, electricity is a crucial topic in the Science curriculum. Students learn about the behavior of electric currents, Ohm’s law, resistance, and the heating effect of electric current. They also study the concepts of parallel and series combinations of resistors, as well as the relationship between power, voltage, current, and resistance.
Q 3. What is electricity and its formula?
Electricity is a form of energy resulting from the movement of electric charge. It involves the flow of electrons through conductive materials like wires. The formula for calculating electrical power (P) is P = VI, where V represents voltage (potential difference) and I denotes current flowing through the conductor.
History 10th Class Chapter-Wise Solutions for NCERT Students
The subject of History 10th class offers students a clear understanding of past events and their significance. Have you ever pondered how modern nations came into existence or how people began to identify themselves as part of a nation?
This sense of national identity evolved gradually over time. In the mid-18th century, people lived within kingdoms, small states, principalities, chiefdoms, and duchies, where the concept of a nation had not fully developed.
Class 10 History delves into the evolution of national sentiments and the development of a shared identity over time. Additionally, you will explore various other captivating topics in History.
To aid your studies, we have provided NCERT Solutions for Class 10 History, making your learning journey more accessible and enjoyable. You must visit at history of class 10th for chapter wise notes.
History 10th Class Chapter-Wise Solutions for NCERT Students

History 10th Class Chapter-Wise Solutions for NCERT Students
The NCERT history 10th class Solutions offer comprehensive answers to all the exercise questions, organized chapter-wise. If students face any difficulties in finding answers to the exercise questions, they can rely on the provided NCERT Solutions.
The table below presents solutions for all the chapters of the History textbook – “India and Contemporary World II.” These solutions serve as a valuable resource to aid students in their understanding and successful completion of the exercises.
| Chapter 1: The Rise of Nationalism in Europe |
| Chapter 2: Nationalism in India |
| Chapter 3: The Making of a Global World |
| Chapter 4: The Age of Industrialisation |
| Chapter 5: Print Culture and the Modern World |
Overview of History 10th Class Chapter-Wise Solutions for NCERT Students
Chapter 1: The Rise of Nationalism in Europe
“The Rise of Nationalism” chapter explores various aspects envisioned by Sorrieu and delves into the diverse processes that gave rise to nation-states and nationalism in nineteenth-century Europe. It covers topics such as the emergence of nationalism in Europe, the influence of the French Revolution, and the interplay between nationalism and imperialism. The 19th century is known as the age of nationalism in Europe, while the 20th century witnessed the proliferation of national movements across Asia and Africa.
Topics Covered in Class 10 History Chapter 1: The Rise of Nationalism in Europe
1. The French Revolution and the Idea of the Nation
2. The Making of Nationalism in Europe
3. The Age of Revolutions: 1830-1848
4. The Making of Germany and Italy
5. Visualizing the Nation
6. Nationalism and Imperialism
Chapter 2: Nationalism in India
“Nationalism in India” traces the origins of nationalism during the French Revolution and highlights its manifestation in India as a result of anti-colonialism. The chapter covers the Indian independence movement, with a focus on the Non-Cooperation and Civil Disobedience Movements that took place during the 1920s.
Topics Covered in Class 10 History Chapter 2: Nationalism in India
1. The First World War, Khilafat, and Non-Cooperation
2. Differing Strands within the Movement
3. Towards Civil Disobedience
4. The Sense of Collective Belonging
List of Map Items in Class 10 History Chapter 2: Nationalism in India
(1918 – 1930) for Locating and Labelling / Identification
1. Indian National Congress Sessions:
a. Calcutta (Sep. 1920)
b. Nagpur (Dec. 1920)
c. Madras (1927)
2. Important Centres of the Indian National Movement
a. Champaran (Bihar) – Movement of Indigo Planters
b. Kheda (Gujarat) – Peasant Satyagrah
c. Ahmedabad (Gujarat) – Cotton Mill Workers Satyagraha
d. Amritsar (Punjab) – Jallianwala Bagh Incident
e. Chauri Chaura (U.P.) – Calling off the Non-Cooperation Movement
f. Dandi (Gujarat) – Civil Disobedience Movement
Chapter 3: The Making of a Global World
“The Making of a Global World” chapter delves into the impact of globalization on the world and the Indian economy. It traces the history of globalization, identifying the causes behind social and economic transformations. The Industrial Revolution in the nineteenth century played a pivotal role in the history of globalization.
Topics Covered in Class 10 History Chapter 3: The Making of a Global World
1. The Pre-modern World
2. The Nineteenth Century (1815-1914)
3. The Inter-war Economy
4. Rebuilding a World Economy: The Post-War Era
Chapter 4: The Age of Industrialisation
“The Age of Industrialisation” chapter begins by examining the pre-Industrial Revolution era and its subsequent changes, including shifts in labor practices and the establishment of factories. It explores industrial growth, the market for goods, and the lives of workers during this transformative period.
