Category: Class 10
Molecular Mass of Na
Molecular Mass of Na: Sodium, with its symbol “Na” derived from the Latin word “natrium,” is a highly reactive chemical element found in group 1 (or Group IA) of the periodic table.
This metal is well-known for its importance in various biological processes and industrial applications. To understand the properties and utility of sodium, it’s essential to explore its molecular mass, also referred to as atomic weight.
In this article, we will delve into the molecular mass of sodium and its significance in both scientific research and practical applications.

Molecular Mass of Na
The Atomic Structure of Sodium
Before delving into the molecular mass of sodium, it’s crucial to understand its atomic structure. Sodium, with the atomic number 11, contains 11 electrons in various energy levels or shells surrounding its nucleus. Its atomic mass is approximately 22.99 atomic mass units (amu). Sodium has only one stable isotope, sodium-23 (Na-23), which accounts for its average atomic mass.
Molecular Mass vs. Atomic Mass
To avoid confusion, it’s important to differentiate between atomic mass and molecular mass. Atomic mass refers to the mass of a single atom of an element, while molecular mass (or molecular weight) is the mass of a molecule, which consists of two or more atoms. In the case of sodium, it typically exists as individual sodium atoms (Na) rather than diatomic molecules.5
Calculating the Molecular Mass of Sodium (Na)
To determine the molecular mass of sodium, we consider the atomic mass of a single sodium atom:
- The atomic mass of sodium (Na) is approximately 22.99 atomic mass units (amu).
- Since sodium is primarily encountered as single atoms, there is no molecular mass to calculate beyond the atomic mass.
Significance of Molecular Mass in Sodium
While the concept of molecular mass is less applicable to sodium, the atomic mass of sodium is crucial for various reasons:
- Chemical Reactions: Sodium is highly reactive and readily forms compounds with other elements and compounds. Its atomic mass plays a vital role in stoichiometry, helping to determine the amounts of reactants and products in chemical reactions.
- Electrolytes in Biology: Sodium ions (Na+) are essential electrolytes in biological systems, playing a critical role in nerve function, muscle contraction, and maintaining fluid balance in the body.
- Industrial Applications: Sodium and its compounds are used in various industrial processes, including the production of chemicals, metallurgy, and as coolants in nuclear reactors.
- Salt Production: Sodium chloride (table salt) is a ubiquitous seasoning and food preservative, and the atomic mass of sodium is a key factor in determining the salt’s composition and properties.
- Energy Storage: Sodium-ion batteries, a potential alternative to lithium-ion batteries, rely on the chemistry of sodium. The atomic mass of sodium is important for designing efficient energy storage systems.
Conclusion
Sodium, a versatile and reactive element, has an atomic mass of approximately 22.99 atomic mass units (amu). While sodium primarily exists as individual atoms (Na) rather than molecules, its atomic mass is fundamental to its chemical properties and various applications. Understanding the atomic mass of sodium is essential for predicting its behavior in chemical reactions, its roles in biological systems, and its utility in industries ranging from metallurgy to energy storage. Sodium’s significance in both scientific research and practical applications underscores its importance in our modern world.
Read More
- Chemical Properties of Acid
- Molar Mass of Benzene
- Molar Mass of Calcium
- Electromagnetic Spectrum X Rays
- Electric Circuit Electrical Symbols
Frequently Asked Questions (FAQs) Molecular Mass of Na
What is the molecular mass of sodium (Na)?
Sodium, typically encountered as individual sodium atoms (Na), does not have a molecular mass in the traditional sense. Its atomic mass is approximately 22.99 atomic mass units (amu).
What is the difference between atomic mass and molecular mass?
Atomic mass refers to the mass of a single atom of an element, such as a sodium atom (Na). Molecular mass (or molecular weight) refers to the mass of a molecule, which consists of two or more atoms chemically bonded together. Sodium is primarily found as individual atoms, so its molecular mass is not typically calculated.
Why is the atomic mass of sodium important?
The atomic mass of sodium is crucial in various fields, including chemistry and biology. It plays a significant role in stoichiometry, determining the quantities of reactants and products in chemical reactions. In biology, sodium ions (Na+) are essential for various physiological processes.
Is sodium always found as single atoms (Na) in its natural state?
Sodium is a highly reactive metal and is typically found as individual sodium atoms (Na) in its natural state. It readily forms compounds with other elements and molecules due to its reactivity.
What are some common uses of sodium in industry?
Sodium and its compounds have numerous industrial applications. These include metallurgy (sodium is used as a reducing agent), chemical production (sodium compounds are used in the manufacture of various chemicals), and nuclear reactors (sodium is used as a coolant).
Chemical Properties of Acid
Chemical Properties of Acid: Acids are a class of chemical compounds known for their distinct chemical properties. They play a vital role in various chemical reactions, industrial processes, and natural phenomena.
In this article, we will explore the chemical properties of acids, including their behavior in aqueous solutions, their reactivity, and their importance in chemistry and everyday life.

Chemical Properties of Acid
1. Acids and Their Properties
1.1. Definition of Acids
Acids are substances that can donate protons (H+) or accept pairs of electrons in chemical reactions. This proton-donating characteristic is at the heart of their chemical properties.
1.2. Acidic Taste and pH
Acids often have a sour taste, which is why substances like lemon juice and vinegar taste sour. The acidity of a substance is measured on the pH scale, where acids have pH values less than 7. The lower the pH, the stronger the acid.
2. Behavior of Acids in Aqueous Solutions
2.1. Formation of Hydronium Ions
When acids dissolve in water, they release protons (H+ ions), which immediately bond with water molecules to form hydronium ions (H3O+). This is represented in chemical equations as follows:
Acid (HA) + Water (H2O) → Hydronium Ion (H3O+) + Conjugate Base (A-)
2.2. Strong and Weak Acids
Acids can be classified as strong or weak based on their ability to dissociate in water. Strong acids dissociate almost completely in solution, while weak acids only partially dissociate. The degree of dissociation determines the concentration of hydronium ions in the solution, influencing the acidity.
3. Chemical Reactivity of Acids
3.1. Neutralization Reactions
One of the most well-known chemical properties of acids is their ability to react with bases to form water and salts. This reaction is called neutralization and is represented by the following general equation:
Acid (HA) + Base (BOH) → Water (H2O) + Salt (BA)
For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) results in the formation of water and sodium chloride (table salt):
HCl + NaOH → H2O + NaCl
3.2. Corrosive Properties
Concentrated acids can exhibit corrosive properties when they come into contact with various materials, including metals, due to their ability to donate protons and react with surfaces.
