Category: Class 11
Plant Physiology Class 11
Plant Physiology Class 11: Plant physiology, a branch of biology, delves into the intricate mechanisms that govern the life and functions of plants. It is a fascinating field that explores the inner workings of plants, from their growth and development to their responses to environmental factors.
In this comprehensive article, we will embark on a journey to uncover the captivating world of plant physiology, revealing the remarkable processes that allow plants to thrive and sustain life on Earth.
Plant Physiology Class 11
Understanding Plant Physiology
What Is Plant Physiology?
Plant physiology is the scientific study of how plants function, grow, and respond to their surroundings. It encompasses a wide range of topics, including plant nutrition, photosynthesis, respiration, water uptake, hormone regulation, and plant-microbe interactions. Plant physiologists investigate the molecular, cellular, and ecological aspects of plant life.
The Significance of Plant Physiology
Plant physiology plays a pivotal role in agriculture, horticulture, forestry, and environmental science. Understanding how plants function is essential for improving crop yields, conserving natural ecosystems, and addressing global challenges such as climate change and food security.
Plant Growth and Development
Seed Germination
The journey of a plant’s life begins with seed germination. The process involves the activation of dormant seeds, followed by the emergence of a seedling. Factors such as moisture, temperature, and oxygen levels influence germination.
Growth Hormones
Plant growth is regulated by hormones, including auxins, gibberellins, cytokinins, and abscisic acid. These hormones control cell elongation, root development, flowering, and fruit formation. Understanding their roles is crucial for agriculture and horticulture.
Photomorphogenesis
Plants are highly responsive to light, a phenomenon known as photomorphogenesis. Light signals trigger changes in plant morphology, such as phototropism (bending towards light) and photoperiodism (flowering in response to day length).
Nutrient Uptake and Photosynthesis
Nutrient Absorption
Plants obtain essential nutrients, including nitrogen, phosphorus, and potassium, from the soil. Root structures, such as root hairs, facilitate nutrient uptake. Nutrient deficiency can hinder plant growth.
Photosynthesis
Photosynthesis is the process by which plants convert light energy into chemical energy in the form of glucose. Chloroplasts, containing the pigment chlorophyll, are the sites where photosynthesis occurs. Oxygen is released as a byproduct.
Respiration
Plants also undergo cellular respiration to derive energy from glucose. During respiration, oxygen is consumed, and carbon dioxide is produced. This process takes place in mitochondria.
Water Transport and Transpiration
Water Uptake
The Water is crucial for plant survival, and its uptake is facilitated by roots. The process involves osmosis, where water moves from areas of high soil water potential to areas of lower potential within the plant.
Transpiration
Transpiration is the loss of water vapor from plant leaves through small openings called stomata. It is essential for nutrient transport and cooling the plant. However, excessive transpiration can lead to water stress.
Xylem and Phloem
Plants have specialized tissues for water and nutrient transport. Xylem transports water and minerals from roots to shoots, while phloem carries sugars and other organic compounds throughout the plant.
Responses to Environmental Factors
Tropisms
Tropisms are plant growth responses to environmental stimuli. Phototropism (light), geotropism (gravity), and hydrotropism (water) are examples. These responses help plants adapt to their surroundings.
Environmental Stress
Plants face various stressors, including drought, salinity, and temperature extremes. They employ adaptive mechanisms, such as osmotic adjustments and the production of stress proteins, to survive harsh conditions.
Defense Mechanisms
Plants defend themselves against herbivores and pathogens through chemical compounds, including alkaloids, terpenoids, and phenolics. Some plants also engage in symbiotic relationships with beneficial microbes for protection.
Reproduction and Growth
Flowering
The transition from vegetative growth to flowering is a critical phase in a plant’s life cycle. Environmental factors, photoperiods, and hormonal signals control flowering time.
Pollination
Pollination is the transfer of pollen from the male to the female reproductive structures of a flower. It is crucial for fertilization and seed production. Insects, wind, and other agents aid in pollination.
Seed Production
The fertilized ovule develops into a seed containing the embryo plant and stored nutrients. Dispersal mechanisms, such as wind, animals, and water, ensure that seeds find suitable environments for germination.
Interactions with Microbes
Symbiotic Relationships
Plants form symbiotic relationships with beneficial microbes, such as mycorrhizal fungi and nitrogen-fixing bacteria. These partnerships enhance nutrient uptake and nitrogen availability.
Plant-Microbe Interactions
Plants engage in complex interactions with pathogens, leading to diseases. Understanding these interactions is crucial for developing disease-resistant crop varieties.
Ecological Significance
Ecosystem Services
Plants provide essential ecosystem services, including carbon sequestration, oxygen production, soil stabilization, and habitat creation. Forests, wetlands, and grasslands are integral to biodiversity and climate regulation.
Conservation
Preserving plant diversity is essential for ecosystem health and human well-being. Conservation efforts focus on safeguarding endangered plant species and habitats.
Agricultural Applications
Crop Improvement
Plant physiology informs agricultural practices, from optimizing irrigation to developing drought-resistant crops. Genetic engineering and breeding programs aim to enhance crop yield and nutritional content.
Pest and Disease Management
Understanding plant responses to pests and diseases aids in pest management. Integrated pest management (IPM) strategies minimize the use of pesticides.
Genomics and Molecular Biology
Advances in genomics and molecular biology have allowed scientists to unravel the genetic basis of plant functions. This knowledge enables precise genetic modifications for crop improvement, disease resistance, and stress tolerance.
Climate Change Resilience
Plant physiology research is essential in developing crop varieties that can thrive in changing climate conditions. Scientists are studying how plants respond to elevated temperatures, altered precipitation patterns, and increased carbon dioxide levels.
Sustainable Agriculture
Plant physiologists are exploring sustainable agricultural practices that minimize environmental impacts. This includes optimizing nutrient use efficiency, reducing pesticide reliance, and developing crops with reduced resource requirements.
Conclusion
Plant physiology is a captivating field that unveils the inner workings of plants and their profound impact on our world. From the intricacies of growth and development to the responses to environmental challenges, plants exhibit a remarkable array of adaptations and processes. As we delve deeper into the science of plant physiology, we gain insights not only into the lives of plants but also into the essential interconnections between plants, ecosystems, and human societies. In a rapidly changing world, the study of plant physiology remains vital for addressing global challenges, including food security, climate change, and biodiversity conservation. It underscores the importance of nurturing and preserving the green life that sustains us all.
Read More
- Molecular Weight Of Ammonia
- Molar Mass Of Elements
- Molar Mass Of Zinc
- Differences Between Enthalpy And Entropy
- Periodic Table Class 11
Frequently Asked Questions (FAQs) On Plant Physiology Class 11
1. What is plant physiology?
Plant physiology is the branch of biology that focuses on understanding the life processes and functions of plants, including growth, development, and responses to the environment.
2. Why is plant physiology important?
Plant physiology is crucial for agriculture, horticulture, and environmental science. It helps us improve crop yields, conserve natural ecosystems, and address global challenges like climate change and food security.