Topics Covered in Class 10 History Chapter 4: The Age of Industrialisation
1. Before the Industrial Revolution
2. Hand Labour and Steam Power
3. Industrialisation in the Colonies
4. Factories Come Up
5. The Peculiarities of Industrial Growth
6. Market for Goods
Chapter 5: Print Culture and Modern World
“Print Culture and Modern World” discusses the development of print, from its origins in East Asia to its expansion in Europe and India. This chapter highlights the impact of print technology on social and cultural aspects, considering how it transformed lives and societies.
Topics Covered in Class 10 History Chapter 5: Print Culture and Modern World
1. The First Printed Books
2. Print Comes to Europe
3. The Print Revolution and its Impact
4. The Reading Mania
5. The Nineteenth Century
6. India and the World of Print
7. Religious Reform and Public Debates
8. New Forms of Publication
9. Print and Censorship
By studying these NCERT Solutions for Class 10 History, students can effectively prepare for their board exams and gain a deeper understanding of the subject.
Frequently Asked Questions History on 10th Class Chapter-Wise Solutions for NCERT Students
Q1. Is 10th History easy?
The difficulty level of Class 10 History may vary for different students based on their interests, study habits, and prior knowledge. Some students may find it easy, while others may find it challenging. To excel in the subject, consistent study, understanding of concepts, and practice are essential.
Q2. How can I pass the History exam?
To pass the History exam, follow these tips:
– Understand the syllabus and exam pattern.
– Take organized notes while studying.
– Create a study schedule and cover one topic at a time.
– Practice with previous years’ question papers and sample papers.
– Revise regularly and clarify doubts from teachers or study guides.
Q3. Which is the toughest chapter in Class 10 Social Science?
The perception of the toughest chapter may vary among students. Some common chapters that students often find challenging in Class 10 Social Science are “The Rise of Nationalism in Europe” and “The Making of a Global World.”
Q4. How to score full marks in SST Class 10?
Scoring full marks in Class 10 Social Science requires dedication and smart preparation:
– Thoroughly study each chapter, making sure to understand the concepts.
– Practice answering previous year’s question papers and sample papers.
– Focus on key events, dates, and important names.
– Use flow charts, diagrams, and mind maps to aid in better retention.
– Write clear and concise answers during exams.
Q5. How to complete the SST syllabus in 1 day?
Completing the entire SST syllabus in one day is a daunting task. However, if you have limited time, try to prioritize important topics from each chapter. Quickly go through the main concepts, key events, and dates. Focus on understanding the broader themes and connections between topics. It’s crucial to manage your time efficiently and make the most of the available study time. Keep in mind that deep learning and understanding may not be possible in one day, but a strategic overview can be helpful.
Q 6. How to study for exams?
To study for exams effectively, create a well-structured study schedule, break down the syllabus into manageable portions, and practice with past papers. Utilize active learning techniques like summarizing and making flashcards, and take regular breaks to avoid burnout. Find a quiet environment to study, stay focused, and minimize distractions. Ensure you get enough rest and sleep before the exam to be alert and perform at your best.
Q 7. How can I study hard?
Studying hard involves setting clear goals, maintaining consistency in your study routine, and staying organized. Challenge yourself with difficult concepts, seek help when needed, and maintain a positive attitude towards your studies. Believe in your abilities and be persistent in your efforts to achieve academic success.
Q 8. What is Chapter 2 in History Class 10?
Chapter 2 in Class 10 History is titled “Nationalism in India.” It explores the concept of nationalism, its connection to the French Revolution, and its manifestation in India during the anti-colonial movements. The chapter focuses on the Indian independence movement, highlighting the significance of the Non-Cooperation and Civil Disobedience Movements in the 1920s. These movements played a crucial role in shaping India’s struggle for independence against British colonial rule.
Class 10th How Do Organisms Reproduce Notes: Science Ch. 7
Class 10th How Do Organisms Reproduce Notes: All living beings have the ability to multiply or reproduce, giving rise to offspring of a similar nature. Reproduction is a fundamental process crucial for the survival of a species and the perpetuation of life.
In this chapter, we will delve into the fascinating world of reproduction, exploring the diverse methods employed by both unicellular and multicellular organisms. These include bacteria, algae, plants, animals, and humans. We shall explore the various reproductive structures they possess and the different modes of reproduction they engage in, such as cell division, vegetative reproduction, asexual reproduction, and sexual reproduction.
Class 10th How Do Organisms Reproduce Notes
Class 10th How Do Organisms Reproduce Notes
Reproduction
Reproduction is the natural mechanism through which every organism proliferates and enhances its population size
Asexual Reproduction
Asexual reproduction is a mode of reproduction wherein a single organism is solely responsible for generating two or more offspring. This process is observed in unicellular organisms, some multicellular organisms, and certain plant species.
Fission
Fission is a prevalent form of asexual reproduction observed in many unicellular organisms. Depending on the outcome, it can be classified into two types:
- Binary fission: This type of fission results in the formation of two daughter cells. An example of an organism undergoing binary fission is paramecium.
- Multiple fission: In this case, fission leads to the production of multiple daughter cells. An example of an organism undergoing multiple fission is Plasmodium.
It’s important to note that the planes of fission may vary among different organisms.
Budding
Budding is a form of asexual reproduction characterized by the development of a small cyst-like structure on the parent’s body, from which a new individual emerges.
The bud may either remain attached to the parent’s body, as observed in yeast, or it may eventually separate and become an independent new individual, as seen in hydra.
Regeneration and Fragmentation
Regeneration is the remarkable process through which an organism, such as a lizard, can grow back a lost organ or body part.
On the other hand, fragmentation is a process in which an organism breaks into smaller pieces, and each fragment has the ability to develop into a complete new organism. Examples of organisms capable of fragmentation and subsequent regeneration include Planaria and Hydra.
Spore Formation
Certain organisms, like fungi, employ spores as a means of reproduction. These spores have the potential to develop into entirely new individuals once they are released from their fruiting bodies.
The sporangia are responsible for producing these spores, which are encased in a resilient outer layer, providing protection during challenging conditions.
Under favorable environmental circumstances, spores germinate, initiating their growth and development into new organisms.
Vegetative Propagation
Vegetative propagation is a form of asexual reproduction commonly observed in plants. In this process, various vegetative parts of the plant, such as leaves, stems, and roots, have the ability to give rise to new individual plants.
Natural vegetative propagation occurs through different means, including:
1. Leaves, as seen in plants like bryophyllum.
2. Stems, as observed in plants like turmeric and ginger.
3. Runners or stolons, as found in grass runners and strawberry plants.
4. Bulbs, exemplified by plants like onion and lily.
In addition to natural methods, artificial techniques of vegetative propagation are also practiced, including cutting, grafting, layering, and plant tissue culture. These methods allow for the controlled and efficient propagation of desired plant varieties.
Class 10th Sexual Reproduction Notes
The reproductive mode entails the involvement of two individuals – one male and one female – who produce specialized sex cells or gametes. These gametes then fuse together to give rise to a new organism.
Types of Cell Division
Eukaryotic organisms exhibit two distinct types of cell division:
1. Mitosis:
– Occurs in somatic cells (non-reproductive cells).
– Maintains the original chromosome number.
– Yields two diploid daughter cells (with the same number of chromosomes as the parent cell).
– Essential for asexual reproduction, development, growth, cell replacement, and regeneration.
2. Meiosis:
– Occurs in sex cells (reproductive cells).
– Reduces the number of chromosomes by half through two consecutive divisions.
– Produces four haploid daughter cells (with half the number of chromosomes as the parent cell).
– Crucial for sexual reproduction, specifically in the formation of gametes (sperm and egg cells).
The Reproductive System
Humans exhibit a significant contrast between the male and female reproductive systems. In males, the testes serve as the primary reproductive structure responsible for producing sperm, which are the male gametes.
On the other hand, the ovary is the key reproductive organ in females, where the production of ova (female gametes) takes place. Now, let us delve into a comprehensive exploration of the male and female reproductive systems in humans.
Male Reproductive System: Class 10th How Do Organisms Reproduce Notes
In males, the primary reproductive organs consist of a pair of testes. These testes play a crucial role in producing male sex cells known as sperm, as well as secreting the male sex hormone, testosterone.
Male Primary Reproductive Organ
In males, the primary reproductive organs are a pair of testes, situated within scrotal sacs outside the body. These testes consist of seminiferous tubules, which serve as the structural and functional units responsible for the production of male sex cells, sperms. The maturation of these sperms occurs in the epididymis.
Furthermore, the spaces between the seminiferous tubules contain Leydig cells or interstitial cells, which play a vital role in secreting the hormone testosterone.
Male Accessory Reproductive Organs
The reproductive process is supported by various accessory organs.
Within the reproductive system, the prostate gland and seminal vesicles function as glands responsible for producing semen and providing nourishment to the sperm.
Moreover, the penis, which accommodates the urethra, serves as a copulatory organ facilitating the passage of semen during sexual intercourse.
Male Ducts
Within males, the primary ducts involved in the reproductive process are the vas deferens and the urethra.
Each testis is connected to a single vas deferens, which serves as a conduit for transporting sperm from the testis to the urethra.
The urethra functions as a shared passageway for both semen and urine, facilitating the expulsion of semen during ejaculation and the elimination of urine from the body.
Female Reproductive System: Class 10th How Do Organisms Reproduce Notes
The female reproductive system in humans comprises a set of paired organs, including ovaries and fallopian tubes (oviducts), alongside accessory organs such as the uterus and vagina.
Female Primary Reproductive Organ
In females, the primary reproductive organs are a pair of ovaries. These ovaries play a pivotal role in producing female sex cells known as eggs or ova. Additionally, the ovaries are responsible for producing the female sex hormones, estrogen and progesterone.
Female Accessory Reproductive Organ
In human females, the uterus, oviducts, and vagina function as essential accessory reproductive organs.
The uterus serves as the site for fetal development during pregnancy, while the vagina receives sperm from the male during sexual intercourse.
Furthermore, a pair of oviducts are responsible for carrying the ovum from the ovaries to the uterus, facilitating fertilization and potential pregnancy.
Menstrual Cycle
Menstruation
Menstruation is a cyclical process where the ovum is released from the ovary and expelled from the body if fertilization does not occur.
During menstruation, the blood-rich endometrium lining of the uterus also breaks down and is shed along with the unfertilized ovum.
Various hormones play crucial roles in this process, including two pituitary hormones, LH and FSH, as well as two ovarian hormones, estrogen and progesterone.
In humans, this menstrual cycle typically repeats approximately every 28 days.
Fertilization
Human Reproduction
Humans reproduce sexually. The male produces sperms and the female produces eggs. When the sperm fuses with the egg, it forms a zygote that gives rise to a new progeny.
Contraceptive Methods

Reproductive Health
Reproductive health encompasses efforts aimed at preventing sexually transmitted diseases (STDs) and unwanted pregnancies. Moreover, fostering awareness and understanding of the reproductive system is an integral aspect of promoting reproductive health.
Contraceptives
Contraceptives are tools designed to prevent unwanted pregnancies and aid in the prevention of STDs.
These contraceptives come in various types, including mechanical barriers, hormonal/chemical methods, surgical methods, and more.
Their diverse range provides individuals with options to make informed choices regarding their reproductive health and family planning.
Coitus Interruptus
This method of contraception is highly unreliable and involves interrupting sexual intercourse before the male ejaculates inside the female reproductive tract.
Rhythm Method
Another unreliable method of contraception involves avoiding sexual intercourse when the female is fertile, and the chances of fertilization are significantly elevated.
Condoms:
Among the most effective contraception methods, condoms act as mechanical barriers, preventing semen from entering the female reproductive tract and thus avoiding pregnancy. Additionally, they provide protection against the transmission of STDs.
Diaphragms:
Diaphragms are barriers placed inside the female reproductive tract, effectively blocking semen entry and preventing pregnancy.
Contraceptive Pills:
These are chemical methods of contraception that modify hormone levels in the body, hindering the release of the ovum from the ovaries.
Emergency Pill:
Emergency pills can be taken after sexual intercourse to prevent pregnancy. They swiftly alter hormone levels, hindering successful implantation, even if fertilization has occurred.
IUD (Intrauterine Device):
IUDs are devices inserted into the uterus to alter its shape, effectively preventing the successful implantation of the zygote. They offer long-term contraception for several years.
Sterilization:
Sterilization is a permanent surgical method to achieve infertility. In males, it is called vasectomy, and in females, it is known as tubal ligation.
Reproduction in Plants
Plants employ both asexual and sexual methods for reproduction. Asexual reproduction is achieved through vegetative propagation. Now, let’s explore the process of sexual reproduction in plants.
Sexual Reproduction in Flowering Plants:
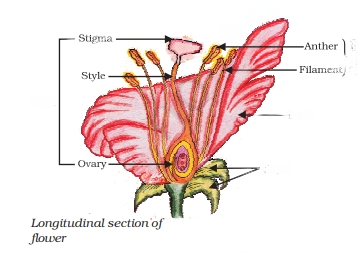
Sexual reproduction in plants occurs through flowers. The androecium and gynoecium are essential whorls of the flower involved in this process.
Non-Essential Parts of Flowers:
Apart from the essential whorls, flowers also have non-essential whorls, including sepals and petals. These parts do not directly participate in reproduction. Sepals protect the inner delicate parts during bud formation and, if green, perform photosynthesis. Petals, when colored, attract insects for pollination.
Essential Whorls of Flowers:
The androecium and gynoecium are considered the essential or reproductive whorls of a flower. The androecium produces pollen grains containing male gametes, while the gynoecium produces ovules, which are female gametes. Flowers may be bisexual (containing both whorls) or unisexual (containing either one).
Pollination:
Pollination is the transfer of pollen grains from anthers to the stigma of a flower, a necessary step for fertilization. It can occur through self-pollination (within the same flower or another flower of the same plant) or cross-pollination (between different flowers). Various agents, such as water, wind, insects, birds, and bats, play roles in cross-pollination.
Fertilization:
Fertilization involves the fusion of male and female gametes. After pollination, pollen grains germinate on the stigma surface, producing two male nuclei. The ovule contains an egg cell and two polar nuclei.
One male nucleus fuses with the polar nuclei to form a triploid endosperm. The other male nucleus fuses with the egg cell to form the zygote, which develops into the embryo and the future plant. After fertilization, the ovary transforms into a fruit, and the ovules develop into seeds, while other flower parts wither away.
Read More:
- Class 10 Notes for Science NCERT
- Chemical Reaction and Equation Notes Class 10 NCERT Sci. Ch.1
- Acids Bases and Salts Class 10 Notes of NCERT Science Ch. 2
- Metals and Non Metals Class 10 NCERT Science Chapter 3 Notes
- Life Process Notes Class 10 NCERT Science Chapter 5
- Control and Coordination Notes Class 10: NCERT Science Ch. 6
- Class 10th Heredity and Evolution Notes: NCERT Science Ch. 8
- Notes on Magnetic Effect of Electric Current Class 10 NCERT
- Human Eye and the Colourful World Notes Chapter 10 Science
- Class 10th Chapter 11 Science Notes for NCERT Students
- Notes of Our Environment Class 10: NCERT Science Chapter 13
Frequently Asked Questions – FAQs on Class 10th How Do Organisms Reproduce Notes
Q 1. What is an example of an organism reproduce?
One example of an organism that reproduces is the honeybee (Apis mellifera). Honeybees engage in sexual reproduction, where the male drone transfers sperm to the queen during mating.
The queen then stores the sperm in her spermatheca and uses it to fertilize eggs throughout her life. Honeybees also exhibit a unique form of asexual reproduction called parthenogenesis.
Unfertilized eggs laid by the queen develop into male drones, while fertilized eggs give rise to female worker bees or potential new queens. This diverse reproductive strategy ensures the continuation of the honeybee colony and enables them to adapt and thrive in various environments.
Q 2. Why do organisms reproduce?
Organisms reproduce to ensure the continuation of their species and the perpetuation of life. Reproduction is a fundamental biological process that allows organisms to pass on their genetic material to the next generation.
Through reproduction, organisms produce offspring with traits that enhance their survival and adaptation to changing environments. It also aids in maintaining biodiversity and ecological balance.
Reproduction is essential for the growth and development of populations, contributing to the stability and sustainability of ecosystems. It is a fundamental drive ingrained in the biological imperative of all living beings, ensuring the survival and success of their species over time.
Q 3. What are the 3 types of reproduction?
The three main types of reproduction are: a) Asexual reproduction: Involves a single parent, and offspring are genetically identical to the parent. b) Sexual reproduction: Involves two parents, and offspring inherit a combination of genetic traits from both parents. c) Parthenogenesis: A form of asexual reproduction where unfertilized eggs develop into offspring without involving males.
Q 4. According to Class 10th How Do Organisms Reproduce Notes why is reproduction important?
Reproduction is vital for the survival and continuity of life on Earth. It ensures the perpetuation of species, the maintenance of genetic diversity, and the adaptation to changing environments. Through reproduction, organisms pass on their genetic material to future generations, allowing them to adapt and evolve over time. It is essential for the growth of populations, the balance of ecosystems, and the functioning of biological communities.
Q 5. Is reproduction a life process?
Yes, reproduction is considered a life process. It is one of the fundamental characteristics of living organisms, distinguishing them from non-living entities. Reproduction is crucial for the continuation of life and is inherent in the biological imperative of all living beings. Without reproduction, species would cease to exist, and life as we know it would not be sustained.
Class 10th Heredity and Evolution Notes: NCERT Science Ch. 8
In this chapter “class 10th heredity and evolution notes“, we delve into the fascinating world of heredity and evolution. Heredity involves the transfer of characteristics from one generation to the next. While evolution encompasses the gradual progression from simple life forms to more complex organisms over multiple generations.
Here in class 10th heredity and evolution notes, we explore the mechanisms behind the creation of variations. Also, how the accumulation of these variations ultimately drives the process of evolution. Get ready to unravel the mysteries of life’s continuity and transformation.
Class 10th heredity and evolution notes of NCERT Science Ch. 8
A cross that involves the consideration of only one character between two organisms is referred to as a monohybrid cross. The resulting ratio of characters in the F2 generation is known as the monohybrid ratio.
For example, when a tall plant (TT) is crossed with a dwarf plant (tt), the F2 generation yields a ratio of 3 tall plants to 1 short plant, which represents the monohybrid ratio of 3:1. In this cross, only the height of the plants is taken into account at a time, illustrating the principles of monohybrid inheritance.
Notes for Class 10th Chapter Heredity and Evolution
Dihybrid cross
A cross that involves the consideration of two characters between two organisms is termed a dihybrid cross. The resulting ratio of characters in the F2 generation is referred to as the dihybrid ratio.

For example, if a plant with round and green peas is crossed with a plant having wrinkled and yellow peas, the first-generation plants would all exhibit round and green peas. In the subsequent F2 generation cross, we would observe four combinations of characters in the ratio of 9:3:3:1.
Thus, the dihybrid ratio is represented as 9:3:3:1. IT is indicating the occurrence of different combinations of traits resulting from the dihybrid cross between the two organisms.
Inheritance
In Biology, inheritance refers to the transmission of traits from one generation to the next.
Laws of Mendel as define in Heredity and Evolution class 10th
The Law of Dominance states that a gene possesses two contrasting alleles, and one of them always expresses itself in the organism. This allele is known as the dominant gene, and it can manifest in any possible combination.
According to the Law of Segregation, traits are completely separated during gamete formation, with no mixing of alleles. Each gamete carries only one allele for a particular trait.
The Law of Independent Assortment reveals that traits can segregate independently of different characters during gamete formation. This means that the inheritance of one trait does not influence the inheritance of another trait. It is allowing for diverse combinations of traits in offspring.
Class 10th Heredity and Evolution Notes
Sex Determination
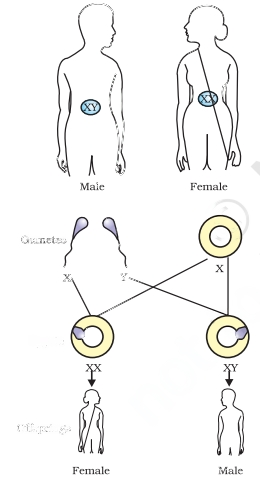
Sex determination is the process of identifying an individual’s sex based on their genetic material composition. In various animals, the sex of an embryo is determined by diverse factors. In humans, sex determination occurs through the presence or absence of the Y chromosome.
A female human is represented by XX chromosomes, while a male is represented by XY chromosomes. An ovum always carries an X chromosome. When the ovum fuses with a sperm carrying a Y chromosome, it results in the birth of a male child. On the other hand, when it fuses with a sperm carrying an X chromosome, it leads to the birth of a female child.
This process of sex determination plays a vital role in defining an individual’s gender. It is governed by specific genetic factors that shape human development and reproduction.
What is Traits in Class 10th Heredity and Evolution
Traits are distinguishing characteristics of an organism, evident either in its visible physical form or in its physiological aspects.
Acquired Characters
Acquired characteristics refer to the traits acquired by an organism during its lifetime. However, these characteristics are not transmitted to the DNA of germ cells and, consequently, are not inherited by the next generation. For instance, traits like muscle loss and reduced weight due to starvation, loss of limbs or tails from injuries, and similar acquired features are not passed on to future generations.
Class 10th Heredity and Evolution Notes
Inherited Characters
Inherited characters refer to the traits passed down from parents to their offspring. These traits are consistently transmitted to the next generation, but their expression depends on whether they are dominant or recessive.
For instance, characteristics like height, skin color, and eye color are examples of inherited traits. While they are reliably inherited, their manifestation in the offspring can vary based on whether the alleles governing these traits are dominant or recessive. The interplay of dominant and recessive alleles determines the observable expression of these inherited characters in the offspring.
Variation
Variation represents the extent of dissimilarity among individuals within the same species. Offspring are not identical to their parents, exhibiting distinct variations. Each member of a population differs from others due to the influence of recombination and mutation. Which serve as the primary causes of these variations.
Sexually reproducing organisms display considerable diversity among individuals of a species, and the long-term accumulation of variations plays a pivotal role in the process of evolution. Environmental factors play a crucial part in evolutionary processes as they select advantageous variants, driving the progression of evolution.
Genetic Variations
Genetic variations refer to the dissimilarities in DNA sequences among organisms, contributing to the diverse gene pool. These variations result in distinct physical characteristics or biochemical pathways, showcasing the unique traits exhibited by each individual.
Natural Selection
Natural selection is the process through which a favorable trait within a species’ population is chosen. Changing environmental conditions exert equal pressure on all existing species. The species or organisms that demonstrate better adaptation to the altering conditions survive and reproduce, signifying their selection by nature.
Conversely, species or organisms that struggle to adapt face elimination, being rejected by nature. Natural selection thus acts as a driving force, shaping the survival and evolution of species in response to dynamic environmental changes.
Class 10th Heredity and Evolution Notes
Speciation
Genetic Drift
In the determination of traits that endure within a population, natural selection assumes a significant role. Nevertheless, random fluctuations in gene variants can be observed on various occasions, which is termed genetic drift. Genetic drift denotes a change in the frequency of an existing allele within a small population.
As a consequence of genetic drift, a gene variant may diminish and eventually disappear from the population, leading to a reduction in genetic variation. This phenomenon highlights the influence of chance events on the genetic makeup of small populations, and it has implications for the evolutionary trajectory of species over time.
Speciation
Speciation is the transformative process through which new species emerge from existing ones, driven by various evolutionary forces such as genetic drift, population isolation, and natural selection. This intricate process gives rise to diversity within ecosystems, and this diversity, in turn, fuels the continued evolution of life forms.
As species adapt to their unique environments and encounter diverse ecological challenges, they undergo changes that set them apart from their ancestral populations. Over time, these modifications accumulate, leading to the formation of distinct species with specialized characteristics. The diverse array of species contributes to the complexity and richness of ecosystems, driving the ongoing process of evolution and perpetuating the fascinating tapestry of life on Earth.
Gene Flow: Class 10th Heredity and Evolution Notes
Gene flow involves the movement of genes from one population to another, often facilitated by migration or the introduction of organisms to new populations. As genes are transferred between populations, the frequencies of certain genes change, leading to alterations in the genetic makeup of the receiving population.
This dynamic process of gene flow helps in the exchange and dissemination of genetic traits among different groups, contributing to genetic diversity and evolution within and across species.
Population
A population refers to a community or a collective of animals, plants, or any living organism capable of reproducing with one another and producing fertile, viable offspring.
Charles Darwin
Charles Darwin, renowned as the “Father of Evolution,” was an English naturalist and biologist. His groundbreaking theory of evolution was shaped during a five-year expedition aboard the HMS Beagle to the Galapagos Islands. This remarkable journey provided him with invaluable insights into the diversity of life and the processes of adaptation and natural selection.
In 1859, Darwin unveiled his comprehensive theory of evolution in his influential book titled “On the Origin of Species.” In this seminal work, he expounded on the concept of natural selection and its role in shaping the development and diversity of species over time. Darwin’s pioneering contributions revolutionized the field of biology and laid the foundation for our modern understanding of the evolutionary processes that govern life on Earth.
Evolution and Fossils
Evolution represents a measurable transformation in the heritable traits of a population across multiple generations. These alterations can lead to the emergence of a new species or enable existing species to adapt and thrive in their environment more effectively.
Class 10th Heredity and Evolution Notes on Origin of Species
Following a fruitful expedition aboard HMS Beagle, Charles Darwin penned a book detailing his observations from the Galapagos Islands. Titled ‘The Origin of Species,’ the book presented a comprehensive theory of evolution, primarily founded on the concept of Natural Selection.
Origin of Life – Haldane’s Theory
JBS Haldane, a distinguished British scientist, postulated the idea that life originated from organic and lifeless matter. This hypothesis was later substantiated by Urey and Miller’s experiment, confirming the validity of his theory, which became known as the theory of abiogenesis.
Evolutionary Evidence – Fossils
Numerous pieces of evidence strongly support the theory of evolution, with fossils being among the most compelling. Fossils are the well-preserved remnants of ancient animals or plants that existed millions of years ago.
These remarkable relics provide invaluable insights into the anatomy and physiology of these organisms, enabling us to comprehend the mechanisms of evolution and how they culminated in the development of the diverse life forms we encounter today.
Fossils serve as crucial time capsules, shedding light on the fascinating history of life on Earth and bolstering our understanding of the gradual transformation and diversification of species over time.
Formation of Fossils
Fossils play a crucial role in providing evidence for evolution and are created through the following steps:
1. Organisms perish and become buried in mud and silt.
2. Soft tissues decompose rapidly, leaving behind the durable bones or shells.
3. Over time, sediment accumulates and solidifies into rock.
4. As the bones decay, minerals gradually infiltrate and replace the cell contents, a process known as petrification.
5. If the bones decompose entirely, they leave behind the impression or cast of the original animal, preserving its form in the fossil record.
Evolutionary Relationships: Class 10th Heredity and Evolution Notes
Studying homologous and analogous organs offers insights into the evolutionary relationships among animals.
Homologous organs exhibit similar structures but serve different functions:
– For instance, the wings of birds and the forelimbs of mammals share similar structures, but they have been modified to suit different functions.
– Similarly, the tendril of a pea plant and the spine of a barberry plant are both modified leaves, yet they perform distinct functions.
Analogous organs, on the other hand, serve similar functions but have different structures and origins:
– The wings of bats, birds, and insects, for example, are all used for flying, but their structures vary significantly.
– Likewise, the leaves of opuntia and peepal both perform photosynthesis, but while the leaves of opuntia are modified stems, the peepal leaves are typical leaves.
Evolution by Stage
It is a gradual and extended process, unfolding over time rather than occurring abruptly. Nearly all present-day animals have undergone several stages of evolution in their journey to their current forms.
The complexities of organisms do not arise suddenly but progress incrementally, with certain traits serving limited purposes during specific stages.
This gradual progression in the evolution of species is known as “evolution by stages.” It involves a step-by-step development, where small changes accumulate over generations, leading to the emergence of new traits and characteristics.
Evolutionary transformations unfold over extended periods, encompassing numerous intermediary stages, reflecting the intricate and fascinating journey that shapes the diversity of life on Earth.
Artificial Selection
Artificial selection can lead to the evolution of multiple distinct species from a single ancestral species. A prime example is the cabbage family, where a common ancestor gave rise to several diverse species through the deliberate selection of various traits.
Through human intervention and cultivation practices, specific desirable traits were favored and perpetuated. Resulting in the emergence of distinct cabbage species with unique characteristics and adaptations. Artificial selection, driven by human influence, demonstrates the remarkable capacity to shape the course of evolution and contribute to the diversity and specialization observed in the natural world.
Molecular Phylogeny
Phylogeny refers to the evolutionary connections between various biological species, giving rise to an evolutionary tree. In molecular phylogeny, these relationships are explored at the molecular level, primarily through the examination of DNA sequences. This entails the analysis of DNA composition and gene comparisons among different species.
By studying the hereditary molecular traits, such as DNA sequences, scientists can unravel the intricate evolutionary history and relationships among diverse organisms. Molecular phylogeny offers valuable insights into the common ancestry and evolutionary divergence of species, shedding light on the dynamic and interconnected nature of life’s evolutionary journey.
Human Evolution
Human Evolution
Humans are categorized within the primate family, and their genetic connection to chimps and other primates is remarkably close. Although the full evolutionary journey from primates to humans remains a mystery, a broader understanding of human evolution has emerged.
The ancestry of humans includes various predecessors like Dryopithecus, Ramapithecus, Australopithecus, Homo erectus, Homo sapiens neanderthalensis, Cro-magnon man, and ultimately, Homo sapiens – us.
The study of these ancestors and their characteristics provides valuable insights into the fascinating path of human evolution. While some aspects of our evolutionary history may remain elusive, the evidence gathered from these ancestral relatives paints a vivid picture of the transformation that led to the emergence of modern humans.
Read Also:
- Class 10 Notes for Science NCERT
- Chemical Reaction and Equation Notes Class 10 NCERT Sci. Ch.1
- Acids Bases and Salts Class 10 Notes of NCERT Science Ch. 2
- Metals and Non Metals Class 10 NCERT Science Chapter 3 Notes
- Life Process Notes Class 10 NCERT Science Chapter 5
- Control and Coordination Notes Class 10: NCERT Science Ch. 6
- Class 10th How Do Organisms Reproduce Notes: Science Ch. 7
- Class 10th Heredity and Evolution Notes: NCERT Science Ch. 8
- Notes on Magnetic Effect of Electric Current Class 10 NCERT
- Human Eye and the Colourful World Notes Chapter 10 Science
- Class 10th Chapter 11 Science Notes for NCERT Students
- Notes of Our Environment Class 10: NCERT Science Chapter 13
Frequently Asked Questions on Class 10th Heredity and Evolution Notes
Q 1. what are the sources of genetic diversity within a population?
The sources of genetic diversity within a population include mutations, which introduce new variations in the DNA sequence. Genetic recombination during sexual reproduction, creating unique combinations of alleles. Gene flow, through migration or interbreeding, brings in new genes from other populations. Natural selection favors advantageous traits, increasing their frequency, while genetic drift, in small populations, causes random changes in gene frequencies. These processes collectively contribute to the variability of traits in a population, enabling adaptation to changing environments and promoting species survival and evolution.
Q 2. From Class 10th Heredity and Evolution Notes How you can explain the evolution diversity of life on Earth?
The Evolution explains the diversity of life on Earth through the gradual process of genetic change and natural selection. Over millions of years, genetic variations arise within populations due to mutations and genetic recombination during reproduction. Natural selection then acts on these variations, favoring advantageous traits that increase an organism’s chances of survival and reproduction in specific environments. As a result, organisms with beneficial traits pass on their genes to future generations, leading to the accumulation of adaptive characteristics and the diversification of species. This continuous process of genetic change and selection has resulted in the vast array of life forms and their adaptations to different ecological niches on Earth.
Q 3. What is the role of adaptation in the survival and success of organisms in various environments?
Adaptation plays a crucial role in the survival and success of organisms in various environments. It refers to the process by which organisms develop advantageous traits that enable them to better suit their surroundings. Organisms with beneficial adaptations have increased chances of survival, reproduction, and passing on their advantageous genes to the next generation. In different environments, such as extreme temperatures, limited resources, or predators, adaptations can provide a competitive advantage. Over time, these advantageous traits become more prevalent in the population, leading to the specialization and diversification of species, ultimately enhancing their ability to thrive and persist in their respective habitats.
Q 4. What is the significance of studying heredity and evolution in fields like medicine and agriculture?
Studying heredity and evolution holds immense significance in fields like medicine and agriculture. In medicine, understanding heredity helps identify genetic factors responsible for various diseases, enabling personalized treatments and genetic counseling. Knowledge of evolution aids in comprehending the origin of diseases and their potential spread. In agriculture, studying heredity assists in breeding programs to develop crops with desired traits, such as higher yield or resistance to pests and diseases. Understanding evolutionary principles aids in combating evolving pests and diseases, improving crop resilience, and developing sustainable agricultural practices. Both fields benefit from the insights gained through the study of heredity and evolution, leading to advancements and better strategies for human health and food security.
Q 5. What is heredity, and how does it influence the transmission of traits from one generation to another?
Heredity is the process by which traits or characteristics are passed from parents to their offspring through genetic information encoded in DNA. It influences the transmission of traits from one generation to another by the inheritance of genes. Offspring inherit a combination of genes from their parents, determining their physical and biochemical traits. Genes come in different forms called alleles. The combination of alleles inherited from each parent influences the expression of traits in the offspring. Some traits may be dominant, and their alleles are expressed over recessive traits. Heredity ensures continuity and variation in populations, allowing the transfer of genetic information and shaping the diversity of life on Earth.