Category: Class 12
Ethylene Glycol Molar Mass
Ethylene Glycol Molar Mass: Ethylene glycol is a common organic compound with a wide range of applications, from its use as a coolant in automobile radiators to its role as a key ingredient in the production of antifreeze.
Understanding the molar mass of ethylene glycol is essential in various chemical processes and industries. In this article, we will explore what molar mass is, how it relates to ethylene glycol, and its significance in practical applications.
Ethylene Glycol Molar Mass
What Is Molar Mass?
Molar mass, also known as molecular weight, is a fundamental concept in chemistry. It refers to the mass of one mole of a substance, expressed in grams per mole (g/mol). A mole is a unit that represents a specific number of atoms, molecules, or ions, approximately 6.022 x 10^23 entities, known as Avogadro’s number. The molar mass of a substance is numerically equal to its atomic or molecular weight.
Ethylene Glycol Composition
Ethylene glycol’s formula, C2H6O2, signifies two carbon (C) atoms, six hydrogen (H) atoms, and two oxygen (O) atoms per molecule. To determine ethylene glycol’s molar mass (C2H6O2), add the atomic masses of each element in its chemical formula together.
The atomic mass of carbon (C) is around 12.01 g/mol, hydrogen (H) is roughly 1.01 g/mol, and oxygen (O) is about 16.00 g/mol. Calculating the mol mass of ethylene glycol is as follows:
To calculate the molar mass of C2H6O2, sum the products of each element’s atomic mass by its respective count.
Molar Mass of C2H6O2 = (2 x 12.01 g/mol) + (6 x 1.01 g/mol) + (2 x 16.00 g/mol) ≈ 62.08 g/mol
Therefore, the mol mass of ethylene glycol is approximately 62.08 grams per mole (g/mol).
Significance of Ethylene Glycol Molar Mass
- Antifreeze Formulation: Ethylene glycol is a primary component of antifreeze, which prevents freezing and overheating in vehicle cooling systems. Accurate molar mass knowledge is essential for formulating effective and safe antifreeze products.
- Chemical Reactions: Mol mass plays a crucial role in chemical reactions involving ethylene glycol, such as its reaction with acids to produce esters or its use as a reactant in the synthesis of various organic compounds.
- Industrial Processes: Ethylene glycol is used in various industrial applications, including as a heat transfer fluid, deicing agent, and in the production of polyester fibers. Understanding its molar mass is vital for precise manufacturing processes.
- Environmental Considerations: Ethylene glycol’s molar mass is critical in evaluating its environmental impact, especially upon release, facilitating the assessment of ecological consequences.
Conclusion
The mol mass of ethylene glycol is a fundamental concept in chemistry, significant in various practical applications, including automotive maintenance and industrial processes. Understanding and applying this concept enables scientists and professionals to responsibly use ethylene glycol in various industries, promoting safety, efficiency, and environmental responsibility.
Read More
- Organisms And Population Class 12
- Difference Between Capacitor And Inductor
- Van De Graaff Generator
- Dielectric Polarization In Polar And Nonpolar Material And Dielectric Constant
- Chemistry In Everyday Life
Frequently Asked Questions (FAQs) On Ethylene Glycol Molar Mass
What is molar mass, and why is it important in chemistry?
Mol mass, often called molecular weight, represents the mass of one mole of a substance in grams per mole (g/mol). This concept is foundational in chemistry, assisting in stoichiometry—determining substance quantities in chemical reactions and various practical applications.
What is the chemical formula of ethylene glycol?
Ethylene glycol’s formula, C2H6O2, reveals two carbon (C), six hydrogen (H), and two oxygen (O) atoms per molecule.
How is the molar mass of ethylene glycol calculated?
To calculate the mol mass of ethylene glycol (C2H6O2), add the atomic masses of carbon (C), hydrogen (H), and oxygen (O) in its chemical formula: To find the molar mass of C2H6O2, add twice the atomic mass of carbon, six times the atomic mass of hydrogen, and twice the atomic mass of oxygen.
What is the molar mass of ethylene glycol?
The mol mass of ethylene glycol (C2H6O2) is approximately 62.08 grams per mole (g/mol).
Why is the molar mass of ethylene glycol important in antifreeze formulation?
Ethylene glycol is a key component of antifreeze used in vehicle cooling systems. Its molar mass is essential in formulating antifreeze to ensure the proper performance and safety of the product.
Organisms And Population Class 12
Organisms And Population Class 12: The natural world is a dynamic tapestry of life where countless organisms coexist, interact, and evolve. At the heart of this biological complexity lies the fundamental concepts of organisms and populations.
Organisms are the individual living beings, while populations represent groups of organisms of the same species living in a particular area. In this article, we delve into the intricate relationships between organisms and populations and their crucial roles in shaping ecosystems.
Organisms And Population Class 12
Organisms: The Building Blocks of Life
Living entities form the fundamental components of life, representing a broad spectrum of species, each intricately tailored to its specific habitat. These life forms showcase a wide range of dimensions, configurations, conduct, and physiological characteristics, encompassing everything from minute bacteria to towering trees and from nimble predators to placid herbivores. This biological diversity spans the entirety of the natural world.
Key characteristics of organisms:
- Individuality: Each organism is a distinct entity with its own genetic makeup, physical structure, and unique characteristics. It is a product of evolution, shaped by natural selection and environmental factors.
- Reproduction: Organisms reproduce, ensuring the continuation of their species. Reproductive strategies vary, including asexual reproduction in bacteria and sexual reproduction in many animals and plants.
- Interactions: Organisms interact with each other and their environment. They compete for resources such as food, water, and shelter, and they form complex symbiotic relationships, like mutualism, parasitism, and commensalism.
- Adaptation: Organisms evolve over time through a process of adaptation to better suit their surroundings. This adaptation can lead to the development of new traits and behaviors that enhance an organism’s chances of survival and reproduction.
Populations: The Collective Expression of Life
Populations are groups of organisms of the same species that inhabit a specific geographical area. They represent the collective dynamics of individuals within a particular ecosystem. Understanding populations is vital in ecology, as they are the cornerstone of biodiversity and ecosystem health.
Key characteristics of populations:
- Size: Population size refers to the number of individuals of a species in a given area at a particular time. This size can fluctuate due to births, deaths, immigration, and emigration.
- Distribution: The spatial arrangement of individuals within a population is known as population distribution. It can be clumped, random, or uniform, depending on various ecological factors.
- Growth: Population growth rate is influenced by birth rates and death rates. A population with more births than deaths experiences growth, while a population with more deaths than births declines.
- Interactions: Interactions between individuals within a population can include competition for resources, mating, social structures, and cooperation.
The Interplay Between Organisms and Populations
The relationship between organisms and populations is intricate and mutually influential. Organisms are the building blocks of populations, and their behaviors, adaptations, and interactions directly impact population dynamics. Conversely, population-level factors such as resource availability, predation, and disease prevalence shape the characteristics and survival strategies of individual organisms.
Understanding these interactions is crucial for various reasons:
- Conservation: Studying populations helps in the conservation of endangered species by identifying critical habitats and population trends.
- Ecosystem Management: Understanding population dynamics aids in managing ecosystems sustainably by ensuring the health of all constituent species.
- Scientific Research: Research into the relationship between organisms and populations provides insights into evolution, genetics, and ecology, contributing to scientific knowledge.
- Human Impact: Human activities, such as deforestation, pollution, and overfishing, can disrupt the delicate balance between organisms and populations, underscoring the importance of responsible environmental stewardship.
Challenges and Implications
While the relationship between organisms and populations is fascinating, it also presents challenges and implications that extend beyond the realm of biology and ecology.
- Climate Change: Rapid changes in climate can disrupt the delicate balance of ecosystems, affecting both individual organisms and populations. Species may need to adapt quickly to survive, and those unable to do so may face extinction.
- Invasive Species: The introduction of invasive species can have a devastating impact on native populations. Organisms that have not evolved alongside the new species often struggle to compete for resources or defend against predators, leading to population declines.
- Biodiversity Loss: The loss of biodiversity, driven by factors like habitat destruction and pollution, has profound implications for both organisms and populations. Diminished genetic diversity can weaken populations’ ability to adapt to changing environments.
- Human Population Growth: The exponential growth of the human population has led to increased resource consumption and habitat destruction, affecting countless species. Human activities also introduce pollutants and invasive species into ecosystems.
- Conservation Efforts: Understanding the dynamics between organisms and populations is pivotal for successful conservation efforts. Conservationists need to consider the needs of both individual organisms and the populations to effectively protect threatened species.
- Economic Impact: The health of populations directly impacts various human industries, such as agriculture, fisheries, and forestry. Overexploitation of populations can lead to economic challenges.
The Future of Organisms and Populations
As we face global challenges like climate change, habitat loss, and the decline of biodiversity, our understanding of the intricate relationship between organisms and populations becomes even more crucial. Science, technology, and conservation efforts play pivotal roles in mitigating the negative impacts on ecosystems and preserving the planet’s natural balance.
Research into genetic diversity, adaptation, and the interplay between species within populations can guide efforts to protect vulnerable ecosystems and species. Sustainable practices in agriculture, forestry, and fisheries aim to balance human needs with those of the natural world, ensuring the health of both organisms and populations.
Ultimately, our ability to address the challenges facing organisms and populations hinges on a deep appreciation for the complexity and interconnectedness of life on Earth. By recognizing that our actions have far-reaching consequences, we can work toward a future where organisms and populations thrive alongside humanity, creating a more harmonious and sustainable planet for all.
Conclusion
Organisms and populations are the cornerstones of life on Earth. They represent the intricate web of interactions and dependencies that sustain ecosystems. By studying and understanding these fundamental concepts, scientists, ecologists, and conservationists can work to protect and preserve the rich biodiversity that makes our planet a remarkable and thriving place. Recognizing the interconnectedness of organisms and populations is essential for the continued harmony of the natural world.
Read More
- Difference Between Capacitor And Inductor
- Van De Graaff Generator
- Dielectric Polarization In Polar And Nonpolar Material And Dielectric Constant
- Chemistry In Everyday Life
- Difference Between Diffraction And Interference
Frequently Asked Questions (FAQs) Organisms And Population
What is the difference between an organism and a population?
An organism is an individual living being, while a population is a group of organisms of the same species living in a specific area. An organism is a single unit of life, whereas a population represents a collection of individuals of the same species.
Why is it important to study populations in ecology?
Studying populations is essential in ecology because populations are the basic units that drive ecological processes. Understanding population dynamics helps ecologists assess biodiversity, make conservation decisions, and predict the impacts of environmental changes.
What factors influence the size of a population?
The size of a population is influenced by several factors, including birth rates, death rates, immigration (individuals moving into the population), and emigration (individuals moving out of the population).
How do populations interact with each other in an ecosystem?
Populations interact within ecosystems through various ecological relationships, such as competition (when two or more populations compete for the same resources), predation (when one population feeds on another), mutualism (when populations benefit each other), parasitism (when one population benefits at the expense of another), and commensalism (when one population benefits without affecting the other).
What is population distribution, and why does it matter?
Population distribution refers to the spatial arrangement of individuals within a population. It can be clumped, random, or uniform. The distribution pattern can provide insights into resource availability, social behavior, and environmental factors influencing the population.
Difference Between Capacitor And Inductor
Difference Between Capacitor And Inductor: In the realm of electronics and electrical engineering, two fundamental passive components stand out: capacitors and inductors. These components play pivotal roles in shaping electrical circuits, but they do so in distinct ways.
Understanding the differences between capacitors and inductors is essential for designing and analyzing electronic circuits. In this article, we will explore the key disparities between these two components, their properties, and their applications.

Difference Between Capacitor And Inductor
Capacitors:
1. What Is a Capacitor?
A capacitor is an electronic component that stores electrical energy in the form of an electric field. It consists of two conductive plates separated by an insulating material, known as a dielectric. When a voltage is applied across the plates, electric charge accumulates on them, creating an electric field between the plates. This stored energy can be quickly released when needed.
2. Key Properties of Capacitors:
- Capacity (Capacitance): Capacitance is the measure of a capacitor’s ability to store electrical charge. It is measured in farads (F).
- Voltage Rating: Capacitors have a maximum voltage they can withstand before breaking down.
- Charge and Discharge: Capacitors can store and release energy quickly, making them suitable for applications such as smoothing power supplies and timing circuits.
- Frequency Dependency: Capacitors’ impedance (resistance to the flow of alternating current) decreases as the frequency of the signal increases.
3. Applications of Capacitors:
- Filtering: Capacitors are used to filter out noise and stabilize voltage levels in power supplies.
- Timing: They play a crucial role in timing circuits, oscillators, and signal delay applications.
- Energy Storage: Capacitors store energy in devices like camera flashes and defibrillators.

Inductors:
1. What Is an Inductor?
An inductor is a passive electrical component that stores energy in the form of a magnetic field when current flows through it. It typically consists of a coiled wire or a wound conductor. The magnetic field generated by the current induces a voltage, opposing the change in current flow. This property is described by Faraday’s law of electromagnetic induction.
2. Key Properties of Inductors:
- Inductance: Inductance is the measure of an inductor’s ability to store energy in a magnetic field. It is measured in henrys (H).
- Resistance (DC): Inductors resist changes in current flow and act as a short circuit for direct current (DC).
- Reactance (AC): Inductors have reactance, which is an opposition to alternating current (AC) flow, and it increases with frequency.
- Energy Storage: Inductors store energy in magnetic fields and can release it gradually.
3. Applications of Inductors:
- Filtering: Inductors are used in power supplies and filters to reduce high-frequency noise.
- Signal Processing: They can be found in analog circuits, such as amplifiers and analog filters.
- Energy Storage: Inductors are used in transformers for voltage conversion and inductors store energy in some power supplies.
Key Differences:
- Storage Mechanism: Capacitors store energy in an electric field, whereas inductors store energy in a magnetic field.
- Impedance: Capacitors have decreasing impedance with increasing frequency (opposite to inductors).
- Reactance: Capacitors block DC and allow AC, while inductors block AC and allow DC.
- Applications: Capacitors are commonly used for energy storage, filtering, and timing. Inductors are often used in signal processing, energy storage, and voltage conversion.
- Physical Structure: Capacitors have two conductive plates separated by a dielectric. Inductors consist of wound coils of wire.

In summary, capacitors and inductors are crucial components in electronics, each with distinct properties and applications. Understanding their differences is essential for designing and troubleshooting electronic circuits effectively. Whether you’re building a power supply, designing an amplifier, or working on a complex electronic system, capacitors and inductors will play vital roles in shaping your circuits.
Read More
- Van De Graaff Generator
- Dielectric Polarization In Polar And Nonpolar Material And Dielectric Constant
- Chemistry In Everyday Life
- Difference Between Diffraction And Interference
- Alcohols Phenols And Ethers Class 12
- Alcohols Phenols And Ethers
Frequently Asked Questions (FAQs) Difference Between Capacitor And Inductor
1. What is the fundamental difference between capacitors and inductors?
The fundamental difference lies in how they store energy. Capacitors store energy in an electric field, while inductors store energy in a magnetic field.
2. How is capacitance different from inductance?
Capacitance (measured in farads) is a measure of a capacitor’s ability to store electrical charge, while inductance (measured in henrys) is a measure of an inductor’s ability to store energy in a magnetic field.
3. Do capacitors and inductors behave differently with direct current (DC) and alternating current (AC)?
Yes, capacitors and inductors have opposite behaviors with DC and AC. Capacitors block DC and allow AC to pass, while inductors block AC and allow DC to pass.
4. How do capacitors and inductors affect the frequency of an AC signal?
Capacitors exhibit a decrease in impedance (resistance to the flow of AC) as the frequency increases, whereas inductors experience an increase in impedance with rising frequency.
5. What are some common applications of capacitors?
Capacitors find application in energy storage, noise filtering within power supplies, and precision timing circuits. They are also commonly incorporated into devices such as camera flashes.
Van De Graaff Generator 12th physics
Van De Graaff Generator: The Van de Graaff generator is a remarkable device in the realm of physics and electrical engineering. It stands as a testament to the ingenuity of scientists and inventors who have pushed the boundaries of our understanding of electricity and electrostatics. Named after its creator, Robert J. Van de Graaff, this machine is a captivating instrument that generates high voltages and produces awe-inspiring electrical sparks.