3. What are some key topics in plant physiology?
Key topics include seed germination, growth hormones, photosynthesis, respiration, water transport, responses to environmental factors (tropisms and stress responses), reproduction, and interactions with microbes.
4. How do plants absorb nutrients from the soil?
Plants absorb essential nutrients from the soil through their root systems. Root hairs and specialized transport mechanisms, like ion channels and pumps, facilitate nutrient uptake.
5. What is photosynthesis, and where does it occur in plants?
Photosynthesis is the process by which plants convert light energy into chemical energy (glucose) using chlorophyll. It occurs primarily in the chloroplasts of plant cells.
Molecular Weight Of Ammonia
Molecular Weight Of Ammonia: Ammonia, with the chemical formula NH3, is a compound that holds immense significance in various fields, from agriculture to industrial chemistry.
Understanding its molecular weight is essential for grasping its properties, applications, and how it behaves in chemical reactions. In this article, we will explore the molecular weight of ammonia, its calculation, and its relevance in different scientific and industrial contexts.

Molecular Weight Of Ammonia
What is Molecular Weight?
Molecular weight, also known as molecular mass or molar mass, is the sum of the atomic masses of all the atoms present in a molecule. It can be denoted in atomic mass units (amu) or grams per mole (g/mol). Molecular weight serves as a bridge between the microscopic world of atoms and molecules and the macroscopic world we can observe and measure.
Molecular Weight of Ammonia (NH3)
To determine the molecular weight of ammonia (NH3), we need to consider the atomic masses of its constituent elements:
- Nitrogen (N) has an atomic mass of approximately 14.01 amu.
- Hydrogen (H) has an atomic mass of approximately 1.008 amu.
Now, let’s calculate the mole weight of ammonia (NH3):
mole weight of NH3 = (1 * 14.01 amu for nitrogen) + (3 * 1.008 amu for hydrogen) = 14.01 amu + 3.024 amu ≈ 17.034 amu.
Therefore, the mole weight of ammonia (NH3) is approximately 17.034 amu or 17.034 g/mol.
Relevance in Agricultural and Industrial Applications
- Fertilizer Production: Ammonia is a primary component in the production of nitrogen-based fertilizers. Understanding its molecular weight helps in determining the appropriate amount to use, ensuring optimal crop growth.
- Refrigeration: In refrigeration systems, ammonia is used as a refrigerant. Its molecular weight is crucial in designing and maintaining efficient cooling systems.
- Cleaning Agents: Ammonia-based cleaning products rely on ammonia’s properties. Molecular weight helps manufacturers in formulating effective cleaning solutions.
- Chemical Reactions: In chemical reactions involving ammonia, its molecular weight is employed to determine the amounts of reactants and products, allowing precise control in diverse industrial processes.
- Environmental Monitoring: In environmental studies, knowing the mole weight of ammonia is vital for measuring its concentration in air and water, particularly in cases of pollution or in the context of atmospheric chemistry.
Conclusion
The mole weight of ammonia (NH3) is a fundamental parameter that underpins its properties, applications, and behavior in various contexts. It facilitates the accurate calculation of reactant and product quantities in chemical reactions and plays a significant role in industries such as agriculture and refrigeration. By understanding the mole weight of ammonia, scientists and engineers can harness its potential and address diverse challenges, from feeding the world’s population to creating efficient cooling systems.
Read More
- Molar Mass Of Elements
- Molar Mass Of Zinc
- Differences Between Enthalpy And Entropy
- Periodic Table Class 11
- Molecular Weight Of Sodium Carbonate
Frequently Asked Questions (FAQs) On Molecular Weight Of Ammonia
1. What is the molecular weight of ammonia (NH3)?
The mole weight of ammonia (NH3) is approximately 17.034 atomic mass units (amu) or 17.034 grams per mole (g/mol).
2. How can you determine the molecular weight of ammonia?
The mole weight of ammonia is determined by summing the atomic masses of its elements: nitrogen (N) and hydrogen (H). Nitrogen has an atomic mass of approximately 14.01 amu, while hydrogen has an atomic mass of around 1.008 amu. To find the mole weight of ammonia (NH3), add 1 * 14.01 amu (for nitrogen) and 3 * 1.008 amu (for hydrogen).
3. Why is knowing the molecular weight of ammonia important?
Understanding the mole weight of ammonia is crucial in various applications, including agriculture, refrigeration, chemical reactions, and environmental monitoring. It helps determine quantities, reactions, and behaviors related to ammonia.
4. What is the role of ammonia in agriculture, and how is its molecular weight relevant in this agricultural context?
Ammonia is a key component in nitrogen-based fertilizers. Its molecular weight is essential for determining the appropriate amount to use, ensuring optimal crop growth and nutrient balance in soil.
5. How is ammonia employed in refrigeration, and how is its molecular weight relevant in this context?
Ammonia serves as a refrigerant in diverse cooling systems. Its molecular weight is significant in the design and upkeep of efficient refrigeration systems, impacting the refrigeration cycle and heat transfer characteristics.
Molar Mass Of Elements
Molar Mass Of Elements: In the world of chemistry, the concept of molar mass is fundamental and indispensable. It serves as a bridge between the macroscopic and microscopic worlds, allowing scientists to quantify and understand the behavior of atoms and molecules on a scale that is both practical and meaningful.
Molar mass plays a crucial role in stoichiometry, the study of chemical reactions and their quantitative aspects. In this article, we will delve into the concept of molar mass, why it’s important, how it’s calculated, and its significance in various chemical applications.

Molar Mass Of Elements
What is Molar Mass?
Molar mass, often denoted as “M,” is a fundamental property of an element or compound. It represents the mass of one mole of a substance, which is equal to 6.022 x 10^23 particles (Avogadro’s number). These particles can be atoms, molecules, ions, or any other discrete entities that make up matter. The unit of molar mass is grams per mole (g/mol). In essence, molar mass allows chemists to relate the mass of a substance to the number of constituent particles it contains.
Why is Molar Mass Important?
Stoichiometry: Molar mass is central to stoichiometry, a branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It enables chemists to determine the exact amounts of reactants required and products produced in a reaction, allowing for precise experimental design and product yield prediction.
- Molecular Weight: In the realm of chemistry, substances are often referred to by their molecular weight, which is the molar mass expressed in atomic mass units (amu). This helps chemists compare and contrast the masses of different molecules, facilitating the understanding of chemical structures and properties.
- Formulating Chemical Equations: When writing chemical equations, balancing the number of atoms and molecules on each side is crucial. Molar mass helps ensure that the equation is balanced correctly, as it provides a reliable basis for comparing the masses of reactants and products.
Calculating Molar Mass
The molar mass of an element is determined by summing the atomic masses of all its constituent atoms, taking into account the number of each type of atom present. For compounds, it is the sum of the molar masses of all the atoms within the molecule, weighted by their respective subscripts in the chemical formula.
To calculate the molar mass of a compound, follow these steps:
- Write down the chemical formula of the compound.
- Find the atomic masses of each element in the formula from the periodic table.
- Multiply each element’s atomic mass by the number of atoms of that element in the compound.
- Sum up all these products to obtain the molar mass of the compound.
For example, let’s calculate the molar mass of water (H2O):
- Hydrogen (H) has an atomic mass of approximately 1 amu.
- Oxygen (O) has an atomic mass of approximately 16 amu.
Molar mass of H2O = (2 * 1 amu for hydrogen) + (1 * 16 amu for oxygen) = 2 amu + 16 amu = 18 amu.
The molar mass of water is 18 g/mol.
Significance in Chemical Applications
Molar mass finds applications in various branches of chemistry, including:
- Analytical Chemistry: In analytical techniques like mass spectrometry, molar mass helps identify and quantify compounds in complex mixtures by comparing experimental data to known molar masses.
- Chemical Reactions: In stoichiometric calculations, molar mass aids in determining the limiting reactant, the amount of product formed, and the efficiency of a reaction.
- Gas Laws: In gas law equations such as the ideal gas law (PV = nRT), molar mass plays a role in determining the quantity of moles within a gas sample.
- Chemical Formulas: Molar mass is instrumental in determining empirical and molecular formulas of compounds.
Conclusion
Molar mass is a fundamental concept in chemistry, serving as a key to understanding and quantifying the behavior of substances at the atomic and molecular levels. It plays a pivotal role in stoichiometry, chemical analysis, and many other aspects of the science. By knowing the molar mass of elements and compounds, chemists can unlock the secrets of matter, enabling them to predict and manipulate the outcomes of chemical reactions and explore the properties of substances with remarkable precision.
Read More
- Molar Mass Of Zinc
- Differences Between Enthalpy And Entropy
- Periodic Table Class 11
- Molecular Weight Of Sodium Carbonate
- Difference Between Isothermal And Adiabatic Process
Frequently Asked Questions (FAQs) On Molar Mass Of Elements
1. What is molar mass?
Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It represents the mass of a specific number of atoms, molecules, or ions, and it’s a fundamental concept in chemistry.
2. Why is molar mass important in chemistry?
Molar mass is crucial as it enables chemists to establish a connection between a substance’s mass and its constituent particles. It is crucial in stoichiometry, chemical equations, and various calculations in chemistry.
3. How do you calculate the molar mass of an element?
To calculate the molar mass of an element, find its atomic mass on the periodic table. This value is expressed in atomic mass units (amu). The molar mass in grams per mole (g/mol) is numerically equivalent to the atomic mass in amu.
4. How is the molar mass of a compound calculated?
To find a compound’s molar mass, sum the atomic masses of its constituent atoms, respecting subscripts as atom quantities in the formula. Multiply the atomic mass of each element by its subscript and then sum these values.
5. What is the molar mass of water (H2O)?
The molar mass of water (H2O) is approximately 18.015 g/mol. It is calculated by adding the molar masses of two hydrogen atoms (2 * 1.008 g/mol) and one oxygen atom (1 * 15.999 g/mol).
Molar Mass Of Zinc
Molar Mass Of Zinc: In the world of chemistry, understanding the molar mass of elements is fundamental. It’s a concept that plays a crucial role in various chemical calculations, from determining the amount of a substance in a reaction to understanding its physical properties. In this article, we explore the molar mass of zinc (Zn), a vital element with a wide range of applications.

Molar Mass Of Zinc
What is Molar Mass?
Molar mass, also known as molecular weight, is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). In the realm of chemistry, a mole serves as a foundational unit for quantifying entities like atoms, molecules, or ions. This number is approximately 6.022 x 10^23, known as Avogadro’s number.
Molar mass is significant because it allows chemists to relate the weight of a substance to the number of moles present, which is essential for performing stoichiometric calculations and understanding the behavior of substances in chemical reactions.
Calculating the Molar Mass of Zinc (Zn)
To determine the molar mass of zinc (Zn), we follow these steps:
- Find the atomic mass: The atomic mass of an element is the average mass of its atoms, taking into account the relative abundance of its isotopes. For zinc, the atomic mass is approximately 65.38 atomic mass units (amu).
- Multiply by the molar mass constant: The molar mass constant, which relates atomic mass units to grams per mole, is 1 g/mol. So, to find the molar mass of zinc, we multiply the atomic mass by this constant.
Mol Mass of Zn = Atomic Mass of Zn x Molar Mass Constant Molar Mass of Zn ≈ 65.38 g/mol
Hence, the mol mass of zinc (Zn) is approximately 65.38 g/mol.
Significance of Zinc’s Molar Mass
Understanding the mol mass of zinc holds significant importance across various applications:
- Chemical Reactions: In chemical reactions involving zinc, its molar mass is vital for accurately calculating the amount of zinc needed or produced, ensuring the reaction’s efficiency.
- Galvanization: In industry, zinc is commonly used to coat steel in a process known as galvanization. Knowledge of its molar mass is essential for quality control and determining the appropriate amount of zinc coating.
- Pharmaceuticals: Zinc is an essential mineral for human health and is used in various pharmaceutical formulations. Understanding its molar mass aids in dosage calculations.
- Alloys: Zinc alloys, such as brass and bronze, have numerous engineering applications. Molar mass information is crucial for alloy composition and properties.
- Corrosion Protection: Zinc is employed in sacrificial anodes to protect metal structures from corrosion in marine and industrial environments. Its molar mass is vital for designing effective corrosion protection systems.
In conclusion, the mol mass of zinc (Zn) is a fundamental concept in chemistry that has wide-ranging implications in various fields, including industry, medicine, and materials science. It enables precise measurements and calculations, making it an indispensable tool for understanding and utilizing this essential element in our daily lives.
Read More
- Differences Between Enthalpy And Entropy
- Periodic Table Class 11
- Molecular Weight Of Sodium Carbonate
- Difference Between Isothermal And Adiabatic Process
- Simple Harmonic Motion Examples
Frequently Asked Questions (FAQs) On Molar Mass Of Zinc
1. What is the molar mass of zinc, and why is it important?
The mol mass of zinc (Zn) is approximately 65.38 grams per mole (g/mol). It’s crucial because it allows chemists to relate the weight of zinc to the number of moles, aiding in various chemical calculations.
2. How is the molar mass of zinc calculated?
The mol mass of zinc is calculated by multiplying its atomic mass by the mol mass constant, which is approximately 1 g/mol.
- Mol Mass of Zn = Atomic Mass of Zn x Molar Mass Constant
3. What is the atomic mass of zinc, and how is it determined?
The atomic mass of zinc is approximately 65.38 atomic mass units (amu). The determination of zinc’s atomic mass involves considering the isotopes’ relative abundances and their corresponding atomic masses.
4. Why is the molar mass of zinc important in chemical reactions?
In chemical reactions involving zinc, its mol mass is crucial for calculating the precise amount of zinc needed or produced. This accuracy is essential for reaction efficiency.
5. What are some common applications of zinc that rely on its molar mass?
Zinc finds application in diverse fields, including galvanization for steel corrosion protection, pharmaceuticals for health benefits, and various alloy formulations. Its molar mass is essential for quality control, dosage calculations, and alloy composition.
Differences Between Enthalpy And Entropy
Differences Between Enthalpy And Entropy: In the realm of thermodynamics, two fundamental concepts play pivotal roles in understanding and describing the behavior of matter and energy: enthalpy and entropy.
These concepts are often used in chemistry, physics, and engineering to analyze and predict the outcomes of various processes. While both enthalpy and entropy are thermodynamic properties, they have distinct definitions and applications.
In this article, we will explore the key differences between enthalpy and entropy and how they contribute to our understanding of the physical world.

Differences Between Enthalpy And Entropy
Enthalpy: The Measure of Heat Content
Enthalpy (H) is a thermodynamic property that represents the total heat content of a system at constant pressure. It includes the internal energy of a system and the energy required to maintain the constant pressure during various processes, such as chemical reactions. Enthalpy is often used to describe heat transfer in chemical reactions and phase changes.
Key characteristics of enthalpy:
- Heat Exchange: Enthalpy accounts for the heat exchanged between a system and its surroundings. In exothermic reactions, the enthalpy change is negative, indicating a release of heat energy. In endothermic reactions, the enthalpy change is positive, signifying an absorption of heat energy.
- Constant Pressure: Enthalpy is particularly useful at constant pressure because it directly relates to the heat exchanged in open systems, like those commonly encountered in chemistry laboratories.
- State Function: Enthalpy is a state function, meaning it depends solely on the initial and final states of a system, not on the path taken to reach those states.
Entropy: The Measure of Disorder
Entropy (S) is another thermodynamic property, but it deals with the degree of disorder or randomness within a system. It is a measure of the system’s tendency to move from a state of order to a state of disorder. Entropy is used to explain why natural processes tend to favor disorder over order.
Key characteristics of entropy:
- Disorder: Entropy is a measure of the randomness or disorder within a system. Higher entropy values indicate a more disordered state, while lower values indicate a more ordered state.
- Second Law of Thermodynamics: The second law states that in any energy transfer or transformation, the total entropy of an isolated system always increases over time. This principle underlines the irreversibility of natural processes.
- Microscopic Level: Entropy is a microscopic property that relates to the statistical distribution of particles and their energy in a system.

Comparison
Now, let’s summarize the key differences between enthalpy and entropy:
1. Definition:
- Enthalpy (H): Measures the total heat content of a system at constant pressure.
- Entropy (S): Measures the degree of disorder or randomness within a system.
2. Focus:
- Enthalpy focuses on heat exchange during chemical reactions and phase changes.
- Entropy focuses on the tendency of natural processes to move towards greater disorder.
3. Symbol:
- Enthalpy is represented by H.
- Entropy is represented by S.
4. Unit:
- Enthalpy is typically measured in joules (J) or kilojoules (kJ).
- Entropy is typically measured in joules per kelvin (J/K).
5. State Function:
- Enthalpy is a state function.
- Entropy is a state function.
Conclusion
Enthalpy and entropy are essential concepts in thermodynamics that provide valuable insights into the behavior of matter and energy in various systems. Enthalpy deals with heat content and exchange, particularly at constant pressure, while entropy quantifies the degree of disorder within a system and explains why natural processes tend towards greater randomness. Understanding the differences between these two properties is crucial for scientists and engineers working in fields where thermodynamics plays a significant role, as it enables them to make predictions and optimize processes more effectively.
Read More
- Periodic Table Class 11
- Molecular Weight Of Sodium Carbonate
- Difference Between Isothermal And Adiabatic Process
- Simple Harmonic Motion Examples
- Molecular Mass Of Calcium
Frequently Asked Questions (FAQs) Differences Between Enthalpy And Entropy
What is enthalpy, and how does it differ from entropy?
Enthalpy (H) is a thermodynamic property that represents the total heat content of a system at constant pressure, including the internal energy and energy required to maintain constant pressure during processes. Entropy (S), on the other hand, measures the degree of disorder or randomness within a system. Enthalpy deals with heat transfer, while entropy deals with the tendency of natural processes to become more disordered over time.
What are the main units of measurement for enthalpy and entropy?
Enthalpy is typically measured in joules (J) or kilojoules (kJ), whereas entropy is usually measured in joules per kelvin (J/K).
How do enthalpy and entropy relate to chemical reactions?
Enthalpy is crucial in describing the heat exchange that occurs during chemical reactions. Exothermic reactions release heat energy, leading to a decrease in enthalpy (ΔH < 0), while endothermic reactions absorb heat energy, resulting in an increase in enthalpy (ΔH > 0). Entropy, on the other hand, explains why chemical reactions tend to move towards states of greater disorder or randomness.
Are enthalpy and entropy related to each other?
Yes, enthalpy and entropy are related through the Gibbs free energy equation (ΔG = ΔH – TΔS), where ΔG is the change in Gibbs free energy, ΔH is the change in enthalpy, ΔS is the change in entropy, and T is the temperature in kelvin. This equation helps determine whether a process is spontaneous (ΔG < 0) or non-spontaneous (ΔG > 0).
Are enthalpy and entropy state functions?
Yes, both enthalpy and entropy are state functions. This means that their values depend only on the initial and final states of a system and are independent of the specific path taken to reach those states.
Periodic Table Class 11
Periodic Table Class 11: The periodic table is one of the foundational pillars of chemistry, and for Class 11 students embarking on their chemistry journey, it is a crucial topic to understand.
This comprehensive guide aims to provide Class 11 students with an in-depth exploration of the periodic table, covering its history, organization, periodic trends, and the significance of this iconic arrangement of elements.
Periodic Table Class 11
1. The Historical Journey of the Periodic Table
1.1 The Early Days of Chemistry:
- The study of elements and their properties dates back to ancient times, with philosophers like Aristotle contemplating the nature of substances.
- Alchemists in the Middle Ages contributed to the understanding of materials, although their methods were often mystical.
1.2 Mendeleev’s Periodic Table:
- Dmitri Mendeleev, a Russian chemist, is credited with creating the first periodic table in 1869.
- Mendeleev organized elements based on their atomic mass, noticing that elements with similar properties occurred at regular intervals.
- He left gaps for undiscovered elements and accurately predicted their properties.
1.3 The Modern Periodic Table:
- The modern periodic table is organized by increasing atomic number, which is the number of protons in an atom’s nucleus.
- Elements are arranged into periods (horizontal rows) and groups (vertical columns).
- The periodic table accommodates all known elements, including synthetic ones.
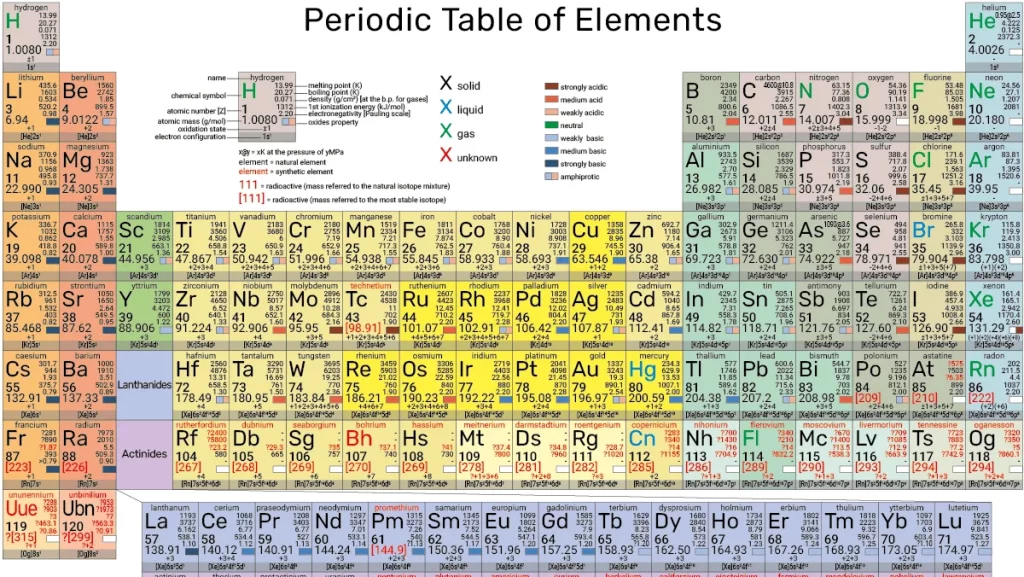 Periodic Table
Periodic Table
2.1 Periods and Groups:
- A period is a horizontal row on the periodic table, and there are seven periods in total.
- A group (or family) is a vertical column, and there are 18 groups.
- Elements in the same group share similar chemical properties due to their similar electron configurations.
2.2 Atomic Number and Atomic Mass:
- The atomic number of an element is its unique identifier, representing the number of protons in its nucleus.
- The atomic mass (or atomic weight) is the average mass of an element’s isotopes.
2.3 Electronic Configuration:
- The arrangement of electrons in an atom’s energy levels determines its chemical behavior.
- Understanding electronic configurations helps predict how elements will bond and react.
3. Elements and Their Properties
3.1 Metals, Non-Metals, and Metalloids:
- The periodic table can be divided into three main categories: metals, non-metals, and metalloids.
- Metals are typically shiny, conductive, and have high melting and boiling points.
- Non-metals are often dull, poor conductors, and have lower melting and boiling points.
- Metalloids exhibit properties of both metals and non-metals.
3.2 Representative and Transition Elements:
- Representative elements are found in the “s” and “p” blocks of the periodic table.
- Transition elements are located in the “d” and “f” blocks and are known for their variable oxidation states and colorful compounds.
4. Periodic Trends
4.1 Atomic Radius:
- Atomic radius is the size of an atom and decreases across a period and increases down a group.
- This trend is due to the effective nuclear charge and the number of electron energy levels.
4.2 Ionization Energy:
- Ionization energy is the energy required to remove an electron from an atom.
- It increases across a period and decreases down a group due to electron shielding and effective nuclear charge.
4.3 Electronegativity:
- Electronegativity is an element’s ability to attract electrons in a chemical bond.
- It increases across a period and decreases down a group.
4.4 Electron Affinity:
- Electron affinity is the energy change when an atom gains an electron.
- It generally increases across a period and decreases down a group.
5. The Significance of the Periodic Table
5.1 Predicting Element Properties:
- The periodic table allows scientists to predict the properties of elements, even those not yet discovered.
- It provides insights into an element’s reactivity, bonding behavior, and physical characteristics.
5.2 Understanding Chemical Bonding:
- The arrangement of elements in the periodic table explains how chemical bonds form.
- Elements in the same group often share similar bonding tendencies.
5.3 Analyzing Chemical Reactions:
- The periodic table helps chemists understand and predict the outcomes of chemical reactions.
- It guides reaction stoichiometry and the formation of products.
5.4 Applications in Real Life:
- The periodic table has practical applications in various fields, from materials science and medicine to environmental science and energy production.
- It is essential for designing new materials and understanding their properties.
6. Key Concepts and Practical Tips for Class 11 Students
As you study the periodic table in your Class 11 chemistry curriculum, here are some key concepts and practical tips to help you grasp this fundamental topic effectively:
6.1 Understand Periods and Groups:
- Pay close attention to the structure of the periodic table, with its rows (periods) and columns (groups).
- Recognize that elements in the same group have similar properties due to their electron configurations.
6.2 Atomic Number vs. Atomic Mass:
- Differentiate between the atomic number (Z) and atomic mass (A) of elements.
- The atomic number defines the element, while the atomic mass accounts for isotopes.
6.3 Electronic Configurations:
- Learn how to write electronic configurations for elements, as they play a crucial role in understanding reactivity and bonding.
6.4 Periodic Trends:
- Master the periodic trends of atomic radius, ionization energy, electronegativity, and electron affinity.
- Recognize how these trends change across periods and down groups.
6.5 Chemical Bonding:
- Understand how elements’ positions in the periodic table influence the type of chemical bonds they form.
- This knowledge is vital for predicting bond types and reactions.
6.6 Practice Problem-Solving:
- Solve a variety of problems related to the periodic table to reinforce your understanding.
- Work on exercises that involve predicting element behavior and calculating trends.
6.7 Real-World Applications:
- Explore the practical applications of the periodic table in various fields.
- Consider how elements are used in everyday life, technology, and industry.
7. Resources for Further Learning
To deepen your understanding of the periodic table, consider using additional resources:
- Textbooks: Consult your Class 11 chemistry textbook for in-depth explanations and practice problems related to the periodic table.
- Online Tutorials: Explore online chemistry tutorials and video lessons that provide visual explanations and demonstrations.
- Interactive Periodic Tables: Use interactive periodic tables available online or through chemistry apps. These tools allow you to explore element properties and trends interactively.
- Periodic Table Games: Engage in educational games and quizzes that make learning about the periodic table fun and interactive.
- Chemistry Forums: Join online chemistry forums or communities where you can ask questions, share knowledge, and learn from fellow students and experts.
- Laboratory Experiments: If possible, participate in chemistry laboratory experiments that involve elements from the periodic table. Hands-on experience can enhance your understanding.
8. Study Strategies for Success
To excel in your Class 11 chemistry studies, particularly when delving into the periodic table, consider the following study strategies:
8.1 Consistent Practice:
- Regularly review and practice the concepts related to the periodic table. Consistency is key to mastering this fundamental topic.
8.2 Flashcards:
- Create flashcards with element names, symbols, atomic numbers, and key properties. Use these flashcards for quick and effective revision.
8.3 Group Study:
- Collaborate with classmates for group study sessions. Discussing and teaching each other can reinforce your understanding.
8.4 Mnemonics:
- Use mnemonics or memory aids to remember the order of elements in a group or other important periodic table information.
8.5 Visual Aids:
- Utilize visual aids, such as color-coded periodic tables and diagrams, to help you grasp the organization and trends effectively.
8.6 Online Resources:
- Explore online resources, including educational websites, interactive periodic tables, and YouTube tutorials that offer alternative explanations and examples.
8.7 Regular Review:
- Periodically revisit previously learned concepts to ensure retention. This can be particularly useful for mastering periodic trends.
9. Realizing the Beauty of Chemistry
While the periodic table may seem like a static chart of elements, it holds the key to understanding the dynamic world of chemistry. As you progress through your Class 11 chemistry course, you’ll come to appreciate how this elegant arrangement of elements simplifies complex chemical phenomena.
Moreover, the periodic table transcends classroom boundaries; it’s a universal language understood by scientists worldwide. It’s a testament to human ingenuity and the power of collaboration among scientists over centuries.
Remember, the periodic table is not merely a chart—it’s a gateway to exploring the mysteries of matter, the wonders of chemical reactions, and the boundless possibilities of science. Embrace your journey into the world of chemistry, and let the periodic table be your guide.
Your curiosity and dedication will unlock a realm of knowledge that will not only enrich your academic pursuits but also deepen your appreciation for the beauty of the natural world. Happy studying!
10. Classroom and Beyond
As you progress through your Class 11 chemistry curriculum and explore the periodic table, keep in mind that chemistry extends far beyond the classroom. Here are some ways to engage with chemistry in your everyday life:
10.1 Observing the Elements:
- Look around you and identify elements in your environment. Consider the metals used in your electronics, the gases in the air you breathe, and the compounds in household items.
10.2 Chemistry in Nature:
- Explore the chemistry of the natural world. Learn about the chemical processes that occur in plants, animals, and geological formations.
10.3 Chemistry in Technology:
- Investigate how chemistry drives technological advancements. Discover how elements and compounds are used in cutting-edge innovations.
10.4 Chemistry in Medicine:
- Delve into the role of chemistry in healthcare. Learn about pharmaceuticals, medical diagnostics, and the chemistry behind disease prevention and treatment.
10.5 Environmental Chemistry:
- Explore the impact of human activities on the environment and the role of chemistry in addressing environmental challenges.
10.6 Chemistry in Art and Culture:
- Appreciate the connection between chemistry and art, from pigments used in paintings to the chemistry of cuisine.
12. Seek Guidance and Stay Curious
Remember that chemistry, including the pd table, can be a challenging subject at times, but it’s also incredibly rewarding. Don’t hesitate to seek guidance from your teachers, classmates, or online resources when you encounter difficulties. Chemistry is a subject that rewards curiosity and perseverance.
Stay curious and inquisitive. Ask questions, conduct experiments if possible, and explore chemistry-related topics that pique your interest. The pd table is just the beginning of a fascinating journey into the world of chemistry, where countless discoveries await.
As you continue your studies in Class 11 and beyond, keep in mind that chemistry is not confined to textbooks and classrooms. It’s a vibrant field that impacts every aspect of our lives. Whether you aspire to be a scientist, engineer, healthcare professional, or simply a curious learner, chemistry will provide you with valuable insights and a deeper understanding of the world around you.
Embrace the pd table as your roadmap, and let your curiosity drive your exploration of the captivating realm of chemistry. With dedication and a thirst for knowledge, you’ll find that chemistry opens doors to endless possibilities and a lifelong appreciation for the wonders of the natural world. Enjoy your journey into the world of chemistry!
The pd table is a foundational concept in chemistry that serves as a roadmap to the world of elements and their properties. As a Class 11 student, your journey through chemistry will be greatly enriched by a solid grasp of the pd table and its associated principles. Embrace the pd table as a tool that will empower you to unravel the mysteries of matter, and enjoy the adventure of discovery as you explore the fascinating world of chemistry.
Conclusion
The pd table is a foundational concept in chemistry that serves as a roadmap to the world of elements and their properties. As a Class 11 student, your journey through chemistry will be greatly enriched by a solid grasp of the pd table and its associated principles. Embrace the pd table as a tool that will empower you to unravel the mysteries of matter, and enjoy the adventure of discovery as you explore the fascinating world of chemistry.
Read More
- Molecular Weight Of Sodium Carbonate
- Difference Between Isothermal And Adiabatic Process
- Simple Harmonic Motion Examples
- Molecular Mass Of Calcium
- Molecular Weight of Chlorine
Frequently Asked Questions (FAQs) Periodic Table
1. What is the periodic table?
The pd table is a tabular arrangement of chemical elements, organized by their atomic number, which represents the number of protons in an atom’s nucleus. It groups elements with similar properties in columns called groups or families.
2. Who developed the first periodic table, and when was it created?
The first pd table was developed by Dmitri Mendeleev, a Russian chemist, in 1869. He organized elements based on their atomic masses and predicted the properties of undiscovered elements.
3. How are elements arranged in the periodic table?
Elements are arranged by increasing atomic number from left to right and top to bottom. They are grouped into periods (rows) and groups (columns) based on similar electronic configurations and properties.
4. What are periods and groups in the periodic table?
Periods are the horizontal rows in the pd table, numbered from 1 to 7. Groups, also known as families, are the vertical columns, numbered from 1 to 18.
5. What are representative elements and transition elements?
Representative elements, also called main group elements, are found in the “s” and “p” blocks of the pd table. Transition elements are located in the “d” and “f” blocks.
Molecular Weight Of Sodium Carbonate
Molecular Weight Of Sodium Carbonate: Sodium carbonate, commonly known as soda ash or washing soda, is a chemical compound with the molecular formula Na2CO3.
Understanding its molecular weight is fundamental in various fields, including chemistry, industry, and environmental science.
In this article, we delve into the concept of molecular weight and explore the significance of knowing the molecular weight of sodium carbonate.

Molecular Weight Of Sodium Carbonate
Definition of Molecular Weight
Molecular weight, also known as molar mass or molecular mass, is a measure of the mass of a molecule. It is defined as the sum of the atomic weights (masses) of all the atoms in a molecule. The unit of molecular weight is the atomic mass unit (amu) or unified atomic mass unit (u). For convenience, molecular weights are often expressed in grams per mole (g/mol).
Calculating the Molecular Weight of Sodium Carbonate
To calculate the molecular weight of sodium carbonate (Na2CO3), we need to sum the atomic weights of all the constituent atoms:
- Sodium (Na) has an atomic weight of approximately 22.99 amu.
- Carbon (C) has an atomic weight of roughly 12.01 amu.
- Oxygen (O) has an atomic weight of about 16.00 amu.
Now, let’s calculate the molecular weight:
Molecular Weight of Na2CO3 = (2 * Atomic Weight of Na) + Atomic Weight of C + (3 * Atomic Weight of O)
Molecular Weight of Na2CO3 = (2 * 22.99 amu) + 12.01 amu + (3 * 16.00 amu)
Mole Weight of Na2CO3 ≈ 45.98 amu + 12.01 amu + 48.00 amu
Mole Weight of Na2CO3 ≈ 105.99 amu (or g/mol)
So, the molecular weight of sodium carbonate is approximately 105.99 g/mol.
Significance of Mole Weight of Sodium Carbonate
Understanding the mole weight of sodium carbonate holds several practical implications:
- Chemical Formulation: The mole weight provides the exact mass of one mole of sodium carbonate molecules. This is essential for precise chemical formulations and reactions in laboratories and industries.
- Stoichiometry: In chemical reactions, the molecular weight plays a crucial role in stoichiometry, helping scientists and engineers determine the quantities of reactants and products.
- Dosage Calculation: In various applications, such as water treatment and detergents, the molecular weight is vital for calculating the appropriate dosage of sodium carbonate needed to achieve specific results.
- Environmental Impact: In environmental science, understanding the molecular weight aids in assessing the impact of sodium carbonate on ecosystems and water quality when it is used or released.
- Quality Control: Industries that produce sodium carbonate products rely on accurate molecular weight data to maintain quality control standards.
In conclusion, the mole weight of sodium carbonate, approximately 105.99 g/mol, is a crucial parameter in various scientific, industrial, and environmental contexts. It enables precise calculations, formulations, and assessments, ultimately contributing to the efficient and responsible use of this important chemical compound.
Read More
- Difference Between Isothermal And Adiabatic Process
- Simple Harmonic Motion Examples
- Molecular Mass Of Calcium
- Lumen Meaning In Biology
- Molar Mass of Chlorine
Frequently asked questions (FAQs) On Mole Weight Of Sodium Carbonate
1. What is the mole formula of sodium carbonate?
The mole formula of sodium carbonate is Na2CO3, indicating that each molecule contains two sodium (Na) atoms, one carbon (C) atom, and three oxygen (O) atoms.
2. What is the mole weight of sodium carbonate?
The mole weight of sodium carbonate (Na2CO3) is approximately 105.99 grams per mole (g/mol).
3. Why is it important to know the mole weight of sodium carbonate?
Knowing the mole weight is essential for accurate chemical calculations, including determining reactant and product quantities in chemical reactions, dosage calculations in various applications, and quality control in industries.
4. How is the mole weight of sodium carbonate calculated?
The mole weight of sodium carbonate is calculated by summing the atomic weights of all the constituent atoms in its chemical formula: 2 * Atomic Weight of Sodium (Na) + Atomic Weight of Carbon (C) + 3 * Atomic Weight of Oxygen (O).
5. What are some common uses of sodium carbonate in everyday life?
Sodium carbonate has a wide range of applications, including as a cleaning agent (in laundry detergents and household cleaners), in water treatment, as a food additive, and in various industrial processes.
Difference Between Isothermal And Adiabatic Process
Difference Between Isothermal And Adiabatic Process: Certainly! Here’s a concise summary of the difference between isothermal and adiabatic processes:

Difference Between Isothermal And Adiabatic Process
Isothermal Process:
- Temperature: Temperature remains constant throughout the process.
- Heat Transfer: Heat is exchanged with the surroundings to maintain temperature.
- Efficiency: Isothermal processes are more efficient due to constant temperature.
- Applications: Used in refrigeration and precise chemical reactions.
- Mathematical Representation: Follows the equation PV = constant for ideal gases.
Adiabatic Process:
- Temperature: Temperature can change significantly during the process.
- Heat Transfer: No heat exchange with the surroundings; the process is thermally isolated.
- Efficiency: Adiabatic processes are generally less efficient due to temperature changes.
- Applications: Common in gas compression and expansion, like internal combustion engines.
- Mathematical Representation: Follows the equation PV^γ = constant, where γ is the ratio of specific heats.
In summary, the key distinction lies in temperature behavior and heat transfer. Isothermal processes maintain constant temperature with heat exchange, while adiabatic processes have temperature changes without heat exchange.
Read More
- Simple Harmonic Motion Examples
- Molecular Mass Of Calcium
- Molecular Weight of Chlorine
- Molecular Weight Of Benzene
- Lumen Meaning In Biology
Frequently Asked Questions (FAQs) Difference Between Isothermal And Adiabatic Process
1. What is an isothermal process?
An isothermal process is one in which the temperature of a system remains constant throughout the process. Heat is continuously added or removed to maintain this constant temperature.
2. What characterizes an adiabatic process?
An adiabatic process is defined by the absence of heat exchange with the surroundings. It occurs in thermally isolated systems where no heat is added to or removed from the system.
3. How does temperature change in an isothermal process?
In an isothermal process, the temperature remains constant, so there is no significant change in temperature.
4. Can you explain how temperature changes in an adiabatic process?
In an adiabatic process, temperature can change significantly. When work is done on or by the system, it can lead to temperature increases (compression) or decreases (expansion) without any heat exchange with the surroundings.
5. Which process is more efficient, isothermal or adiabatic?
Isothermal processes are generally more efficient because they maintain a constant temperature, allowing for maximum energy transfer. Adiabatic processes can be less efficient due to temperature changes.
Simple Harmonic Motion Examples
Simple Harmonic Motion Examples: Simple Harmonic Motion (SHM) is a fundamental concept in physics that describes the repetitive, oscillatory motion of an object around an equilibrium position.
It is a common phenomenon in various natural and artificial systems. In this article, we will explore some examples of simple harmonic motion to better understand this important concept and its real-world applications.

Simple Harmonic Motion Examples
1. Pendulum Motion
One of the most classic examples of simple harmonic motion is the motion of a pendulum. A pendulum consists of a mass (known as the bob) attached to a string or rod of fixed length. When the bob is displaced from its equilibrium position and released, it swings back and forth in a regular, repeating pattern. The motion of the pendulum is SHM as long as the angle of displacement is small.
2. Mass-Spring Systems
Another common example of SHM is a mass-spring system. This system consists of a mass (M) attached to a spring with a spring constant (k). When the mass is displaced from its equilibrium position and then released, it oscillates back and forth around the equilibrium point. The motion of the mass in a mass-spring system is SHM, and the frequency of oscillation depends on the mass and the spring constant.
3. Vibrating Guitar String
When a guitar string is plucked or strummed, it vibrates back and forth to produce sound. The motion of the vibrating guitar string is a classic example of simple harmonic motion. The pitch of the sound is determined by the frequency of the string’s vibration, which can be adjusted by changing the tension in the string or altering its length.
4. Simple Pendulum Clocks
Historically, many clocks used a simple pendulum as the timekeeping mechanism. The oscillation of the pendulum created a regular and accurate timing reference. Although modern clocks often use electronic oscillators, the principle of simple harmonic motion in pendulums remains an important part of the history of timekeeping.
5. Vibrations in Car Suspensions
Car suspensions use springs and shock absorbers to provide a smooth ride by absorbing road bumps and vibrations. When a car encounters a rough road, the springs in the suspension system undergo simple harmonic motion as they compress and expand to absorb the shock. This motion helps improve ride comfort.
6. Molecular Vibrations
In the realm of molecular chemistry, simple harmonic motion can be observed in molecular vibrations. Molecules consist of atoms connected by chemical bonds, and these bonds act like springs. When a molecule absorbs energy, it undergoes vibrational motion, which can be described using SHM principles. Molecular vibrations play a crucial role in various chemical processes, including infrared spectroscopy.
7. Electrical Oscillations in Circuits
Electrical circuits containing components like capacitors and inductors can exhibit simple harmonic motion when an electrical charge or current oscillates back and forth. Oscillations in electrical circuits are fundamental in various electronic devices, such as radios, oscillators, and antennas.
Conclusion
Simple Harmonic Motion (SHM) is a pervasive concept in the physical world, and these examples illustrate its presence in various systems and phenomena. Understanding SHM is crucial in physics and engineering. It allows scientists and engineers to analyze and design systems that involve oscillatory behavior. Whether it’s the swinging of a pendulum, the vibrations in a guitar string, or the oscillations in electronic circuits, SHM provides valuable insights into the dynamics of countless systems in our everyday lives.
Read More
- Molecular Mass Of Calcium
- Molecular Weight of Chlorine
- Molecular Weight Of Benzene
- Lumen Meaning In Biology
- Molecular Weight Of Sucrose
Frequently Asked Questions (FAQs) Simple Harmonic Motion Examples
What is Simple Harmonic Motion (SHM)?
Simple Harmonic Motion is a type of periodic motion in which an object oscillates back and forth around an equilibrium position. The motion is characterized by a sinusoidal (sine or cosine) pattern. It occurs when a restoring force is proportional to the displacement from the equilibrium.
Why is SHM important in physics and engineering?
SHM is essential because it provides a fundamental understanding of oscillatory behavior. which is prevalent in various natural and artificial systems. It is a key concept in physics and engineering, allowing scientists and engineers to analyze and design systems involving periodic motion.
What are some everyday examples of SHM?
Everyday examples of SHM include pendulum motion (e.g., a swinging pendulum or a grandfather clock), mass-spring systems, vibrations in guitar strings, car suspensions, and electrical oscillations in circuits.
How does a pendulum exhibit SHM?
A pendulum exhibits SHM when a mass (bob) is suspended from a fixed point and allowed to swing freely. The restoring force is provided by gravity. the motion follows a sinusoidal pattern as the pendulum oscillates back and forth.
What determines the frequency of oscillation in SHM?
The frequency of oscillation in SHM is determined by factors such as the mass of the object, the spring constant (in mass-spring systems), or the length of the pendulum. It is a measure of how many cycles (complete oscillations) occur per unit of time.
Molecular Mass Of Calcium
Mol Mass Of Calcium: Calcium, with its symbol Ca and atomic number 20, is a fundamental element in the periodic table. It is renowned for its crucial role in biological processes, as well as its various applications in industry, agriculture, and everyday life.
Understanding the mol mass of calcium is essential for comprehending its behavior and significance in the world of chemistry. In this article, we delve into the concept of molecular mass, its calculation for calcium, and the implications of this fundamental property.

Mol Mass Of Calcium
The Nature of Calcium
Calcium is a metallic element found in group 2 (formerly group IIA) of the periodic table, along with other alkaline earth metals. It is a silvery-white, soft metal that reacts vigorously with water and oxygen, forming compounds like calcium oxide (CaO) and calcium hydroxide (Ca(OH)2). In its natural state, calcium is often found in the form of calcium carbonate (CaCO3), which makes up materials like limestone, chalk, and shells.
Importance of Molecular Mass
Mol mass, also known as molar mass or molecular weight, is the mass of a molecule or compound expressed in atomic mass units (amu) or grams per mole (g/mol). It is a fundamental concept in chemistry and has several key implications:
- Stoichiometry: Molecular mass is crucial for calculating the molar ratio of reactants and products in chemical reactions, aiding in the determination of reaction yields and limiting reagents.
- Synthesis and Formulation: Chemists use molecular mass to precisely measure and combine reactants when synthesizing compounds or formulating chemical solutions.
- Analytical Chemistry: Molecular mass is essential in the identification and quantification of compounds using techniques like mass spectrometry.
- Physical Properties: Molecular mass influences a compound’s physical properties, including melting and boiling points, density, and solubility.
Calculating the Molecular Mass of Calcium
To calculate the molecular mass of calcium (Ca), you consider the atomic mass of a single calcium atom. The atomic mass of calcium is approximately 40.08 atomic mass units (amu).
Now, let’s calculate the molecular mass of calcium (Ca):
Molecular Mass of Calcium (Ca) = Atomic Mass of Calcium Molecular Mass of Calcium (Ca) = 40.08 amu
The molecular mass of calcium is approximately 40.08 amu.
Implications of Calcium’s Molecular Mass
The molecular mass of calcium, approximately 40.08 amu, has several implications in the field of chemistry and various applications:
- Quantitative Analysis: In analytical chemistry, calcium’s molecular mass is essential for accurately determining its concentration in samples, such as in water quality testing.
- Chemical Reactions: Calcium’s molecular mass plays a key role in stoichiometric calculations for chemical reactions involving calcium compounds, ensuring precise measurements and predictions.
- Biological Role: In biology, calcium ions (Ca2+) are vital for numerous physiological processes, including muscle contraction, nerve signaling, and blood clotting.
- Industrial Applications: Calcium and its compounds are used in diverse industrial applications, including the production of steel, cement, and fertilizers, as well as in the food and pharmaceutical industries.
Conclusion
The molecular mass of calcium, approximately 40.08 amu, is a fundamental property that plays a pivotal role in chemistry and various scientific disciplines. It is indispensable for stoichiometric calculations, accurate measurements, and an in-depth understanding of calcium’s chemical and physical properties. As a versatile element with significant applications in industry, agriculture, and biology, calcium’s molecular mass continues to be a critical parameter for researchers and professionals working with this essential element.
Read More
- Molecular Weight Of Sucrose
- Molecular Mass Of Carbon
- Molar Mass Of Phosphorus
- Molar Mass of Chlorine
- Electrical Energy And Power
Frequently Asked Questions (FAQs) Molecular Mass Of Calcium
Q1: What is molecular mass or molecular weight?
A1: Molecular mass, also known as molar mass or molecular weight, is the mass of a molecule or compound expressed in atomic mass units (amu) or grams per mole (g/mol). It is a fundamental property used in chemistry to quantify the mass of chemical substances.
Q2: What is the chemical symbol for calcium?
A2: The chemical symbol for calcium is Ca.
Q3: Is calcium a metal or non-metal?
A3: Calcium is a metal. It belongs to the group of metals known as alkaline earth metals.
Q4: Why is calcium important in biology?
A4: Calcium plays a crucial role in biology as a key intracellular signaling ion. It is involved in various physiological processes, including muscle contraction, nerve transmission, blood clotting, and cell communication.
Q5: How is the molecular mass of calcium calculated?
A5: To calculate the molecular mass of calcium (Ca), you consider the atomic mass of a single calcium atom, which is approximately 40.08 atomic mass units (amu).