3.3. Reactivity with Carbonates
Acids react vigorously with carbonates to produce carbon dioxide gas, water, and a salt. For example, when hydrochloric acid (HCl) is added to calcium carbonate (CaCO3), the following reaction occurs:
2HCl + CaCO3 → CO2 + H2O + CaCl2
4. Importance of Acids in Everyday Life and Industry
4.1. Food and Beverage Industry
Acids are commonly used in the food and beverage industry to impart tartness and flavor to various products. Citric acid, for instance, is found in citrus fruits and used as a food additive.
4.2. Pharmaceuticals
Acids are essential in pharmaceuticals for drug formulation and chemical synthesis. They play a role in adjusting the pH of medications and are used in the production of various pharmaceutical compounds.
4.3. Chemical Manufacturing
Acids are crucial in the chemical manufacturing industry for processes like polymerization, catalysis, and the production of fertilizers, explosives, and dyes.
4.4. Environmental Impact
Acids can have a significant environmental impact, especially when they are released into water bodies. Acid rain, which results from the presence of sulfuric acid (H2SO4) and nitric acid (HNO3) in the atmosphere, can harm ecosystems, damage buildings, and affect aquatic life.
Conclusion
Acids exhibit a wide range of chemical properties that make them essential in various scientific, industrial, and everyday applications. From their ability to donate protons and react with bases to their role in adjusting pH and their impact on the environment, the chemical properties of acids play a crucial role in shaping the world of chemistry and our daily lives. Understanding these properties is fundamental for both scientists and the general public.
Read More
- Molar Mass of Benzene
- Molar Mass of Calcium
- Electromagnetic Spectrum X Rays
- Electric Circuit Electrical Symbols
- Difference Between Watts And Volts
Frequently Asked Questions (FAQs) Chemical Properties of Acid
Q1: What is the pH scale, and how does it relate to the chemical properties of acids?
A1: The pH scale is a measure of the acidity or alkalinity of a substance. It ranges from 0 to 14, here pH 7 is neutral, values below 7 indicate acidity (with lower numbers indicating stronger acidity), and values above 7 indicate alkalinity (with higher numbers indicating stronger alkalinity). Acids have pH values below 7, with lower pH values indicating stronger acidity.
Q2: Can you provide examples of common acids found in everyday life?
A2: Certainly! Common acids found in everyday life include:
- Hydrochloric acid (HCl) in gastric juice.
- Citric acid in citrus fruits like lemons and oranges.
- Acetic acid (vinegar) used in cooking.
- Sulfuric acid (H2SO4) used in car batteries.
- Carbonic acid (H2CO3) in carbonated beverages.
Q3: What is the difference between a strong acid and a weak acid in terms of their chemical properties?
A3: The main difference between strong acids and weak acids is their degree of dissociation in water. Strong acids almost completely dissociate into ions (e.g., H+ and A-) in aqueous solutions, resulting in high concentrations of hydronium ions (H3O+). In contrast, weak acids only partially dissociate, leading to lower concentrations of hydronium ions.
Q4: How do acids contribute to corrosion, and what are some examples of corrosive acids?
A4: Acids can contribute to corrosion by reacting with metals to produce metal salts and hydrogen gas. For example, hydrochloric acid (HCl) can corrode metals like iron, zinc, and aluminum. Sulfuric acid (H2SO4) is another extremely corrosive acid employed across a wide range of industrial applications.
Q5: What happens when an acid reacts with a carbonate substance?
A5: When an acid reacts with a carbonate substance, it produces carbon dioxide gas (CO2), water (H2O), and a salt. This reaction is characterized by effervescence or the release of bubbles of carbon dioxide gas. For example, the reaction between hydrochloric acid (HCl) and calcium carbonate (CaCO3) results in the formation of carbon dioxide, water, and calcium chloride (CaCl2).
Molar Mass of Benzene
Molar Mass of Benzene: Benzene, a six-carbon, six-hydrogen ring structure, is a quintessential compound in the world of organic chemistry. This article will explore the molar mass of benzene (C6H6) and its significance in various chemical applications.

Molar Mass of Benzene
The Significance of Benzene
Benzene is an aromatic hydrocarbon and one of the fundamental building blocks in organic chemistry. Its hexagonal ring structure and resonance contribute to its stability, making it a cornerstone of various chemical reactions and the synthesis of countless organic compounds. Benzene is also well-known for its unique odor and its presence in many natural sources like crude oil and gasoline.
Molecular Structure of Benzene
Before delving into the molar mass of benzene, it’s essential to understand its molecular structure. A benzene molecule consists of six carbon (C) atoms and six hydrogen (H) atoms, arranged in a symmetrical hexagonal ring. Each carbon atom forms a sigma bond with one hydrogen atom and two sigma bonds with neighboring carbon atoms. This arrangement results in a highly stable, planar, and symmetrical molecule.
Molar Mass of Benzene
The molar mass of a compound is defined as the mass of one mole of that compound, measured in grams per mole (g/mol). To calculate the molar mass of benzene (C6H6), we sum the atomic masses of its constituent atoms.
- Carbon (C) has an atomic mass of approximately 12.01 g/mol.
- Hydrogen (H) has an atomic mass of approximately 1.01 g/mol.
Now, let’s calculate the molar mass of benzene:
Molar Mass of Benzene (C6H6) = (6 × Atomic Mass of Carbon) + (6 × Atomic Mass of Hydrogen)
Molar Mass of Benzene (C6H6) = (6 × 12.01 g/mol) + (6 × 1.01 g/mol)
Molar Mass of Benzene (C6H6) ≈ 72.06 g/mol
So, the molar mass of benzene is approximately 72.06 grams per mole.
Significance of Molar Mass in Chemistry
Understanding the molar mass of benzene is crucial for several reasons. It is a fundamental parameter for stoichiometry, allowing chemists to determine the number of moles of benzene involved in a chemical reaction. This, in turn, helps in calculating reactant and product quantities, which is essential for formulation and balancing of chemical equations.
Additionally, the molar mass of benzene is integral in various applications, such as in gas chromatography and mass spectrometry, where it is used to identify and quantify compounds in complex mixtures.
Conclusion
The mol mass of benzene, approximately 72.06 g/mol, is a key piece of information for chemists and scientists working with this iconic aromatic compound. It facilitates precise measurements, stoichiometry calculations, and a deeper understanding of benzene’s role in the vast realm of organic chemistry. Whether in the laboratory or industrial settings, a grasp of benzene’s molar mass is indispensable for harnessing the versatility of this fundamental organic molecule.
Read More:
- Molar Mass of Calcium
- Electromagnetic Spectrum X Rays
- Electric Circuit Electrical Symbols
- Difference Between Watts And Volts
- Molar Mass Of Sulphur
Frequently Asked Questions (FAQs) mol Mass of Benzene
Q1: What is the mol mass of benzene?
A1: The mol mass of benzene (C6H6) is approximately 72.06 grams per mole (g/mol).
Q2: Why is the mol mass of benzene important in chemistry?
A2: The mol mass of benzene is crucial because it allows chemists to relate the mass of benzene molecules to the number of molecules present. It is essential for stoichiometry, which involves determining reactant and product quantities in chemical reactions, as well as for identifying and quantifying compounds in analytical techniques like gas chromatography and mass spectrometry.
Q3: What is the molecular structure of benzene?
A3: Benzene has a hexagonal ring structure consisting of six carbon (C) atoms and six hydrogen (H) atoms. Each carbon atom forms a sigma bond with one hydrogen atom and two sigma bonds with neighboring carbon atoms, resulting in a highly stable and symmetrical molecule.
Q4: How is the mol mass of benzene calculated?
A4: The mol mass of benzene is calculated by adding the atomic masses of its constituent atoms. Carbon (C) has an atomic mass of approximately 12.01 g/mol, and hydrogen (H) has an atomic mass of approximately 1.01 g/mol. To find the mol mass of benzene, you sum the masses of the six carbon atoms and six hydrogen atoms in its chemical formula.
Q5: What are some practical applications of benzene in chemistry and industry?
A5: Benzene is a versatile compound used in the synthesis of various organic chemicals. Its applications include the production of plastics, synthetic fibers, dyes, drugs, and pesticides. It is also a key component in the production of gasoline and other fuels. However, due to its toxicity and carcinogenicity, its use has become more restricted in recent years, and safer alternatives are sought in many applications.
Molar Mass of Calcium
Molar Mass of Calcium: Calcium is an element that plays a pivotal role in various aspects of our lives, from maintaining healthy bones and teeth to its widespread use in industrial applications.
In this article, we will explore the molar mass of calcium (Ca) and delve into its significance in the realm of chemistry.

Molar Mass of Calcium
The Importance of Calcium
Calcium is a chemical element with the atomic number 20 and the symbol Ca. It is an essential nutrient for living organisms, including humans. In our bodies, calcium is primarily found in our bones and teeth, where it provides structural support and strength. Beyond its biological importance, calcium also has significant industrial applications, making it a valuable element in many industries.
Atomic Structure of Calcium
To understand the molar mass of calcium, we need to explore its atomic structure. A calcium atom contains 20 electrons, arranged in four energy levels or electron shells. The electron configuration of calcium is 2-8-8-2, indicating that it has two electrons in the first shell, eight electrons in the second and third shells, and two electrons in the fourth shell. The outermost shell, with its two electrons, is known as the valence shell.
Calcium’s atomic number, 20, signifies that it has 20 protons in its nucleus, giving it a positive charge. To maintain electrical neutrality, calcium also has 20 electrons. In addition to protons and electrons, the nucleus of a calcium atom contains 20 neutrons, which are electrically neutral particles with a mass similar to that of protons.
Molar Mass of Calcium
The molar mass of an element is defined as the mass of one mole of atoms of that element, expressed in grams per mole (g/mol). It is a fundamental concept in chemistry, as it enables chemists to link the mass of a substance to the number of atoms or molecules it contains.
To calculate the molar mass of calcium, we consider the contribution of its isotopes. Calcium has several isotopes, with calcium-40 being the most abundant, followed by calcium-44, calcium-42, and a few others. The molar mass of calcium is determined by taking a weighted average of the masses of these isotopes, using their natural abundance as the weighting factor:
Molar Mass of Calcium (Ca) = (Fractional Abundance of Ca-40 × Mass of Ca-40) + (Fractional Abundance of Ca-44 × Mass of Ca-44) + …
In practice, the calculation considers all isotopes and their abundances, resulting in the molar mass of approximately 40.08 g/mol for calcium.
The Significance of Molar Mass
The molar mass of calcium is a critical value in chemistry, serving as a cornerstone for various chemical calculations. It is indispensable in determining the amount of calcium required for specific chemical reactions, stoichiometry calculations, and the formulation of chemical equations. In industries such as metallurgy, agriculture, and construction, knowing the molar mass of calcium is vital for precise measurements and formulations.
In conclusion, the molar mass of calcium, approximately 40.08 g/mol, underlines the importance of this element in both biological and industrial contexts. Its role in maintaining our health and contributing to technological advancements makes understanding its atomic properties and molar mass an essential part of our scientific knowledge.
Read More
- Electromagnetic Spectrum X Rays
- Electric Circuit Electrical Symbols
- Difference Between Watts And Volts
- Molar Mass Of Sulphur
- Electronics In Daily Life
Frequently Asked Questions (FAQs) Molar Mass of Calcium
Q1: What is the mol mass of calcium?
A1: The mol mass of calcium (Ca) is approximately 40.08 grams per mole (g/mol).
Q2: Why is the mol mass of calcium important in chemistry?
A2: The mol mass of calcium is crucial in chemistry because it allows chemists to relate the mass of calcium atoms or molecules to the number of atoms or molecules present. This value is fundamental for stoichiometry calculations, chemical equations, and precise measurements in various industries.
Q3: How is the molar mass of calcium calculated?
A3: The mol mass of calcium is calculated by taking a weighted average of the masses of its isotopes, considering their natural abundances as weighting factors. Calcium has several isotopes, with calcium-40 being the most abundant. The calculation accounts for all isotopes and their abundances.
Q4: What are the main applications of calcium in industry and daily life?
A4: Calcium has numerous applications, including:
- In daily life: It is essential for the formation and maintenance of healthy bones and teeth.
- In agriculture: Calcium is used as a soil conditioner and in fertilizers to enhance plant growth.
- In construction: Calcium is used in the production of cement and concrete.
- In metallurgy: Calcium is employed to extract various metals from their ores.
- In the food industry: Calcium is added as a dietary supplement and preservative.
Q5: Are there any health implications associated with calcium’s molar mass?
A5: Calcium’s molar mass itself doesn’t have direct health implications. However, maintaining an adequate intake of calcium through diet or supplements is important for overall health, particularly for bone health, muscle function, and nerve signaling.
Electromagnetic Spectrum X Rays
Electromagnetic Spectrum X Rays: The electromagnetic spectrum is a vast continuum of electromagnetic waves, each with its own unique properties and applications. X-rays occupy a crucial position within this spectrum, known for their ability to penetrate matter and provide valuable insights into the inner workings of our world.
In this article, we’ll dive into the world of X-rays, exploring their characteristics, generation, applications, and safety considerations.
Electromagnetic Spectrum X Rays
Table of Contents:
Introduction to the Electromagnetic Spectrum
- The Diversity of Electromagnetic Waves
- Where X-Rays Reside in the Spectrum
What Are X-Rays
- The Discovery of X-Rays
- Characteristics of X-Rays
Generation of X-Rays
- Production Methods
- X-Ray Tubes and Sources
Applications of X-Rays
- Medical Imaging
- Industrial Inspection and Non-Destructive Testing
- Security Screening
- Astrophysics and Astronomy
Safety and Risks Associated with X-Rays
- Radiation Exposure and Protection
- Medical X-Ray Safety
- Industrial and Research X-Ray Safety
Advancements in X-Ray Technology
- Digital X-Ray Imaging
- X-Ray Crystallography
Conclusion
- The Ongoing Impact of X-Rays
1. Introduction to the Electromagnetic Spectrum
The Diversity of Electromagnetic Waves: The electromagnetic spectrum encompasses a wide range of waves, from radio waves with long wavelengths to gamma rays with incredibly short wavelengths. Each type of wave has its unique characteristics and applications.
Where X-Rays Reside in the Spectrum: X-rays fall within the spectrum between ultraviolet (UV) radiation and gamma rays. They have shorter wavelengths than visible light and carry higher energy, making them suitable for a range of applications.
2. What Are X-Rays?
The Discovery of X-Rays: X-rays were discovered by Wilhelm Conrad Roentgen in 1895 while he was experimenting with cathode rays. His discovery earned him the first Nobel Prize in Physics in 1901.
Characteristics of X-Rays: X-rays are high-energy electromagnetic waves characterized by their ability to penetrate matter. They can ionize atoms, causing electrons to be ejected, and are invisible to the human eye. X-rays have applications in imaging and various scientific fields.
3. Generation of X-Rays
Production Methods: X-rays are produced through processes that involve the acceleration of charged particles, typically electrons, and their interaction with matter. This interaction results in the emission of X-rays.
X-Ray Tubes and Sources: X-ray tubes are commonly used devices for generating X-rays. They consist of a cathode and an anode within a vacuum tube. When high-speed electrons from the cathode strike the anode, X-rays are produced. Synchrotrons and X-ray generators are other sources of X-rays.
4. Applications of X-Rays
Medical Imaging: X-ray imaging is widely used in medicine for diagnostic purposes. It allows healthcare professionals to visualize the internal structures of the body, detect fractures, identify diseases, and guide surgical procedures.
Industrial Inspection and Non-Destructive Testing: X-rays are employed in industrial settings to inspect welds, detect defects in materials, and ensure the quality of manufactured products. This non-destructive testing technique is crucial for safety and quality control.
Security Screening: X-ray scanners are used for security purposes, such as at airports and border crossings. They can detect concealed weapons, explosives, and other contraband items.
Astrophysics and Astronomy: X-rays provide valuable insights into the universe. X-ray telescopes in space capture X-ray emissions from distant celestial objects, revealing the high-energy processes occurring in stars, galaxies, and black holes.
5. Safety and Risks Associated with X-Rays
Radiation Exposure and Protection: X-rays can pose health risks when exposure levels are excessive. Radiation protection measures, such as lead shielding and limiting exposure time, are essential to mitigate these risks.
Medical X-Ray Safety: In medical settings, practitioners follow strict safety protocols to minimize patient and staff exposure to X-rays. Lead aprons, collimators, and dose monitoring are commonly used safeguards.
Industrial and Research X-Ray Safety: Industrial and research facilities adhere to safety guidelines to protect workers from radiation exposure. Protective barriers, dosimetry, and training are crucial for safety.
6. Advancements in X-Ray Technology
Digital X-Ray Imaging: Digital X-ray imaging has replaced traditional film-based radiography in many applications. It offers faster image acquisition, lower radiation doses, and the ability to enhance and share images digitally.
X-Ray Crystallography: X-ray crystallography is a powerful technique used to determine the atomic and molecular structure of crystals. It has profound applications in chemistry, biology, and materials science.
Conclusion
X-rays have revolutionized various fields, from healthcare to industry and astrophysics. Their ability to penetrate matter and reveal hidden details continues to drive advancements in technology and scientific understanding. As technology evolves, X-rays will remain a valuable tool for exploring the world around us and the mysteries of the universe.
Read More
- Electric Circuit Electrical Symbols
- Difference Between Watts And Volts
- Molar Mass Of Sulphur
- Electronics In Daily Life
- Molecular Weight Of Hcl
Frequently Asked Questions (FAQs) Electromagnetic Spectrum X Rays
1. What is the electromagnetic spectrum?
The electromagnetic spectrum is the entire range of electromagnetic waves, including radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays. It spans from low-energy, long-wavelength waves to high-energy, short-wavelength waves.
2. Where do X-rays fit in the electromagnetic spectrum?
X-rays fall between ultraviolet (UV) radiation and gamma rays in the electromagnetic spectrum. They have shorter wavelengths and higher energy compared to visible light.
3. How were X-rays discovered?
X-rays were discovered by Wilhelm Conrad Roentgen in 1895 while he was conducting experiments with cathode rays. His discovery was accidental and led to the first X-ray image of the human body.
4. What are the characteristics of X-rays?
X-rays are high-energy electromagnetic waves.
- They can penetrate matter, including human tissues and materials.
- X-rays are invisible to the human eye.
- They can ionize atoms, causing the ejection of electrons.
5. How are X-rays generated?
X-rays are typically generated by accelerating electrons and directing them at a target material, usually made of metal like tungsten. When high-speed electrons strike the target, X-rays are produced as a result of interactions between electrons and atoms in the target.
Electric Circuit Electrical Symbols
Electric Circuit Electrical Symbols: Creating and understanding electrical circuits is a fundamental skill in the field of electrical engineering and electronics.
These circuits are composed of various components that work together to perform specific tasks, from powering your household appliances to controlling complex industrial systems. To design and analyze circuits effectively, engineers and technicians use a standardized set of electrical symbols to represent components and connections.
Electric Circuit Electrical Symbols
Table of Contents:
Introduction to Electrical Symbols
- The Importance of Standardized Symbols
- Historical Development of Electrical Symbols
Common Electrical Symbols
- Passive Components
- Active Components
- Power Sources and Generators
- Connectors and Wires
- Switches and Relays
- Measuring Instruments
- Transformers and Inductors
- Capacitors and Batteries
- Diodes and Transistors
Understanding Electrical Circuit Diagrams
- Schematic Diagrams
- Wiring Diagrams
- Block Diagrams
How to Read Electrical Symbols
- Identifying Components and Their Functions
- Analyzing Circuit Connections
Applications and Industries
- Residential Electrical Wiring
- Automotive Electronics
- Industrial Control Systems
- Telecommunications
- Aerospace and Avionics
Designing Electrical Circuits
- Circuit Design Software
- Prototyping and Testing
Safety Considerations
- Electrical Hazards
- Safety Symbols
Conclusion
- The Ubiquity of Electrical Circuits
- The Role of Electrical Symbols in Engineering
1. Introduction to Electrical Symbols
Electrical symbols are graphical representations of electrical and electronic components used in circuit diagrams. These symbols serve as a universal language for engineers, electricians, and technicians to communicate circuit designs and concepts efficiently. Standardized symbols ensure that anyone familiar with electrical diagrams can understand and work with them regardless of their geographic location or language.
The Importance of Standardized Symbols: Standardized symbols simplify the design, analysis, and troubleshooting of electrical circuits. They eliminate ambiguity and reduce the chances of errors in circuit diagrams. This consistency is crucial in industries where safety and reliability are paramount.
Historical Development of Electrical Symbols: The history of electrical symbols dates back to the early days of electrical engineering. Engineers and scientists like Charles Wheatstone, Michael Faraday, and Samuel Morse developed rudimentary symbols to represent electrical components in their diagrams. Over time, these symbols evolved and were standardized by organizations like the International Electrotechnical Commission (IEC) and the Institute of Electrical and Electronics Engineers (IEEE).
2. Common Electrical Symbols
Electrical symbols encompass a wide range of components, including passive and active components, power sources, connectors, switches, measuring instruments, and more. Here’s an overview of some common electrical symbols:
Passive Components:
- Resistor (R): Represents a passive component that resists the flow of electrical current.
- Capacitor (C): Symbolizes a device used to store electrical energy.
- Inductor (L): Represents a component that stores energy in a magnetic field.
Active Components:
- Voltage Source: Depicts a source of electrical voltage, such as a battery.
- Current Source: Represents a source that provides a constant current.
- Transistor (NPN and PNP): Symbols for NPN and PNP bipolar junction transistors.
- Operational Amplifier (Op-Amp): Symbolizes a versatile analog component used in amplification and signal processing.
Power Sources and Generators:
- Battery: Represents a battery or a collection of batteries.
- Generator: Symbolizes a power generator, such as a turbine or alternator.
Connectors and Wires:
- Connector: Signifies a connection point or terminal.
- Wire: Represents a conductor used to connect components.
- Ground: Symbol for electrical ground, typically connected to the Earth.
Switches and Relays:
- Switch (SPST, SPDT, DPST, DPDT): Various symbols for switches with different configurations.
- Relay: Represents an electromagnetic switch controlled by a separate circuit.
Measuring Instruments:
- Voltmeter: Symbol for a device used to measure voltage.
- Ammeter: Symbol for a device used to measure current.
- Ohmmeter: Represents a device used to measure resistance.
Transformers and Inductors:
- Transformer: Symbolizes a device used to change the voltage level in an AC circuit.
- Mutual Inductor: Represents a transformer with multiple windings.
Capacitors and Batteries:
- Polarized Capacitor: Symbolizes a capacitor with polarity.
- Non-Polarized Capacitor: Represents a capacitor without polarity.
- Cell (Battery): Symbol for an individual cell or battery.
Diodes and Transistors:
- Diode (Rectifier, Zener, Light-Emitting Diode): Various symbols for diodes with different functions.
- Field-Effect Transistor (FET): Symbolizes a type of transistor.
- These symbols form the building blocks of electrical and electronic circuit diagrams, allowing engineers and technicians to represent complex systems succinctly.
3. Understanding Electrical Circuit Diagrams
- Electrical circuit diagrams, also known as schematics, are graphical representations of electrical circuits using standardized symbols. These diagrams convey the relationships between components and the flow of electrical current within a circuit. There are different types of circuit diagrams, including schematic diagrams, wiring diagrams, and block diagrams.
Schematic Diagrams:
- Schematic diagrams provide a detailed representation of individual components and their connections within a circuit.
- They are commonly used for complex electronic circuits, control systems, and integrated circuits.
- Schematic diagrams are essential for circuit design, analysis, and troubleshooting.
Wiring Diagrams:
- Wiring diagrams focus on the physical layout of components and their interconnections within a system.
- They are often used in the automotive industry, household electrical wiring, and building control systems.
- Wiring diagrams help technicians understand how to physically wire a circuit.
Block Diagrams:
- Block diagrams provide a high-level overview of a system or circuit.
- They use blocks to represent major components or subsystems and arrows to indicate the flow of signals or information between them.
- Block diagrams are useful for understanding the functional relationships within a complex system.
4. How to Read Electrical Symbols
- Reading electrical symbols and understanding their meanings is crucial for interpreting circuit diagrams. Here are some key principles for reading electrical symbols effectively:
- Identifying Components and Their Functions:
- Familiarize yourself with common electrical symbols and their corresponding components.
- Understand the functions of each component, including passive and active elements, sources, and connectors.
Analyzing Circuit Connections:
- Follow the lines and connections in the diagram to trace the path of electrical current.
- Note the direction of arrowheads, which indicate the flow of signals or currents.
- Pay attention to the labels and annotations to understand component values, such as resistance or voltage.
Referencing a Key:
- Some circuit diagrams include a key or legend that provides explanations for symbols used in the diagram.
- Refer to the key when encountering unfamiliar symbols or components.
Consider Context:
- Analyze the overall context of the circuit and the specific function it is designed to perform.
- Take into account the purpose of the circuit, whether it’s for amplification, control, signal processing, or power distribution.
5. Applications and Industries
- Electrical circuit diagrams and symbols find applications in various industries and sectors, each with its specific requirements and standards:
Residential Electrical Wiring:
- In residential settings, electrical symbols are used in house wiring diagrams to plan and install electrical systems safely.
- These diagrams ensure that electrical connections meet building codes and safety standards.
Automotive Electronics:
- Electrical symbols are prevalent in automotive wiring diagrams, enabling technicians to diagnose and repair electrical issues in vehicles.
- Modern automobiles rely on complex electrical systems for engine control, infotainment, lighting, and safety features.
Industrial Control Systems:
- Industrial circuits often employ complex control systems with sensors, actuators, and programmable logic controllers (PLCs).
- Electrical symbols help engineers design and maintain industrial control systems used in manufacturing and process industries.
Telecommunications:
- The telecommunications industry uses circuit diagrams for designing and troubleshooting communication systems, including network infrastructure and mobile devices.
Aerospace and Avionics:
- Avionics systems on aircraft, spacecraft, and drones heavily rely on electrical symbols and diagrams to ensure reliable communication, navigation, and control.
6. Designing Electrical Circuits
- Designing electrical circuits involves creating schematics, selecting components, and ensuring the circuit meets specific requirements. Engineers use specialized software tools for circuit design and simulation. Some common steps in designing electrical circuits include:
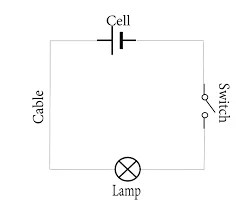
Circuit Design Software:
- Engineers use software tools like AutoCAD Electrical, Eagle, OrCAD, and KiCad to create schematic diagrams, simulate circuit behavior, and generate Bill of Materials (BOM).
Prototyping and Testing:
- After designing a circuit on paper or in software, engineers often build prototypes to test and verify its functionality.
- Prototyping involves selecting physical components, creating a physical circuit, and conducting tests.
Iterative Design:
- Engineers may go through multiple iterations of circuit design and testing to optimize performance, reduce costs, or address specific requirements.
7. Safety Considerations
- Working with electrical circuits presents inherent hazards, including electrical shock, fire, and equipment damage. To ensure safety, electrical diagrams and components may include safety symbols and labels. These symbols warn about potential dangers and provide guidance on safe practices.
Electrical Hazards:
- Electrical hazard symbols indicate the presence of electrical energy and warn individuals to take precautions.
- Symbols for high voltage, electrical shock, and grounding are common safety symbols.
Safety Symbols:
Safety symbols on electrical components or equipment may include information on safe operation, such as voltage limits and safety instructions.
Safety Precautions:
- When working with electrical circuits, always follow safety protocols, wear appropriate personal protective equipment (PPE), and disconnect power sources before handling electrical components.
8. Conclusion
- Electrical symbols play a crucial role in electrical engineering, electronics, and many other industries where electrical circuits are designed, analyzed, and maintained. These symbols provide a standardized and universally understood means of representing components and connections within circuits, simplifying communication among professionals worldwide.
- From the residential wiring in our homes to the intricate systems powering advanced technologies, electrical symbols and circuit diagrams are at the heart of modern electrical and electronic systems. As technology continues to advance, the need for clear and standardized electrical symbols remains vital in ensuring the safety, reliability, and efficiency of electrical circuits and systems.
Read More
- Difference Between Watts And Volts
- Molar Mass Of Sulphur
- Electronics In Daily Life
- Molecular Weight Of Hcl
- Asteroid And Comet Difference
Frequently Asked Questions (FAQs) Electric Circuit Electrical Symbols
1. What are electric circuit electrical symbols, and why are they important?
Electric circuit electrical symbols are graphical representations of electrical and electronic components used in circuit diagrams. They are essential for communicating circuit designs and concepts effectively. Standardized symbols ensure clarity, consistency, and precision in circuit diagrams.
2. How do electric circuit electrical symbols simplify circuit design and analysis?
Electric circuit electrical symbols simplify circuit design and analysis by providing a universal language for representing components. Engineers and technicians can quickly understand and work with circuit diagrams, making it easier to design, troubleshoot, and maintain electrical systems.
3. Where can I find a comprehensive list of electric circuit electrical symbols and their meanings?
You can find comprehensive lists of electric circuit electrical symbols, along with their meanings, in electrical engineering textbooks, online resources, and dedicated symbol libraries in circuit design software. These resources typically include symbols for passive components, active components, power sources, connectors, switches, and more.
4. Are there international standards for electric circuit electrical symbols?
Yes, there are international standards for electric circuit electrical symbols. Organizations like the International Electrotechnical Commission (IEC) and the Institute of Electrical and Electronics Engineers (IEEE) have established standardized symbols to ensure consistency in circuit diagrams worldwide. These standards facilitate cross-border communication and understanding.
5. How do I interpret circuit diagrams that use electric circuit electrical symbols?
Interpreting circuit diagrams involves understanding the meanings of electrical symbols and tracing the path of electrical current through the diagram. Key principles include identifying components, recognizing connections, following the flow of signals or currents, and considering the context and purpose of the circuit.
Difference Between Watts And Volts
Difference Between Watts And Volts: Understanding the Difference Between Watts and Volts
Watts And Volts
Introduction
In the realm of electricity and power, two fundamental concepts that often lead to confusion are watts and volts. While they both play critical roles in the world of electrical systems, they represent different aspects of electricity. This article aims to clarify the distinctions between watts and volts, helping you better comprehend their significance and practical applications.
Watts (W) – The Measure of Power
Watts, denoted by the symbol ‘W,’ are the units used to measure power in an electrical circuit. Power is essentially the rate at which energy is transferred, converted, or used. In simpler terms, it tells us how quickly work is being done in an electrical system. The relationship between watts, voltage (volts), and current (amperes) is described by the formula:
In this equation:
- P represents power in watts.
- V represents voltage in volts.
- I represents current in amperes.
In practical terms, watts are a measure of how much energy is consumed or produced per unit of time. For instance, a 100-watt light bulb consumes 100 watts of electrical power while it’s illuminated. Similarly, a 1,000-watt (1 kilowatt) space heater consumes 1 kilowatt-hour of energy in one hour.
Volts (V) – The Measure of Electrical Potential
Volts, denoted by the symbol ‘V,’ represent electrical potential or voltage. Voltage is a measure of the electric potential energy per unit charge at a given point in an electrical circuit. In simpler terms, it tells us the force that drives electric charge through a conductor. A higher voltage means that electrons have more potential energy and will move with more force through the circuit.
Voltage is often compared to water pressure in a plumbing system. Just as higher water pressure causes water to flow more forcefully through pipes, higher voltage causes electric current to flow more forcefully through wires.
Practical Examples
To better understand the difference between watts and volts, consider the following examples:
- Light Bulb: A 100-watt light bulb requires 100 watts of power to produce light. However, it operates at a standard household voltage of 120 volts in the United States. To calculate the current flowing through it, you can use the formula I = P / V, which in this case is I = 100W / 120V ≈ 0.83 amperes.
- Battery: A common AA battery typically supplies 1.5 volts. The voltage represents the potential difference between the battery’s positive and negative terminals, which drives the flow of electric current. The power (in watts) the battery can deliver depends on the device it’s connected to and the current it draws.
- Electric Kettle: A 1,500-watt electric kettle heats water quickly. When you plug it into a standard 120-volt outlet, it draws approximately 12.5 amperes of current to produce that 1,500 watts of power, creating the heat needed to boil water.
Conclusion
In summary, watts and volts are distinct but interrelated concepts in the world of electricity. Watts measure power, which is the rate of energy transfer or consumption, while volts measure voltage, which is the electrical potential that drives the flow of electric current.
Understanding these differences is crucial for effectively managing and using electrical systems, from household appliances to industrial machinery. Whether you’re troubleshooting electrical issues at home or designing complex electrical circuits, a clear grasp of the distinction between watts and volts is essential for safe and efficient operation.
Read More
- Molar Mass Of Sulphur
- Electronics In Daily Life
- Molecular Weight Of Hcl
- Asteroid And Comet Difference
- Molecular Weight Of Nitrogen
Frequently Asked Questions (FAQs) Watts And Volts
What is the fundamental difference between watts and volts?
Watts measure power, which is the rate of energy transfer or consumption, while volts measure voltage, which is the electrical potential that drives the flow of electric current.
What do watts and volts represent in electrical terms?
Watts represent the amount of work done or the rate of energy transfer in an electrical circuit. Volts represent the electrical potential difference that drives the flow of electric charge.
How are watts and volts related in an electrical circuit?
Watts can be calculated by multiplying volts by amperes (W = V × I), where (V) represent the voltage and amperes (I) represent the current.
Can you provide a practical example illustrating the difference between W and V ?
Sure, consider a 100-W light bulb. The 100 watts (W) represent the power it consumes to produce light, while the standard household voltage of 120 V provides the electrical potential needed for the bulb to function.
What happens if you increase the voltage while keeping the wattage constant in an electrical circuit?
Increasing the voltage while keeping the wattage constant will result in an increase in the current (amperes). This can lead to higher power consumption and may affect the operation of electrical devices.
Molar Mass Of Sulphur
Molar Mass Of Sulphur: Sulfur, represented by the chemical symbol S, is a fundamental element in the periodic table, known for its diverse chemical properties and essential roles in various natural and industrial processes.
Understanding the molar mass of sulfur is a crucial aspect of chemistry, providing insight into its atomic weight and its applications in a wide range of scientific and industrial fields. In this article, we delve into the concept of the molar mass of sulfur, its significance, and practical implications.
 Molar Mass Of Sulphur
Molar Mass Of Sulphur
The Atomic Structure of Sulphur
Before exploring the molar mass of sulfur, it’s essential to appreciate its atomic structure. A sulfur atom has 16 electrons, 16 protons, and varying numbers of neutrons, depending on its isotope.
The most common naturally occurring isotope, sulfur-32 (32S), has 16 neutrons. Sulfur typically forms stable molecules consisting of eight atoms, resulting in molecules with the chemical formula S8.
Calculating the Molar Mass of Sulphur
The molar mass of sulfur is the mass of one mole of sulfur atoms or molecules. To calculate it, we consider the atomic masses of the constituent atoms. The atomic mass of sulfur, as listed in the periodic table, is approximately 32.06 atomic mass units (amu).
One mole of sulfur consists of Avogadro’s number of sulfur atoms, which is approximately 6.022 x 10^23 atoms. Therefore, to find the molar mass of sulfur, we use the atomic mass as follows:
Molar Mass of Sulfur = Atomic Mass of Sulfur x Avogadro’s Number
Molar Mass of Sulfur ≈ 32.06 amu x 6.022 x 10^23 atoms/mol
Molar Mass of Sulfur ≈ 32.06 grams per mole (g/mol)
Thus, the molar mass of sulfur is approximately 32.06 g/mol, which means that one mole of sulfur atoms has a mass of 32.06 grams.
Significance and Applications
The molar mass of sulfur holds significant importance in various scientific and industrial contexts:
1. Chemical Reactions: In chemistry, the molar mass of sulfur is used in stoichiometric calculations to determine the amount of sulfur required or produced in chemical reactions. It is essential for balancing chemical equations.
2. Industry: Sulfur finds extensive use in the chemical industry, including the production of sulfuric acid, fertilizers, and various chemicals. Understanding its molar mass is crucial in manufacturing processes.
3. Agriculture: Sulfur is an essential nutrient for plant growth, and sulfur-containing fertilizers are used to enhance crop yields. Accurate molar mass information aids in fertilizer formulation.
4. Environmental Monitoring: Monitoring sulfur emissions, such as sulfur dioxide (SO2) from industrial processes and volcanic eruptions, is critical for assessing air quality and environmental impact.
5. Pharmaceuticals: Sulfur compounds are employed in pharmaceuticals, and the molar mass of sulfur is essential for precise dosing and formulation.
6. Petrochemicals: Sulfur content in petroleum products can affect their properties and environmental impact. Determining sulfur molar mass is crucial for quality control in the petroleum industry.
Conclusion
The molar mass of sulfur, approximately 32.06 g/mol, is a fundamental parameter in chemistry and various industrial sectors. It guides chemical reactions, facilitates manufacturing processes, and plays a pivotal role in agriculture, environmental monitoring, and pharmaceuticals. As a versatile element with diverse applications, sulfur’s molar mass is a cornerstone in our understanding of chemical processes and their practical implications, contributing to advancements in science and industry alike.
Read More
- Difference Between Diode And Rectifier
- Difference Between AM And FM
- Dielectric Material And Dipole Moment
- Difference Between Centre Of Gravity And Centroid
- Kinetic Gas Equation Derivation
Frequently Asked Questions (FAQs) Molar Mass Of Sulphur
What is the molar mass of sulfur?
The molar mass of sulfur is approximately 32.06 grams per mole (g/mol). This value represents the mass of one mole of sulfur atoms or molecules.
Why is the molar mass of sulfur important?
The molar mass of sulfur is essential for various chemical calculations, including stoichiometry, determining the amount of sulfur in compounds, and balancing chemical equations. It is also crucial in industrial applications and environmental monitoring.
How is the molar mass of sulfur calculated?
The molar mass of sulfur is calculated by considering the atomic mass of sulfur (approximately 32.06 atomic mass units) and multiplying it by Avogadro’s number, which is approximately 6.022 x 10^23.
What is the significance of sulfur in chemistry and industry?
Sulfur is a versatile element used in various chemical processes and industries. It is a key component in the production of sulfuric acid, fertilizers, and various chemicals. Sulfur compounds also have applications in pharmaceuticals and petrochemicals.
How is sulfur used in agriculture?
Sulfur is an essential nutrient for plant growth. Sulfur-containing fertilizers are used to provide plants with the necessary sulfur for healthy development and improved crop yields.
Electronics In Daily Life
Electronics In Daily Life: From smartphones and laptops to household appliances and transportation systems, electronics have permeated every aspect of modern living. Here, we explore the pervasive role of electronics in our daily lives.

Electronics In Daily Life
Communication
- Smartphones: Perhaps the most ubiquitous electronic device, smartphones have transformed the way we communicate. They allow us to make calls, send texts, access the internet, and use countless apps for various purposes, from social networking to navigation.
- Computers and Laptops: Personal computers and laptops are essential tools for work, education, and entertainment. They enable us to perform tasks like research, writing, and data analysis with ease.
- Tablets: These portable touchscreen devices are popular for reading, gaming, and media consumption. They bridge the gap between smartphones and computers.
- Email and Messaging Apps: Electronic communication platforms like email, instant messaging, and video conferencing have made global communication effortless and instantaneous.
Entertainment
- Television: Modern televisions are sophisticated electronic devices that bring a world of entertainment into our homes. Smart TVs allow us to stream movies, shows, and access the internet directly.
- Gaming Consoles: Gaming consoles offer immersive gaming experiences with high-definition graphics and online multiplayer capabilities.
- Music Players: Portable music players, streaming services, and digital music libraries have revolutionized how we listen to music.
- E-Readers: Electronic book readers like the Kindle have made it convenient to carry and read an extensive library of books in one device.
Information and Education
- Internet: The internet is the backbone of information sharing and research. It provides access to vast databases, educational resources, and online courses.
- E-Learning: Online platforms and courses have made education accessible to people worldwide. Students can learn from anywhere and at their own pace.
- E-Books: Electronic books and textbooks are environmentally friendly and convenient for both students and avid readers.
Home and Lifestyle
- Smart Home Devices: Electronics enable us to automate and control our homes, from thermostats and lighting to security systems and appliances.
- Kitchen Appliances: Microwaves, ovens, refrigerators, and coffee makers have become smarter and more energy-efficient thanks to electronic controls.
- Wearable Technology: Devices like smartwatches and fitness trackers monitor our health, track physical activities, and provide notifications on the go.
Transportation
- GPS Navigation: GPS technology in smartphones and dedicated GPS devices has transformed how we navigate, making it easy to find directions and locate places.
- Electric Vehicles (EVs): EVs are powered by advanced electronics, offering eco-friendly alternatives to traditional gasoline-powered cars.
- Public Transportation: Trains, buses, and subways rely heavily on electronics for navigation, scheduling, and passenger information systems.
Healthcare
- Medical Devices: Electronics play a crucial role in medical equipment, such as MRI machines, pacemakers, and insulin pumps, enhancing diagnosis and treatment.
- Telemedicine: Remote patient monitoring and video consultations have become more accessible through electronic platforms, especially in recent times.
Conclusion
Electronics have transformed the way we live, work, and interact with the world. They continue to advance rapidly, shaping our daily experiences and opening up new possibilities for communication, entertainment, education, and innovation. As technology evolves, electronics will undoubtedly play an even more significant role in our future daily lives.
Read More
- Molecular Weight Of NaOH
- Difference Between Force And Pressure
- Difference Between Diode And Rectifier
- Difference Between AM And FM
- Dielectric Material And Dipole Moment
Frequently Asked Question (FAQs) Electronics In Daily Life
What are electronics?
Electronics are devices or systems that use electrical circuits and components to control and manipulate electrical currents for various purposes, such as communication, computation, and automation.
How have smartphones changed our daily lives?
Smartphones have revolutionized daily life by providing communication tools, internet access, productivity apps, entertainment, and navigation services all in one handheld device. They have made information and connectivity more accessible than ever.
What are some benefits of using e-readers for reading books?
E-readers are portable and lightweight, allowing readers to carry a library of books in a single device. They often feature adjustable font sizes and built-in lighting for comfortable reading in various conditions.
How do wearable technologies like smartwatches benefit daily life?
Smartwatches and fitness trackers provide real-time health monitoring, notifications, and fitness tracking. They help users stay connected, track their physical activity, and manage their daily schedules.
What is the Internet of Things (IoT) and how does it impact daily life?
IoT refers to the network of interconnected everyday objects and devices that can communicate and share data. It impacts daily life by enabling smart homes, automation, and remote control of appliances and devices for added convenience and efficiency.
Molecular Weight Of Hcl
Molecular Weight Of Hcl: The molecular weights of hydrochloric acid (HCl) can be calculated by adding the atomic masses of its constituent elements:

Molecular Weight Of Hcl
Calculating the Molecular Weight of HCl:
Hydrochloric acid (HCl) consists of two elements: hydrogen (H) and chlorine (Cl). To calculate its molecular weights, we add the atomic masses of these elements:
- Hydrogen (H) has an atomic mass of approximately 1.01 g/mol.
- Chlorine (Cl) has an atomic mass of approximately 35.45 g/mol.
Now, let’s compute the molecular weight of HCl:
Molecular Weights of HCl = Atomic Mass of Hydrogen + Atomic Mass of Chlorine
Molecular Weights of HCl = 1.01 g/mol + 35.45 g/mol
Molecular Weights of HCl ≈ 36.46 g/mol
So, the molecular weights of hydrochloric acid (HCl) is approximately 36.46 grams per mole.
Significance of HCl’s Molecular Weight:
Understanding the molecular weights of HCl is crucial for several reasons:
- Stoichiometry: Molecular weights plays a vital role in chemical calculations, such as stoichiometry, where it helps determine the quantity of reactants and products in chemical reactions involving HCl.
- Concentration: In analytical chemistry and laboratory work, the molecular weights of HCl is used to calculate the concentration of solutions accurately. This is essential for preparing solutions of known strength.
- Industrial Applications: In various industrial processes, HCl is used for tasks like cleaning, pickling metals, and manufacturing chemicals. Knowledge of its molecular weight is necessary for quality control and production procedures.
- Environmental Monitoring: HCl is a common pollutant in industrial emissions. Monitoring and regulating its presence in the environment require precise measurements, often based on its molecular weight.
- Safety: Handling HCl, a highly corrosive acid, necessitates a comprehensive understanding of its properties, including molecular weight, to ensure safe storage and usage.
Conclusion:
The molecular weights of hydrochloric acid (HCl), approximately 36.46 g/mol, is a critical parameter in the world of chemistry. It facilitates accurate measurements, precise calculations, and the efficient use of HCl in various scientific, industrial, and environmental applications. By understanding its molecular weights, scientists and engineers can harness the potential of HCl while ensuring its safe and responsible use.
Read More
- Molecular Weight Of Nitrogen
- Molecular Weight Of NaOH
- Difference Between Force And Pressure
- Difference Between Diode And Rectifier
- Difference Between AM And FM
Frequently Asked Question (FAQs) Molecular Weight Of Hcl
What is the molecular weight of hydrochloric acid (HCl)?
The molecular weights of hydrochloric acid (HCl) is approximately 36.46 grams per mole (g/mol).
Why is knowing the molecular weight of HCl important in chemistry?
Knowing the molecular weights of HCl is crucial for various chemical calculations and laboratory procedures. It helps determine the amount of HCl in chemical reactions, the concentration of HCl solutions, and plays a role in quality control and safety considerations.
How is the molecular weight of HCl calculated?
The molecular weights of HCl is calculated by adding the atomic masses of its constituent elements: hydrogen (H) and chlorine (Cl). The atomic mass of hydrogen is approximately 1.01 g/mol, and the atomic mass of chlorine is approximately 35.45 g/mol.
Can you explain how molecular weight is used in stoichiometry with respect to HCl?
In stoichiometry, molecular weights is used to determine the molar ratios of reactants and products in a chemical reaction. By knowing the molecular weights of HCl, you can calculate how many moles of HCl are involved in a reaction and relate it to other reactants and products.
Are there practical applications of HCl’s molecular weight in industry?
Yes, HCl is used in various industrial processes, such as metal pickling, chemical manufacturing, and water treatment. Its molecular weights is crucial for accurately measuring and controlling the amount of HCl used in these applications.