Van De Graaff Generator 12th physics
1. Introduction
1.1 What is a Van de Graaff Generator?
The Van de Graaff generator is a captivating electrostatic contraption engineered for the production of high voltages. It is purpose-built to illustrate and explore the intricacies of electrostatic principles and frequently serves as an educational instrument in physics laboratories. Bearing the name of its creator, Robert J. Van de Graaff, this apparatus possesses the remarkable capability to generate mesmerizing electrical sparks and stands as a vital instrument across an array of disciplines, encompassing nuclear physics and particle acceleration.
1.2 How Does It Work?
The operation of a Van de Graaff generator is based on the principles of static electricity. It involves the continuous transfer of electric charge, typically through a moving belt, to a large hollow metal sphere. This process accumulates electric potential on the sphere, creating a high voltage difference between the sphere and the ground. As a result, the generator can produce impressive electrical sparks.
2. History of the Van de Graaff Generator
2.1 The Inventor – Robert J. Van de Graaff
The Van de Graaff gen was invented by Robert J. Van de Graaff in the early 1930s. Van de Graaff, an American physicist, developed this device while conducting research on nuclear physics at Princeton University. His goal was to create a machine capable of producing high-energy particles for use in nuclear experiments.
2.2 Development and Advancements
Over the years, Van de Graaff generators have seen significant improvements and adaptations. These generators have been upsized to accommodate various applications, such as particle accelerators and X-ray production machinery. In contemporary times, advanced iterations of the generator find extensive use in research institutions, universities, and laboratories around the globe.
3. The Components of a Van de Graaff Generator
3.1 The Generator’s Structure
A typical Van de Graaff gen consists of a metal column that supports various components. At the top of the column, there is a large metal sphere, which is one of the key elements of the generator.
3.2 The Motor
The motor, usually located at the base, drives the generator. It powers the continuous movement of the belt, which plays a critical role in charge accumulation.
3.3 The Belt
The belt in a Van de Graaff generator is typically made of an insulating material, such as rubber or a synthetic polymer. It is looped around two pulleys – one at the base and one at the top – and continuously moves in a single direction.
3.4 The Comb
Near the bottom of the generator, a small comb with sharp points is positioned close to the belt. This comb collects charges from the belt as it moves.
3.5 The Terminal
The terminal, connected to the large sphere at the top, is where the accumulated charge is stored. It can be used to discharge the high voltage or to produce electrical sparks.
4. Operation of a Van de Graaff Generator
4.1 Charging Process
The operation of a Van de Graaff gen begins with the motor turning the lower pulley, which drives the insulating belt. As the belt moves upwards, it passes near the comb at the bottom. The comb’s sharp points ionize the air and transfer electrons onto the moving belt, making it negatively charged.
4.2 The Role of Insulation
The insulating belt prevents charge from escaping and allows it to accumulate on the large sphere. Since like charges repel each other, the accumulated negative charge on the sphere repels electrons from the ground (or any nearby object), creating a strong electric field.
4.3 Maximum Voltage and Spark Length
The maximum voltage a Van de Graaff generator can produce depends on various factors, including the size of the sphere, the speed of the belt, and the surrounding conditions. When the electric field strength becomes high enough, it can ionize the surrounding air, allowing sparks to jump from the sphere to nearby objects, creating a visually stunning display of electrical discharge.
5. Applications of Van de Graaff Generators
5.1 Particle Acceleration
Van de Graaff generators find application in particle accelerators where they create and propel charged particles like protons and electrons to high energy levels. These accelerated particles play pivotal roles in nuclear physics experiments and medical applications, including cancer therapy.
5.2 Nuclear Physics Research
In the field of nuclear physics, Van de Graaff generators assume a crucial role in the examination of atomic nuclei and their characteristics. They are instrumental in producing particle beams that are directed towards target nuclei, allowing scientists to observe the resulting reactions and gather valuable data.
5.3 X-ray Production
Certain Van de Graaff generators are customized for X-ray production, a technology with diverse applications encompassing medical imaging and non-destructive testing in various industrial sectors.
5.4 Educational Demonstrations
Van de Graaff generators are frequently employed in educational environments to instruct students about the principles of electrostatics and the characteristics of electric charges. They provide captivating demonstrations of electrical phenomena.
6. Challenges and Limitations
6.1 Maintenance
Maintaining a Van de Graaff generator can be challenging, as the insulating belt may degrade over time, and mechanical components require periodic upkeep.
6.2 Safety Concerns
Due to the high voltages they can produce, Van de Graaff generators pose safety risks. Operators must take precautions to avoid electric shocks and sparks. Additionally, the discharge of high voltages can damage electronic equipment.
Conclusion
The Van de Graaff gen, an invention born out of curiosity and scientific exploration, has found its place in various fields, from fundamental physics research to medical applications. With its ability to generate high voltages and produce striking electrical displays, it continues to captivate and educate students and researchers alike. While it comes with maintenance challenges and safety considerations, its contributions to the advancement of science and technology are undeniable. Robert J. Van de Graaff’s creation continues to be a source of inspiration and innovation in the world of physics and beyond.
Read More
- Dielectric Polarization In Polar And Nonpolar Material And Dielectric Constant
- Chemistry In Everyday Life
- Difference Between Diffraction And Interference
- Alcohols Phenols And Ethers Class 12
- Alcohols Phenols And Ethers
Frequently Asked Questions (FAQs) Van De Graaff Generator
1. What is a Van de Graaff Generator?
A Van de Graaff generator is an electrostatic apparatus created with the purpose of generating high voltages. Its functioning is based on the principles of static electricity, and it is commonly employed for educational and experimental purposes in physics laboratories.
2. Who Invented the Van de Graaff Generator?
The Van de Graaff generator was the brainchild of American physicist Robert J. Van de Graaff, conceived in the early 1930s during his research pursuits in the realm of nuclear physics at Princeton University.
3. How Does a Van de Graaff Generator Work?
A Van de Graaff gen operates by continuously transferring electric charge, usually through a moving insulating belt, to a large hollow metal sphere. This accumulation of electric potential on the sphere results in a high voltage difference between the sphere and the ground, which can produce electrical sparks.
4. What Are the Key Components of a Van de Graaff Generator?
The main components of a Van de Graaff gen include the metal sphere, the motor, the insulating belt, the comb, and the terminal. The motor drives the belt, which transfers charge to the sphere. The comb helps collect charges, and the terminal stores the accumulated charge.
5. What Are Some Applications of Van de Graaff Generators?
Van de Graaff generators find applications across a wide spectrum of fields, including particle acceleration, nuclear physics research, X-ray production, and educational demonstrations. Their unique capability to generate high-energy particles contributes to experiments and medical treatments.
Dielectric Polarization In Polar And Nonpolar Material And Dielectric Constant
Dielectric Polarization In Polar And Nonpolar Material And Dielectric Constant: In the world of electromagnetism and materials science, the concept of dielectric polarization plays a central role.
It is a phenomenon that occurs when electric charges within a material shift and create an electric dipole moment, resulting in various intriguing effects. In this article, we will delve into dielectric polarization, exploring its occurrence in both polar and nonpolar materials, and discuss the important parameter known as the dielectric constant.

Dielectric Polarization In Polar And Nonpolar Material And Dielectric Constant
1. Understanding Dielectric Polarization
1.1 What is Dielectric Polarization?
Dielectric polarization is the result of applying an electric field to a material, prompting the movement of its electric charges, which typically involve electrons and nuclei, from their original equilibrium positions. This movement leads to the formation of electric dipoles within the material, wherein positive and negative charges become separated, while the material itself maintains an overall electrical neutrality. The induced dipoles align with the electric field, and this alignment contributes to the material’s response to the applied field.
1.2 Electric Dipoles
An electric dipole consists of two equal and opposite electric charges of magnitude, +q and -q, separated by a distance, denoted as “d.” The electric dipole moment, represented by the vector “p,” is defined as the product of the charge and the distance between them: p = qd. The direction of the dipole moment points from the negative charge to the positive charge.
1.3 Dielectric Polarization Mechanisms
Dielectric polarization can occur through several mechanisms, including:
- Electronic Polarization: In polar materials, such as water, the electron cloud around atoms or molecules shifts under the influence of an external electric field.
- Ionic Polarization: In ionic solids, like table salt (sodium chloride), positive and negative ions are displaced within the crystal lattice.
- Orientational Polarization: In some materials, like ferroelectric crystals, the entire molecules or ions can rotate in response to an electric field.
2. Dielectric Polarization in Polar Materials
2.1 Polar and Nonpolar Materials
Materials can be broadly categorized as polar or nonpolar based on their molecular structure and symmetry.
- Polar Materials: These materials have an uneven distribution of charge due to an asymmetrical arrangement of atoms or molecules. Examples include water (H2O) and hydrogen fluoride (HF).
- Nonpolar Materials: These materials have a symmetrical charge distribution, resulting in no net dipole moment. Examples include oxygen (O2) and nitrogen (N2).
2.2 Dielectric Polarization in Polar Materials
Polar materials exhibit prominent dielectric polarization because of their inherent dipole moments. When an external electric field is imposed, these dipoles align with the field’s direction, amplifying the material’s dielectric polarization. Consequently, polar materials typically possess a higher dielectric constant (ε) in comparison to nonpolar materials.
3. Dielectric Polarization in Nonpolar Materials
3.1 Dielectric Polarization in Nonpolar Materials
In nonpolar materials, dielectric polarization still occurs, but it is typically weaker compared to polar materials. In these materials, electrons within atoms and molecules are displaced, albeit to a smaller extent. While the induced dipoles are less pronounced, they still contribute to the overall dielectric polarization.
3.2 Effect of Temperature
The degree of dielectric polarization in nonpolar materials can be influenced by temperature. As temperature increases, thermal motion disrupts the alignment of induced dipoles, leading to a decrease in dielectric constant.
4. Dielectric Constant (Relative Permittivity)
4.1 Definition and Significance
The dielectric constant, often denoted as ε or εr (relative permittivity), is a dimensionless quantity that represents how effectively a material can store electrical energy in electric field. It is a crucial parameter in understanding the behavior of dielectric materials.
The dielectric constant is defined as the ratio of the electric field (E) in a vacuum (or air) to the electric field (E) within the dielectric material when the same voltage is applied. Mathematically, it is expressed as:
The dielectric constant provides insight into the material’s ability to store electrical energy by forming induced dipoles. Higher dielectric constants indicate better dielectric properties, as they signify a more effective response to an applied electric field.
4.2 How Dielectric Constant is Measured
Dielectric constant measurements involve placing a dielectric material between two conductive plates and applying a known voltage across them. The resulting electric field within the material is compared to the field in a vacuum or air. This comparison allows for the calculation of the dielectric constant using the formula mentioned earlier.
Dielectric constant values vary widely among different materials. For example, the dielectric constant of a vacuum is defined as exactly 1, while common dielectric materials like water have higher values (around 78.5 at room temperature).
4.3 Influence on Capacitance
The dielectric constant plays a pivotal role in the design and operation of capacitors. A capacitor consists of two conductive plates separated by a dielectric material. The capacitance (C) of the capacitor is directly proportional to the dielectric constant (ε) and the surface area of the plates (A) and inversely proportional to the distance between the plates (d):
By using dielectric materials with higher dielectric constants, the capacitance of a capacitor can be significantly increased, allowing it to store more electrical charge for a given voltage.
5. Applications of Dielectric Polarization
5.1 Capacitors
One of the most common applications of dielectric polarization is in capacitors. Capacitors are essential electronic components used in a wide range of devices, including radios, computers, and power supplies. Dielectric materials are placed between the capacitor plates to enhance capacitance, which, in turn, affects the device’s energy storage and discharge characteristics.
5.2 Dielectric Resonators
Dielectric resonators are utilized in microwave and radio frequency (RF) applications as components that selectively filter specific frequencies. The dielectric properties of these resonators, including their dielectric constant and loss tangent, determine their performance in various communication and radar systems.
5.3 Insulators and Electrical Conductors
Dielectric materials, both polar and nonpolar, serve as insulators in electrical and electronic systems. They prevent the flow of electric current and are essential for electrical safety. In contrast, conductive materials, renowned for their low dielectric constants, facilitate efficient electrical conduction and are extensively applied in electrical wiring and circuits.
6. Future Developments in Dielectric Materials
In recent years, researchers have been actively exploring new dielectric materials and pushing the boundaries of dielectric performance. Some promising areas of development include:
6.1 High-K Dielectrics:
Materials with high dielectric constants (K values) are of interest because they can lead to smaller and more energy-efficient electronic devices. Innovations in high-K dielectrics have the potential to revolutionize the semiconductor industry.
6.2 Organic Dielectrics:
Researchers are exploring organic materials as dielectric substances due to their affordability, flexibility, and promising suitability for applications in the realm of organic electronics. Organic dielectrics are important in the development of flexible displays, organic solar cells, and thin-film transistors.
6.3 Ferroelectric and Multiferroic Materials:
Ferroelectric materials, exemplified by substances like lead zirconate titanate (PZT), display inherent spontaneous polarization and find utility in diverse applications, including non-volatile memory devices. Multiferroic materials, which possess both ferroelectric and ferromagnetic properties, are also under exploration for advanced technologies.
6.4 Dielectric Metamaterials:
Researchers are currently investigating metamaterials designed to possess distinctive dielectric characteristics for potential applications in optics, electromagnetic cloaking, and superlensing.
6.5 Energy Storage:
Dielectric materials are being studied for their potential use in energy storage applications, including dielectric capacitors for high-energy density storage and energy harvesting devices.
Conclusion
Dielectric polarization is a fundamental phenomenon that shapes the behavior of materials in response to electric fields. Whether in polar or nonpolar materials, dielectric polarization influences the properties of materials and finds applications in various fields, from electronics to energy storage.
Read More
- Chemistry In Everyday Life
- Difference Between Diffraction And Interference
- Alcohols Phenols And Ethers Class 12
- Alcohols Phenols And Ethers
- Effect Of Magnet On Current Carying Wire
Frequently Asked Questions (FAQs) Dielectric Polarization In Polar And Nonpolar Material And Dielectric Constant
1. What is dielectric polarization?
Dielectric polarization is the phenomenon where electric charges within a material shift and create electric dipoles in response to an applied electric field. It results in the alignment of electric dipoles within the material.
2. What are electric dipoles?
Electric dipoles consist of two equal and opposite electric charges separated by a distance. They create a net dipole moment, represented by a vector pointing from the negative charge to the positive charge.
3. How does dielectric polarization occur in materials?
Dielectric polarization can occur through various mechanisms, including electronic polarization (electron cloud shift), ionic polarization (displacement of ions), and orientational polarization (rotation of molecules or ions).
4. What are polar and nonpolar materials?
Polar materials have an uneven distribution of charge due to an asymmetrical arrangement of atoms or molecules. Nonpolar materials have a symmetrical charge distribution, resulting in no net dipole moment.
5. Does dielectric polarization exhibit greater strength in polar materials when compared to nonpolar materials?
Yes, dielectric polarization is typically stronger in polar materials because they already possess inherent dipole moments due to their asymmetrical structure.
Chemistry In Everyday Life
Chemistry In Everyday Life: Chemistry, often referred to as the central science, plays a crucial role in shaping the world around us. It is the science that explores the properties, composition, and transformations of matter.
While many people associate chemistry with laboratories and complex equations, the truth is that chemistry is an integral part of our daily lives, influencing everything from the food we eat to the medicines we take and the materials that surround us.
In this article, we will delve into the fascinating world of chemistry in everyday life, exploring its applications, impact, and significance.

Chemistry In Everyday Life
The Chemistry of Food: From Farm to Fork
Let’s begin our journey by exploring the role of chemistry in the food we consume every day. Chemistry is intimately connected to food production, preservation, and flavor enhancement.
1. Food Production: The journey of food from farm to table involves various chemical processes. Farmers rely on chemistry to optimize crop yields, utilizing fertilizers to provide essential nutrients to plants. Pesticides and herbicides are chemical compounds designed to protect crops from pests and weeds.
2. Food Preservation: Chemistry is the backbone of food preservation methods. Refrigeration, freezing, and canning are all based on chemical principles that slow down or inhibit the growth of microorganisms and enzymes that can spoil food. Additionally, additives like preservatives and antioxidants are used to extend the shelf life of many food products.
3. Flavor Chemistry: The taste and aroma of food are strongly influenced by chemistry. The interaction between various molecules in food and our taste receptors on the tongue creates the sensations of sweetness, sourness, bitterness, saltiness, and umami. Aromas are the result of volatile organic compounds, and the chemistry of spices and seasonings adds depth and complexity to culinary experiences.
Medicine and Healthcare: Healing through Chemistry
Chemistry plays a central role in the field of medicine and healthcare. From the development of life-saving drugs to diagnostic tests and medical imaging, chemistry impacts every aspect of healthcare.
1. Drug Discovery: Pharmaceutical chemistry focuses on the design, synthesis, and testing of drugs. Chemists work to create compounds that target specific diseases by understanding the chemical processes within the human body. This field has led to the development of antibiotics, painkillers, and treatments for chronic illnesses.
2. Diagnostic Chemistry: Clinical chemistry and medical testing rely on chemistry to detect and measure biomarkers, such as blood glucose levels or cholesterol levels, that provide critical information about a patient’s health. Diagnostic tests, such as blood tests and urine analysis, are based on chemical reactions and molecular interactions.
3. Imaging Technologies: Advanced imaging technologies like MRI (Magnetic Resonance Imaging) and CT (Computed Tomography) scans rely on the principles of nuclear magnetic resonance and X-ray technology, which are rooted in chemistry. These technologies enable non-invasive visualization of the human body’s internal structures.
Household Chemistry: Cleanliness and Comfort
In our daily lives, we encounter numerous household products that owe their effectiveness to chemistry. Cleaning agents, personal care products, and materials in our homes all have chemistry at their core.
1. Cleaning Agents: Household cleaners and detergents contain chemical compounds designed to break down and remove dirt, stains, and unwanted microorganisms. Surfactants, enzymes, and disinfectants are examples of chemicals used in cleaning products.
2. Personal Care Products: From shampoo and soap to toothpaste and deodorants, personal care products are formulated using chemistry to ensure product safety, stability, and efficacy. Chemistry is also responsible for the pleasant scents and textures of these products.
3. Materials Science: Chemistry contributes to the development of materials used in construction, electronics, and everyday items. Polymers, composites, and nanomaterials are all products of chemical research and engineering.
Environmental Chemistry: Sustaining Our Planet
As we grapple with environmental challenges, chemistry plays a vital role in addressing pollution, waste management, and the development of sustainable technologies.
1. Pollution Control: Environmental chemistry focuses on understanding and mitigating the impact of pollutants on ecosystems. Chemical processes are used to remove contaminants from air and water, improving environmental quality.
2. Waste Management: Chemistry informs waste reduction and recycling efforts. Chemical reactions and processes can transform waste into valuable resources or safely dispose of hazardous materials.
3. Sustainable Technologies: Green chemistry seeks to develop environmentally friendly products and processes. It emphasizes reducing the use of hazardous substances and minimizing waste in manufacturing and other industries.
Chemistry at Work: Industry and Innovation
Chemistry is a driving force behind industrial processes and innovations. It fuels technological advancements and economic growth.
1. Industrial Chemistry: The chemical industry produces a wide range of products, including chemicals, plastics, pharmaceuticals, and fuels. Chemistry is integral to manufacturing processes, quality control, and product development.
2. Energy Production: Chemistry is at the heart of energy production, from the combustion of fossil fuels to the development of renewable energy technologies such as solar cells and batteries.
3. Research and Innovation: Scientific research, including chemistry, drives innovation across industries. Breakthroughs in chemistry have led to the development of new materials, technologies, and scientific understanding.
Education and Scientific Advancement
Chemistry education is essential for fostering scientific literacy and training the next generation of scientists and innovators. It provides a foundational understanding of the scientific method, critical thinking, and problem-solving skills.
1. Science Education: Chemistry is a fundamental subject in science education curricula worldwide. It introduces students to the scientific method, laboratory techniques, and the principles that govern matter and energy.
2. Scientific Research: Chemistry research contributes to our understanding of the natural world and leads to discoveries that benefit society. Scientific advancements in chemistry have the potential to address global challenges, from climate change to disease prevention.
Conclusion: Embracing the Chemistry of Everyday Life
In conclusion, chemistry is not confined to laboratories or academic textbooks; it is woven into the fabric of our daily lives. From the food we eat to the medicines we take, from the products we use to the air we breathe, chemistry is the invisible force that shapes our world. Embracing the chemistry of everyday life allows us to make informed choices, promote sustainability, and appreciate the beauty of the natural world at the molecular level. It is a reminder that science is not an abstract concept but a tangible and transformative force that surrounds us every day.
Read More
- Difference Between Diffraction And Interference
- Alcohols Phenols And Ethers Class 12
- Alcohols Phenols And Ethers
- Effect Of Magnet On Current Carying Wire
- Simple Harmonic Motion Formulas
Frequently Asked Questions (FAQs) On Chemistry In Everyday Life
1. What is the significance of chemistry in our daily lives?
Chemistry is crucial in understanding and improving various aspects of daily life, including food production, healthcare, cleaning, personal care products, environmental sustainability, and technological advancements.
2. How does chemistry impact the food we consume?
Chemistry influences food production, preservation, flavor enhancement, and nutritional analysis. It plays a role in creating safe, delicious, and nutritious food products.
3. What is the role of chemistry in healthcare and medicine?
Chemistry is vital for drug discovery, diagnostic tests, medical imaging, and the development of pharmaceuticals and medical treatments that save lives and improve health.
4. What household products rely on chemistry for their effectiveness?
Cleaning agents, personal care products (such as shampoos and soaps), and materials used in construction and electronics all incorporate chemistry to function effectively.
5. How does chemistry contribute to environmental sustainability?
Chemistry is essential for addressing pollution, waste management, and the development of sustainable technologies, including renewable energy sources and green chemistry practices.
Difference Between Diffraction And Interference
Difference Between Diffraction And Interference: Diffraction and interference are both wave phenomena that occur when waves interact with obstacles or slits, but they involve distinct mechanisms and produce different effects. Here’s a comparison of the key differences between diffraction and interference:

Difference Between Diffraction And Interference
1. Definition:
- Diffraction: Diffraction is the bending or spreading of waves as they encounter an obstacle or aperture (like a slit). It occurs when waves encounter an obstacle or aperture that is comparable in size to their wavelength.
- Interference: Interference is the interaction of two or more coherent waves (waves with the same frequency and phase) that results in the combination of their amplitudes. It occurs when two or more waves overlap in space and time.
2. Cause:
- Diffraction: Diffraction is caused by the bending of waves around obstacles or through apertures, leading to the wavefronts spreading out after passing through.
- Interference: Interference is caused by the superposition of waves from different sources or the splitting of a wave into multiple waves that then overlap and interfere constructively or destructively.
3. Wave Interaction:
- Diffraction: Diffraction occurs with a single wavefront encountering an obstacle or aperture. It involves the bending or spreading of that single wavefront.
- Interference: Interference involves the combination of multiple waves, often from different sources, that overlap in space and time, creating regions of constructive and destructive interference.
4. Resultant Pattern:
- Diffraction: Diffraction produces a pattern of waves that spread out in various directions after passing through an obstacle or aperture. The resulting pattern may exhibit a central maximum and secondary maxima and minima.
- Interference: Interference produces an interference pattern characterized by alternating regions of high (constructive) and low (destructive) intensity. It can create patterns with bright and dark fringes.
5. Wavelength Dependency:
- Diffraction: Diffraction is strongly dependent on the wavelength of the wave. It is more noticeable when the wavelength is comparable to the size of the obstacle or aperture.
- Interference: Interference is not directly dependent on the wavelength of the waves but on their phase relationship. Waves with different wavelengths can interfere constructively or destructively if their phases align or differ.
6. Examples:
- Diffraction: Examples of diffraction include the spreading of light waves when passing through a narrow slit, the bending of sound waves around obstacles, and the diffraction of water waves when passing through a harbor entrance.
- Interference: Examples of interference include the colorful patterns in soap bubbles and oil films (thin film interference), the creation of diffraction grating spectra, and the formation of interference patterns in double-slit experiments.
In summary, while both diffraction and interference involve wave phenomena, diffraction is primarily concerned with the bending or spreading of waves as they encounter obstacles or apertures, while interference involves the combination of multiple waves to create regions of constructive and destructive interference, leading to interference patterns.
Read More
- Alcohols Phenols And Ethers Class 12
- Alcohols Phenols And Ethers
- Effect Of Magnet On Current Carying Wire
- Simple Harmonic Motion Formulas
- Dipole Uniform Magnetic Field
Frequently Asked Questions (FAQs) Difference Between Diffraction And Interference
1. What is diffraction?
Diffraction is the bending or spreading of waves as they encounter an obstacle or aperture, leading to changes in the direction of the waves.
2. What is interference?
Interference is the interaction of two or more coherent waves (waves with the same frequency and phase) that results in the combination of their amplitudes, leading to regions of constructive and destructive interference.
3. What causes diffraction?
Diffraction is caused by the interaction of a single wavefront with an obstacle or aperture, leading to the bending or spreading of the wave.
4. What causes interference?
Interference is caused by the superposition of multiple waves, often from different sources, that overlap in space and time, resulting in regions of constructive and destructive interference.
5. Can diffraction occur with a single wave?
Yes, diffraction can occur with a single wave front when it encounters an obstacle or aperture comparable in size to its wavelength.
Alcohols Phenols And Ethers Class 12
Alcohols Phenols And Ethers Class 12: Alcohols, phenols, and ethers are fundamental classes of organic compounds that play crucial roles in various chemical, industrial, and biological processes.
These compounds are characterized by the presence of oxygen atoms bonded to carbon atoms, creating a wide range of chemical properties and applications. In this article, we will delve into the structures, nomenclature, properties, and applications of alcohols, phenols, and ethers.

Alcohols Phenols And Ethers
I. Alcohols
Structure and Nomenclature: Alcohols are organic compounds that contain a hydroxyl (-OH) group attached to a carbon atom. The general formula for alcohols is R-OH, where R represents an alkyl or aryl group. Alcohols are named by replacing the -e at the end of the alkane name with -ol. For example, methane becomes methanol when a hydroxyl group is attached.
Properties:
- Alcohols exhibit a wide range of physical properties depending on the size and structure of the alkyl group. They can be liquids or solids at room temperature.
- Hydrogen bonding between the hydroxyl groups makes alcohols highly soluble in water.
- Alcohols can undergo various chemical reactions, including oxidation, esterification, and dehydration.
Applications:
- Methanol and ethanol are widely used as solvents in industries and laboratories.
- Ethanol is a common alcohol used in alcoholic beverages and as a biofuel.
- Alcohols are crucial intermediates in the synthesis of pharmaceuticals, plastics, and perfumes.
II. