Category: Class 10
Asteroid And Comet Difference
Asteroid And Comet Difference: Asteroids and comets are two distinct types of celestial objects that orbit the Sun, and they differ in several ways, including their composition, appearance, and behavior. Here are the key differences between asteroids and comets:

Asteroid And Comet Difference
-
Composition:
- Asteroids: Asteroids are primarily composed of rocky and metallic materials. They are often referred to as “minor planets” because they are similar in composition to the terrestrial planets like Earth.
- Comets: Comets consist of a mixture of water ice, frozen gases (such as carbon dioxide, methane, and ammonia), dust, and rocky materials. They are often called “dirty snowballs” because of their icy nature.
-
Appearance:
- Asteroids: Asteroids typically have a solid and rocky appearance. They do not develop a visible coma (a cloud of gas and dust) or a tail, and they tend to reflect sunlight like rocky surfaces.
- Comets: Comets often display a distinctive appearance when they approach the Sun. As they heat up, their ices start to sublimate, releasing gas and dust, which form a glowing coma and a tail that points away from the Sun due to the solar wind and radiation pressure.
-
Orbital Characteristics:
- Asteroids: Asteroids usually have more circular or elliptical orbits, resembling the orbits of planets. They are found primarily in the asteroid belt, a region between the orbits of Mars and Jupiter.
- Comets: Comets often have highly elliptical orbits that can take them from the distant reaches of the solar system (Oort Cloud or Kuiper Belt) to much closer to the Sun during their active phases.
-
Location:
- Asteroids: Most asteroids are found within the asteroid belt, although some may have orbits that cross Earth’s path, making them potentially hazardous near-Earth objects.
- Comets: Comets can originate from various regions of the solar system, such as the Oort Cloud (comets with long orbital periods) or the Kuiper Belt (short-period comets), and they can approach the inner solar system from these distant locations.
-
Activity:
- Asteroids: Asteroids are generally considered inactive. They do not exhibit the kinds of outgassing and tail formation that comets do when they approach the Sun.
- Comets: Comets become active when they approach the Sun, and their ices start to vaporize and release gas and dust. This activity makes them visible from Earth.
-
Size:
- Asteroids: Asteroids can vary significantly in size, from a few meters to hundreds of kilometers in diameter. Some of the largest asteroids, like Ceres, are considered dwarf planets.
- Comets: Comets are typically smaller than asteroids, with nuclei ranging from a few hundred meters to a few kilometers in diameter.


In summary, while asteroids and comets are both celestial objects in our solar system, they differ in terms of composition, appearance, orbital characteristics, location, activity, and size.
Read More
- Molecular Weight Of Nitrogen
- Molecular Weight Of NaOH
- Difference Between Force And Pressure
- Difference Between Diode And Rectifier
- Difference Between AM And FM
Frequently Asked Question (FAQs) Asteroid And Comet Difference
-
What is the fundamental difference between asteroids and comets?
The fundamental difference lies in their composition and behavior. Asteroids are primarily composed of rocky and metallic materials and do not exhibit cometary activity, such as outgassing and tail formation. In contrast, comets are composed of ice, dust, and rocky materials and display visible tails and comas when they approach the Sun.
-
Where are most asteroids and comets located in the solar system?
Most asteroids are found in the asteroid belt, a region located between the orbits of Mars and Jupiter. Comets can originate from various regions, including the Oort Cloud (long-period comets) and the Kuiper Belt (short-period comets), which are much farther from the Sun.
-
What causes the tails of comets?
The tails of comets are caused by the sublimation (vaporization) of ices in the comet’s nucleus as it approaches the Sun. Solar radiation and the solar wind push the released gas and dust away from the comet, forming a glowing coma and a tail that points away from the Sun.
-
Do asteroids ever have tails?
No, asteroids do not develop tails. They lack the icy compositions that lead to the outgassing and tail formation characteristic of comets.
-
Can comets become asteroids, or vice versa?
While both asteroids and comets can change orbits over time due to gravitational interactions, they typically retain their essential characteristics. For instance, a comet will still have its icy composition even if its orbit changes, and an asteroid will remain rocky and metallic.
Molecular Weight Of Nitrogen
Molecular Weight Of Nitrogen: Nitrogen, a fundamental element found abundantly in our planet’s atmosphere, plays a pivotal role in various aspects of science and industry.
Its molecular weight, a key property, provides valuable insights into its behavior and applications. In this article, we’ll explore the molecular weight of nitrogen and its significance in diverse fields.

Molecular Weight Of Nitrogen:
Molecular Weight: A Fundamental Concept
Before delving into nitrogen’s molecular weight, let’s grasp the concept itself. Molecular weight, also known as molar mass, signifies the sum of the atomic weights of all the atoms present in a molecule. This numerical value is usually expressed in atomic mass units (amu) or grams per mole (g/mol). Molecular weight serves as a foundational parameter in chemical calculations, enabling scientists to understand the properties and interactions of substances at the molecular level.
Calculating the Molecular Weight of Nitrogen
Nitrogen primarily exists as a diatomic molecule in its natural state, with two nitrogen atoms bonded together as N2. To determine its molecular weight, we simply add the atomic weights of these constituent atoms. The atomic weight of a single nitrogen atom (N) is approximately 14.00674 amu. As nitrogen exists as N2, we multiply this value by 2 to obtain the molecular weights:
Molecular Weight of Nitrogen (N2) = 2 × Atomic Weight of Nitrogen (N)
Molecular Weight of Nitrogen (N2) = 2 × 14.00674 amu ≈ 28.01348 amu
Therefore, the molecular weights of nitrogen, when it exists as N2, is approximately 28.01348 amu or 28.01348 g/mol.
Implications of Nitrogen’s Molecular Weight
Understanding the molecular weights of nitrogen is essential for comprehending its behavior and applications:
- Gas Properties: Nitrogen, with a molecular weight close to 28 g/mol, is lighter than many other gases in the atmosphere, making up about 78% of the air we breathe. Its relatively low molecular weight allows it to remain close to the Earth’s surface, playing a vital role in sustaining life.
- Chemical Reactivity: Nitrogen’s molecular weight influences its chemical reactivity. As N2, it possesses a robust and stable triple bond between its nitrogen atoms, making it unreactive under normal conditions. This stability is critical for maintaining the composition of Earth’s atmosphere.
- Industrial Applications: Nitrogen’s low molecular weight and inert nature have led to various industrial applications, including its use in food packaging, preventing oxidation in electronics manufacturing, and as a key component in ammonia production for fertilizers.
- Biological Significance: In biology, nitrogen’s molecular weight is pivotal. It determines the mass of nitrogen-containing biomolecules such as proteins and nucleic acids (DNA and RNA). Understanding nitrogen’s molecular weight aids in quantifying and analyzing these essential biomolecules, which are fundamental to life.
Conclusion
The molecular weight of nitrogen, approximately 28.01348 g/mol when existing as N2, is a critical parameter that influences its behavior and applications in numerous fields.
Read More
- Molecular Weight Of NaOH
- Difference Between Force And Pressure
- Difference Between Diode And Rectifier
- Difference Between AM And FM
- Dielectric Material And Dipole Moment
Frequently Asked Question (FAQs) Molecular Weight Of Nitrogen
-
What is the molecular weight of nitrogen?
The molecular weights of nitrogen, when it exists as a diatomic molecule (N2), is approximately 28.01348 atomic mass units (amu) or 28.01348 grams per mole (g/mol).
-
Why is nitrogen’s molecular weight important?
Nitrogen’s molecular weight is crucial because it influences its behavior as a gas, its chemical reactivity, and its significance in various fields, including biology, industry, and environmental science. Understanding its molecular weight helps scientists and researchers make informed decisions about its use and applications.
-
How is nitrogen’s molecular weight calculated?
To calculate the molecular weights of nitrogen (N2), you sum the atomic weights of the constituent atoms, which are two nitrogen (N) atoms. The atomic weights of a single nitrogen atom is approximately 14.00674 amu. Therefore, the molecular weights of N2 is 2 times the atomic weights of nitrogen.
-
What are the main properties of nitrogen related to its molecular weight?
Nitrogen’s relatively low molecular weight compared to other atmospheric gases makes it lighter, allowing it to stay close to the Earth’s surface. Its stable diatomic form (N2) with a strong triple bond makes it unreactive under normal conditions, contributing to the stability of the Earth’s atmosphere.
-
In what industries is nitrogen commonly used?
Nitrogen has diverse industrial applications. It is used in the food industry for packaging and preservation, in electronics manufacturing to prevent oxidation, and in the production of ammonia for fertilizers, among others. Its inert nature and low molecular weight make it valuable in various processes.
Molecular Weight Of NaOH
Molecular Weight Of NAOH: The Molecular Weight of NaOH (Sodium Hydroxide) Understanding its Significance in Chemistry Chemistry, the study of matter and its properties, often involves the measurement and analysis of substances at the molecular level.

Molecular Weight Of NaOH
Defining Molecular Weight:
Molecular weight is a measure of the mass of a molecule, and it is expressed in atomic mass units (amu) or grams per mole (g/mol). It provides valuable information about the composition of a substance by summing up the atomic masses of all the atoms present in a molecule.
The Composition of NaOH:
Sodium hydroxide (NaOH) is a chemical compound composed of three elements: sodium (Na), oxygen (O), and hydrogen (H). Its chemical formula, NaOH, tells us that each molecule of sodium hydroxide contains one atom of sodium (Na), one atom of oxygen (O), and one atom of hydrogen (H).
Calculating the Molecular Weight of NaOH:
To find the molecular weight of NaOH, we need to add up the atomic weights of its constituent elements:
- Sodium (Na) has an atomic weight of approximately 22.99 g/mol.
- Oxygen (O) has an atomic weight of roughly 16.00 g/mol.
- Hydrogen (H) has an atomic weight of approximately 1.01 g/mol.
Now, let’s calculate the molecular weight of NaOH:
Molecular Weight (NaOH) = Atomic Weight (Na) + Atomic Weight (O) + Atomic Weight (H)
Molecular Weight (NaOH) = 22.99 g/mol + 16.00 g/mol + 1.01 g/mol
Molecular Weight (NaOH) ≈ 40.00 g/mol
The Significance of Molecular Weight:
Understanding the molecular weight of a compound like NaOH is crucial in various aspects of chemistry:
- Stoichiometry: Molecular weight is used to calculate the number of moles of a substance in a given sample. This is essential for determining the proportions of reactants and products in chemical reactions.
- Molar Mass: Molecular weight is also referred to as molar mass, and it represents the mass of one mole of a substance. This value is used extensively in chemical calculations.
- Formulas and Empirical Formulas: Molecular weight helps in determining the chemical formula of a compound and its empirical formula, which represents the simplest whole number ratio of its constituent atoms.
- Concentration Calculations: In solutions, the molecular weight of a solute is used to calculate its concentration in terms of molarity (moles per liter).
- Chemical Analysis: Molecular weight aids in identifying and quantifying substances through techniques like mass spectrometry and chromatography.
Practical Applications of Sodium Hydroxide:
Sodium hydroxide (NaOH) is a versatile chemical with numerous applications in various industries. It is commonly used in:
- Chemical Manufacturing: NaOH is a key ingredient in the production of various chemicals, including soaps, detergents, and pharmaceuticals.
- Food Processing: It is used in food preparation and production, particularly in the manufacture of products like pretzels and chocolate.
- Water Treatment: NaOH is employed to adjust the pH of water and wastewater in water treatment plants.
- Cleaning Products: It is a vital component of household cleaning products, as it effectively dissolves grease and oils.
- Petroleum Industry: NaOH plays a role in refining petroleum products and removing impurities.
- Paper and Pulp Industry: It is used to break down lignin in wood fibers during the papermaking process.
Understanding the molecular weight of NaOH and its properties allows for precise and controlled use in these applications.
In Conclusion:
The moleculars weight of NaOH, approximately 40.00 g/mol, provides a fundamental insight into the composition of this chemical compound.
Read More
- Difference Between Force And Pressure
- Difference Between Diode And Rectifier
- Difference Between AM And FM
- Dielectric Material And Dipole Moment
- Difference Between Centre Of Gravity And Centroid
Frequently Asked Question (FAQs) Molecular Weight Of NaOH
What is the molecular weight of NaOH?
The moleculars weight of sodium hydroxide (NAOH) is approximately 40.00 grams per mole (g/mol). This value is calculated by adding the atomic weights of the constituent elements: sodium (Na), oxygen (O), and hydrogen (H).
Why is it important to know the molecular weight of NaOH?
Knowing the moleculars weight of NaOH is essential in chemistry and science in general because it helps in various calculations, including molar mass, stoichiometry, and determining the amount of a substance in a given sample.
How can I calculate the molecular weight of other compounds?
To calculate the molecular weight of any compound, add up the atomic weight of all the atoms in the compound according to their chemical formula. You can find atomic weights on the periodic table. Multiply the atomic weight of each element by the number of atoms of that element in the compound, and then sum these values.
Is the molecular weight of NaOH the same as its molar mass?
Yes, in chemistry, the terms “molecular weight” and “molar mass” are often used interchangeably. They both refer to the mass of one mole of a substance in grams. So, the moleculars weight of NaOH is also its molar mass.
Why is NaOH commonly referred to as “caustic soda”?
Sodium hydroxide (NaOH) is commonly known as “caustic soda” because it is a highly caustic and corrosive substance. It has the ability to burn or corrode various materials and is used in a wide range of industrial applications, including in the production of soaps, detergents, and cleaning agents.
Difference Between Force And Pressure
Difference Between Force And Pressure: Significant disparities exist between force and pressure, despite both being fundamental concepts in physics. To grasp this contrast, it’s essential to comprehend their definitions and applications.

Force involves push and pull actions leading to alterations in motion and direction, while pressure quantifies the physical force distributed over a specific area.
Definition:
Force: Force is a vector quantity that represents a push or pull exerted on an object due to its interaction with another object. It has both magnitude and direction and is measured in newtons (N).
Pressure: Pressure is a scalar quantity that measures the intensity of a force applied to a specific area. It is the force per unit area and is expressed in units such as pascals (Pa) or pounds per square inch (psi).
Nature:
Force: Force is a fundamental concept in physics and can manifest as a push or pull along a straight line or at an angle. It acts on a specific point or object and can result in motion or deformation.
Pressure: Pressure is the distribution of force over a given area. It doesn’t act on a specific point but rather is spread out uniformly over the surface. It describes how force is distributed over an area.
Vector vs. Scalar:
Force: Force is a vector quantity because it has both magnitude and direction. It is represented by an arrow indicating the direction of the push or pull.
Pressure: Pressure is a scalar quantity as it has magnitude only. It does not have a specific direction associated with it.
Units:
Force: Force is measured in newtons (N) in the International System of Units (SI) and can also be expressed in other units like pounds or dynes.
Pressure: Pressure is measured in pascals (Pa) in SI units. In other systems, it can be measured in pounds per square inch (psi) or atmospheres (atm).
Formula:
Force: The formula for force is F = ma, where F represents force, m is mass, and a is acceleration. It can also be calculated as the rate of change of momentum, F = Δp/Δt.
Pressure: Pressure is calculated using the formula P = F/A, where P represents pressure, F is the force applied, and A is the area over which the force is distributed.
Effect on Objects:
Force: A force can result in the motion of an object or changes in its shape or velocity. It can cause acceleration or deformation.
Pressure: Pressure affects the deformation of an object or the behavior of fluids (liquids and gases) within containers. It does not necessarily cause motion but can lead to changes in the state or shape of matter.
Examples:
Force: Examples of forces include lifting a book, pushing a car, or pulling an object with a rope.
Pressure: Examples of pressure include the pressure exerted by a gas in a closed container, atmospheric pressure on Earth’s surface, and the pressure applied by a thumbtack on a bulletin board.
In summary, force is a vector quantity representing a push or pull with magnitude and direction, while pressure is a scalar quantity representing the distribution of force over a specific area.
Read More
- Class 10 Geography Book NCERT PDF in English Download
- 10th Class History Textbook PDF English Medium NCERT Download
- Class 10 Social Science Book Pdf Download In Hindi
- NCERT Political Science Class 10 Pdf Download In Hindi
- NCERT Books For Class 10 Economics In Hindi Medium PDF
Frequently Asked Question (FAQs)
What is the fundamental difference between force and pressure?
The fundamental difference lies in their nature. Force is a vector quantity with both magnitude and direction, representing a push or pull on an object. Pressure, on the other hand, is a scalar quantity that measures the intensity of force distributed over an area.
How is force measured?
Force is typically measured in newtons (N) in the International System of Units (SI). It can also be expressed in other units like pounds or dynes.
What are some real-world examples of force?
Examples of forces include lifting objects, pushing or pulling a car, gravitational forces, and tension in ropes or cables.
How is pressure calculated?
Pressure is calculated using the formula P = F/A, where P represents pressure, F is the force applied, and A is the area over which the force is distributed.
Is pressure a vector or scalar quantity?
Pressure is a scalar quantity, as it has magnitude but does not have a specific direction associated with it.
Difference Between Diode And Rectifier
Difference Between Diodes and Rectifiers: Diodes and rectifiers are electronic components commonly used in electrical circuits, but they serve different purposes. Here are the key differences between a diode and a rectifier:

Difference Between Diode And Rectifier
Function:
Diode: A diode is a two-terminal semiconductor device that primarily allows the flow of electric current in one direction while blocking it in the other direction.
It serves as a one-way valve for electrical current and is often used for tasks such as voltage regulation, signal demodulation, and signal protection.
Rectifier: A rectifier is a circuit or a device (usually consisting of multiple diodes) that converts alternating current (AC) into direct current (DC).
Rectifiers are specifically designed to rectify or convert the polarity of an AC signal, ensuring that the output current flows predominantly in one direction.
Number of Terminals:
Diode: A diode has two terminals: an anode (positive) and a cathode (negative). It allows current to flow from the anode to the cathode when forward-biased and blocks current when reverse-biased.
Rectifier: A rectifier typically consists of multiple diodes arranged in a specific configuration, such as a bridge rectifier or a full-wave rectifier. These rectifiers can have more than two terminals, depending on their design.
Typical Applications:
Diode: Diodes find applications in a wide range of electronic circuits, including voltage clamping, signal rectification, signal modulation, and protection against reverse voltage.
Rectifier: Rectifiers are specifically used to convert AC to DC. They are essential components in power supplies, battery chargers, and devices that require a constant source of DC voltage.
Direction of Current:
Diode: A diode allows current to flow in one direction (forward bias) while blocking it in the opposite direction (reverse bias).
Rectifier: A rectifier ensures that the output current flows predominantly in one direction, converting the AC input into a DC output.
Symbol:
Diode: The symbol for a diode typically consists of an arrow pointing towards a vertical line, representing the anode and cathode.
Rectifier: Rectifier symbols vary depending on the specific configuration but often include multiple diode symbols connected in a bridge-like pattern.
In summary, a diode is a fundamental two-terminal semiconductor device that allows current flow in one direction, while a rectifier is a circuit or device that utilizes diodes to convert alternating current (AC) into direct current (DC).
Rectifiers are specialized for converting the polarity of AC signals, making them a subset of diodes designed for a specific purpose.
Read More
- Basic And Standard Maths In Class 10 Sample Paper PDF
- CBSE Sample Papers for Class 11 English With Answers PDF
- CBSE Class 10 Basic Maths Question Paper 2020 With Solutions
- CBSE Social Science Class 10 Question Paper 2020 With Answers
- 10th Class All Subject Books Hindi Medium NCERT PDF Download
Frequently Asked Question (FAQs)
What is the primary function of a diode?
The primary function of a diode is to allow electric current to flow in one direction (forward bias) while blocking it in the opposite direction (reverse bias). It acts as an electronic check valve.
Can a diode be used to convert AC to DC like a rectifier?
No, a diode by itself cannot convert AC to DC. While it rectifies AC by allowing only one half of the AC cycle to pass, it does not convert the entire AC waveform into a smooth DC signal. A rectifier, which often includes multiple diodes, is needed for complete AC-to-DC conversion.
What are the terminals of a diode called?
A diode has two terminals: an anode (positive) and a cathode (negative).
What is the primary function of a rectifier?
The primary function of a rectifier is to convert alternating current (AC) into direct current (DC). It ensures that the output current flows predominantly in one direction.
Are rectifiers composed of multiple diodes?
Yes, rectifiers are often constructed using multiple diodes connected in specific configurations, such as bridge rectifiers or full-wave rectifiers. These diodes work together to achieve the AC-to-DC conversion.
Difference Between AM And FM
Difference Between AM and FM: AM (Amplitude Modulation) and FM (Frequency Modulation) are two common methods used for transmitting information through radio waves.
They differ in how they encode and transmit signals. Here’s a comparison of the key differences between AM and Frequency modulation:

Difference Between AM And FM
Modulation Technique:
AM: In AM, the amplitude (strength) of the carrier wave varies in proportion to the instantaneous amplitude of the modulating signal (e.g., audio signal). This means that the amplitude of the carrier wave is modulated to carry the information.
FM: In FM, the frequency of the carrier wave varies in proportion to the instantaneous frequency of the modulating signal. In other words, the frequency of the carrier wave changes as it carries the information.
Sensitivity to Interference:
AM: AM signals are more susceptible to amplitude variations due to interference, such as atmospheric disturbances and electrical noise. This can result in audible static and interference.
FM: Frequency modulation signals are less sensitive to amplitude variations but are more sensitive to frequency variations. As a result, they are generally less prone to interference, providing clearer audio quality.
Bandwidth:
AM: AM signals have a narrower bandwidth compared to Frequency modulation signals. This means that AM stations can be placed closer together on the radio dial but have a limited audio bandwidth.
FM: Frequency modulation signals have a wider bandwidth, allowing for higher-fidelity audio transmission. However, this wider bandwidth requires Frequency modulation stations to be spaced farther apart on the dial to prevent interference.
Signal Quality:
AM: AM signals are known for their simplicity and are suitable for transmitting voice and news. However, they have lower audio quality compared to FM and are more susceptible to noise.
FM: FM signals offer superior audio quality, making them ideal for music transmission. They are less prone to noise and interference, resulting in clear and high-fidelity sound.
Range and Coverage:
AM: AM signals can travel longer distances and provide better coverage at night due to changes in the ionosphere, which reflects AM signals. This makes AM suitable for long-range communication.
FM: FM signals have a shorter effective range and are less affected by changes in the ionosphere. They are typically used for local and regional broadcasting.
Applications:
AM: AM is commonly used for broadcasting news, talk radio, and voice transmissions, especially in AM radio stations.
FM: Frequency modulation is preferred for music broadcasting, including Frequency modulation radio stations, and is used in applications like two-way radio communication.
In summary, AM and FM are two distinct modulation techniques used in radio communication, each with its own set of advantages and disadvantages. AM is known for its longer range and simplicity, while Frequency modulation offers better audio quality and resistance to interference. The choice between AM and Frequency modulation depends on the specific requirements of the communication system and the type of content being transmitted.
Read More
- Dielectric Material And Dipole Moment
- Difference Between Centre Of Gravity And Centroid
- Kinetic Gas Equation Derivation
- Electromagnetic Waves Transverse Nature Class 10
- Difference Between KVA And KW
Frequently Asked Question (FAQs) on Difference Between AM And FM
What is AM and FM?
AM stands for Amplitude Modulation, and Frequency modulation stands for Frequency Modulation. They are two different methods of modulating radio waves to transmit information.
How do AM and FM differ in modulation technique?
In AM, the amplitude of the carrier wave varies with the information signal, while in Frequency modulation, the frequency of the carrier wave changes with the information signal.
Which has better audio quality, AM or FM?
Frequency modulation has better audio quality compared to AM. Frequency modulation signals are less prone to noise and interference, resulting in clearer and higher-fidelity sound.
Are AM and FM signals equally susceptible to interference?
No, AM signals are more susceptible to amplitude variations due to interference, while Frequency modulation signals are less sensitive to amplitude variations but more sensitive to frequency variations.
Why are AM signals more suitable for long-range communication?
AM signals can travel longer distances due to their ability to bounce off the ionosphere, especially at night. This property makes AM suitable for long-range communication.
Dielectric Material And Dipole Moment
Dielectric Material And Dipole Moment: In this piece, we will explore the distinguishing features of polar and non-polar materials.

Dielectric Material And Dipole Moment
Insulators and Dielectric Materials:
Insulators:
Insulators, also known as non-conductors, are materials that impede the flow of electric current. Unlike conductors, which allow the movement of electric charges, insulators have electrons tightly bound to their atoms or molecules.
This strong binding prevents the easy flow of electrons, resulting in a lack of conductivity. Insulators are commonly used to isolate or separate conductive materials, thereby preventing the leakage of electrical current and ensuring safety in various applications.
Examples of insulators include rubber, plastic, glass, ceramic, and wood. These materials are often utilized in electrical wiring, electronics, and construction to prevent unintentional electrical contact.
Dielectric Materials:
Dielectric materials share similarities with insulators, but they have a distinct focus on their behavior in response to electric fields. When placed in an electric field, dielectric materials become polarized, meaning their electrons shift slightly in response to the applied electric field.
This polarization creates temporary dipoles within the material. While dielectric materials hinder the flow of electric current, their ability to polarize in the presence of an electric field is essential in various applications, particularly in capacitors.
Dielectric materials are used to separate the plates of a capacitor, where they store electric energy due to the accumulation of charges on their surfaces.
This stored energy can then be released when needed. Dielectrics increase the capacitance of a capacitor by allowing it to store more charge for a given voltage.
In summary, insulators prevent the flow of electric current by binding electrons tightly to atoms or molecules, while dielectric materials exhibit polarization in response to electric fields, making them valuable for energy storage and manipulation in devices like capacitors. Both insulators and dielectric materials are vital components in modern technology and electrical engineering.
Polar and Non-Polar Molecules:
Polar Molecules:
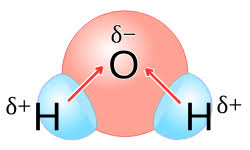
Polar molecules are those in which the distribution of electrical charge is uneven, resulting in a separation of positive and negative charges within the molecule. This charge separation gives rise to a molecular dipole moment, creating a positive end (where the positive charge is concentrated) and a negative end (where the negative charge is concentrated) within the molecule.
Polarity in molecules is primarily influenced by the electronegativity difference between atoms and the molecular geometry. When atoms with differing electronegativities are bonded, the more electronegative atom tends to attract electrons more strongly, leading to an uneven charge distribution. Water (H2O) is a classic example of a polar molecule, with its bent shape and unequal sharing of electrons.
Polar molecules interact strongly with other polar molecules due to the attraction between their opposite charges. This phenomenon is essential in various biological and chemical processes, such as dissolving polar substances in water.
Non-Polar Molecules:
Non-polar molecules are characterized by an even distribution of electrical charge, resulting in no significant dipole moment. This usually occurs when atoms within the molecule have similar or very close electronegativities, leading to a balanced sharing of electrons.
Examples of non-polar molecules include diatomic gases like oxygen (O2) and nitrogen (N2), as well as hydrocarbons like methane (CH4) and ethane (C2H6). These molecules often exhibit symmetrical geometry, and the electronegativity difference between their constituent atoms is negligible.
Non-polar molecules tend to interact weakly with other non-polar molecules due to the absence of significant charge differences. This behavior is evident in processes like the dispersion forces between non-polar molecules, which contribute to the cohesion of substances like oils and gases.
In summary, polar molecules have an uneven distribution of charges, leading to a dipole moment and strong interactions with other polar molecules, while non-polar molecules exhibit an even charge distribution, resulting in weak interactions with other non-polar molecules. Understanding the polarity of molecules is crucial in explaining a wide range of chemical and physical properties.
Read More
- CBSE Class 10 Basic Maths Question Paper 2020 With Solutions
- CBSE Social Science Class 10 Question Paper 2020 With Answers
- 10th Class All Subject Books Hindi Medium NCERT PDF Download
- Class 10 All Subjects Books NCERT PDF Download in English
- CBSE Class 10 Sample Question Paper Social Science PDF
Frequently Asked Question (FAQs)
What is a dielectric material?
A dielectric material is an insulating substance that does not conduct electric current easily. It exhibits polarization when exposed to an electric field, causing shifts in the distribution of charges within the material without allowing the movement of charges.
How do dielectric materials behave in an electric field?
Dielectric materials become polarized when subjected to an electric field. The electrons within the material experience slight displacements, leading to induced dipoles. This polarization effect enhances the ability of dielectric materials to store electric energy in devices like capacitors.
What is the role of dielectric materials in capacitors?
Dielectric materials are used in capacitors to separate the electrically conductive plates. The polarization of the dielectric material increases the capacitance of the capacitor, allowing it to store more electric charge for a given voltage.
How is a dipole moment defined?
A dipole moment is a measure of the separation of positive and negative charges within a molecule or system. It indicates the strength and direction of the electric dipole created due to the charge separation.
What causes the creation of a dipole moment in a molecule?
A dipole moment arises when there is an uneven distribution of electrical charge within a molecule. This can result from differences in electronegativity between atoms within the molecule, leading to partial positive and negative charges.
Difference Between Centre Of Gravity And Centroid
Difference Between Centre Of Gravity And Centroid: The terms “center of gravity” and “centroid” are often used in various fields, such as physics, engineering, and mathematics, to describe different concepts related to the distribution of mass or geometric properties.

Although they might seem similar, they have distinct meanings and applications. Here’s a breakdown of the differences between the two:
Difference Between Centre Of Gravity And Centroid
Definition and Concept:
Centre of Gravity: The center of gravity of an object is the point at which the entire weight of the object can be considered to act. In other words, it is the point around which the object’s weight is evenly balanced in all directions.
It is the point where the gravitational force acts on the object, and if supported at this point, the object will be in equilibrium.
Centroid: The centroid of a geometric shape (such as a two-dimensional figure or a three-dimensional object) is the point at which the shape could be perfectly balanced if it were made of a uniform material.
It’s often considered as the geometric center or average position of all the points that make up the shape, without considering their masses or weights.
Application:
Centre of Gravity: The concept of center of gravity is primarily used in the study of mechanics and engineering to analyze the stability and equilibrium of objects.
For example, in architecture and design, understanding the center of gravity is crucial to ensure the stability of structures and prevent them from toppling over.
Centroid: The concept of centroid is used in mathematics, geometry, and various engineering fields to determine properties such as the geometric center, moment of inertia, and other geometric characteristics of shapes. It’s essential for calculating moments, areas, and volumes in design and analysis.
Calculation:
Centre of Gravity: Calculating the center of gravity involves considering the distribution of mass within an object and determining the point where the weight is balanced.
This calculation can be complex for irregularly shaped objects or those with varying densities.
Centroid: The centroid of a shape is often calculated using specific formulas that take into account the coordinates of the vertices or points that define the shape.
These formulas vary depending on the type of shape being analyzed (e.g., triangles, rectangles, circles).
Symmetry:
Centre of Gravity: The center of gravity may not necessarily coincide with the geometric center of an object, especially if the mass distribution is not uniform or if there are external forces acting on the object.
Centroid: The centroid is often located at the geometric center of regular shapes with uniform distribution of points. In irregular shapes, the centroid can be determined based on the specific properties of the shape.
In summary, the center of gravity relates to the distribution of weight and is essential for understanding the stability of objects, while the centroid focuses on the geometric center of shapes and aids in calculating various geometric properties.
Read More
- Kinetic Gas Equation Derivation
- Electromagnetic Waves Transverse Nature Class 10
- Difference Between KVA And KW
- Difference Between LCD And LED
- Electric Potential of a Point Charge Class 10
Frequently Asked Questions (FAQs)
What is the centre of gravity?
The centre of gravity is the point within an object where the entire weight of the object can be considered to act. It’s the point where the gravitational force effectively acts on the object, determining its equilibrium and stability.
What is the centroid?
The centroid is the geometric center or average position of all the points that make up a shape, without considering their masses or weights. It’s a point used to define the balance and geometric properties of a shape.
How are these concepts used in physics and engineering?
The center of gravity is used in physics and engineering to analyze the stability and balance of objects. It helps determine how external forces will affect an object’s equilibrium. The centroid, on the other hand, is used to calculate various geometric properties of shapes, such as moment of inertia, center of mass, and distribution of areas or volumes.
Are the center of gravity and centroid always the same point?
No, they are not the same. The center of gravity may not coincide with the centroid, especially in cases of non-uniform mass distribution or when external forces are acting on the object. The centroid is primarily a geometric property and may not be affected by mass distribution.
How are the center of gravity and centroid calculated?
Calculating the center of gravity involves considering the distribution of mass within an object and finding the point where the weight is balanced. The centroid of a shape is calculated using specific geometric formulas based on the coordinates of the shape’s vertices or points.
Kinetic Gas Equation Derivation
Kinetic Gas Equation Derivation: The kinetic gas equation, also known as the ideal gas law, describes the behavior of an ideal gas under various conditions.
It relates the pressure, volume, and temperature of a gas to the number of gas molecules and the ideal gas constant. Here, we’ll outline the derivation of the kinetic gas equation step by step.

Kinetic Gas Equation Derivation
Step 1: Understanding Assumptions
The ideal gas law is based on several assumptions:
- Gas molecules are considered to be point masses with no volume.
- Gas molecules undergo elastic collisions with each other and the container walls.
- There are no intermolecular forces between gas molecules.
- The volume occupied by the gas molecules themselves is negligible compared to the container’s volume.
Step 2: Defining Pressure and Force
Pressure (P) is defined as force (F) per unit area (A):
P = F/A
Step 3: Force Due to Collisions
Consider a gas molecule colliding with a container wall. The change in momentum during the collision generates a force on the wall. The average force is given by Newton’s second law as:
Where:
- ∆m is the change in momentum of the molecule,
- v_x is the x-component of the velocity of the molecule, and
- ∆t is the time between successive collisions.
Step 4: Relating Force to Pressure
Substituting the force expression into the pressure definition gives:
Step 5: Relating Velocity and Temperature
The average kinetic energy of a gas molecule is proportional to its temperature (T) in Kelvin: 3/2= 1/2
Where:
- k is the Boltzmann constant,
- m is the mass of a gas molecule,
- v is the root-mean-square velocity of the molecules.
Step 6: Substituting Velocity into Pressure Expression
Substitute the expression for velocity (v) from the kinetic energy equation into the pressure equation:
![]()
Step 7: Molecule Count
The number of molecules per unit volume (n/V) is defined as the number density: Where N is the total number of molecules in the volume V.
Step 8: Combining Equations
Substitute the expression for the number density into the pressure equation:
Step 9: Introducing Ideal Gas Constant
The ideal gas constant (R) is defined as:
Step 10: Arriving at the Kinetic Gas Equation
Substitute the ideal gas constant into the equation:
![]()
This equation simplifies to:
![]()
Since 2 is the average kinetic energy ( = 3/2):
This is the kinetic gas equation, also known as the ideal gas law:
Where:
- P is pressure,
- V is volume,
- n is the number of moles of gas,
- R is the ideal gas constant,
- T is temperature.
The kinetic gas equation describes the behavior of ideal gases under various conditions, providing insights into their macroscopic properties based on the motion of their constituent particles.
Read More
- Electromagnetic Waves Transverse Nature Class 10
- Difference Between KVA And KW
- Difference Between LCD And LED
- Electric Potential of a Point Charge Class 10
- Difference Between In Physics
Frequently Asked Question (FEQs)
What is the kinetic gas equation, also known as the ideal gas law?
The kinetic gas equation, often referred to as the ideal gas law, is a fundamental equation in thermodynamics that describes the relationship between the pressure (P), volume (V), temperature (T), and amount of gas (n) in a system. It is represented by the equation PV = nRT, where R is the ideal gas constant.
Why is the ideal gas law also called the kinetic gas equation?
The ideal gas law is sometimes called the kinetic gas equation because it is derived based on the kinetic theory of gases, which considers the motion of gas molecules and their collisions.
What are the assumptions made in the derivation of the kinetic gas equation?
The derivation of the kinetic gas equation is based on the following assumptions:
- Gas molecules are considered to be point masses with no volume.
- Gas molecules undergo perfectly elastic collisions with each other and the container walls.
- There are no intermolecular forces between gas molecules.
- The volume occupied by the gas molecules is negligible compared to the container’s volume.
How does the kinetic gas equation relate pressure, volume, temperature, and the number of gas molecules?
The kinetic gas equation, PV = nRT, establishes a direct relationship between the pressure, volume, temperature, and number of moles of gas in a system. It implies that for a given amount of gas, an increase in temperature or volume results in a proportional increase in pressure.
What role does the ideal gas constant (R) play in the equation?
The ideal gas constant (R) is a proportionality constant that relates the physical properties of a gas to the equation. It provides a bridge between the microscopic behavior of gas molecules and the macroscopic properties described by pressure, volume, temperature, and amount of gas.
Electromagnetic Waves Transverse Nature
Electromagnetic Waves Transverse Nature: Electromagnetic waves are a fundamental aspect of the electromagnetic spectrum, encompassing phenomena such as radio waves, microwaves, visible light, and more.
One of the distinguishing characteristics of electromagnetic waves is their transverse nature, which sets them apart from other types of waves.

Transverse Waves: A Quick Overview
Transverse waves are a type of wave in which the oscillations of the wave propagate perpendicular to the direction of the wave’s motion. This means that while the wave itself travels in one direction, the individual particles or fields oscillate at right angles to that direction.
Understanding Electromagnetic Waves:
- Electromagnetic Field Oscillations: Electromagnetic waves are composed of electric and magnetic fields that oscillate as the wave propagates. These fields are perpendicular to each other and to the direction of wave motion.
- Perpendicular Oscillations: In an electromagnetic wave, the electric field oscillates in one plane, while the magnetic field oscillates in a plane perpendicular to the electric field. Both fields are orthogonal to the direction of wave propagation.
- Light as an Example: Visible light, which is a form of electromagnetic wave, demonstrates this transverse nature. The oscillating electric and magnetic fields give rise to the characteristic properties of light, such as polarization and interference.
Key Features of Transverse Nature:
- Polarization: The transverse nature of electromagnetic waves allows for polarization. Polarization refers to the orientation of the oscillations of the electric and magnetic fields in a specific direction perpendicular to the wave’s motion.
- Propagation in Empty Space: Electromagnetic waves can propagate through a vacuum, as they do not require a medium for their transmission. This characteristic is a result of their transverse nature.
- Interference and Diffraction: The transverse nature of electromagnetic waves enables them to exhibit interference and diffraction patterns, which are phenomena observed when waves interact with each other or encounter obstacles.
Electromagnetic Waves and Transverse Motion:
- Electric and Magnetic Fields: Electromagnetic waves consist of varying electric and magnetic fields. As the wave travels, these fields oscillate in directions perpendicular to each other and to the direction of the wave.
- Perpendicular Oscillations: The electric field oscillates in a plane perpendicular to the magnetic field, and both are at right angles to the direction of wave propagation. This unique oscillation pattern gives electromagnetic waves their transverse nature.
- Visible Light Example: Consider visible light, a form of electromagnetic wave. The varying electric and magnetic fields give rise to the different colors and properties of light. The transverse oscillations are responsible for phenomena like polarization and color dispersion.
Significance and Characteristics:
- Polarization: The transverse nature allows electromagnetic waves to be polarized. Polarization refers to the alignment of the oscillations in a specific direction. Polarized sunglasses, for instance, filter out certain orientations of light waves to reduce glare.
- Propagation in a Vacuum: Electromagnetic waves can travel through a vacuum, devoid of any material medium. This is due to their transverse nature, which doesn’t require particles to oscillate in the wave’s direction.
- Interference and Diffraction: Transverse waves exhibit interference and diffraction patterns when they interact with each other or encounter obstacles. These patterns result from the superposition of waves with different phases and amplitudes.
Applications:
The transverse nature of electromagnetic waves is at the core of various technological applications, including wireless communication, radio broadcasting, television transmission, and fiber-optic communication. Understanding how these waves oscillate perpendicular to their motion helps engineers and scientists harness their properties for a wide range of purposes.
In summary, the transverse nature of electromagnetic waves is a defining characteristic that governs their behavior, propagation, and applications. This unique feature plays a pivotal role in our ability to communicate, observe the universe, and develop technologies that shape modern society.
Read More
- Kinetic Gas Equation Derivation
- Difference Between KVA And KW
- Difference Between LCD And LED
- Difference Between In Physics
Frequently Asked Question (FAQs)
What does “transverse nature” mean in the context of electromagnetic waves?
In the context of electromagnetic waves, “transverse nature” refers to the perpendicular oscillations of electric and magnetic fields. These oscillations occur at right angles to the direction in which the wave is traveling.
How do the electric and magnetic fields oscillate in electromagnetic waves?
The electric and magnetic fields in electromagnetic waves oscillate perpendicular to each other and to the direction of wave propagation. This perpendicular oscillation gives rise to the unique characteristics of electromagnetic waves.
What is the significance of the transverse nature of electromagnetic waves?
The transverse nature of electromagnetic wave is responsible for various phenomena and applications, such as polarization, interference, diffraction, and the ability to propagate through a vacuum.
How does the transverse nature allow electromagnetic waves to propagate through a vacuum?
Unlike other types of wave that require a medium for propagation, the transverse nature of electromagnetic wave enables them to travel through a vacuum because they don’t rely on the movement of particles for transmission.
What is polarization in the context of transverse electromagnetic waves?
Polarization refers to the alignment of the oscillations of the electric and magnetic fields in a specific direction perpendicular to the wave’s motion. This property has applications in areas like optics and communication.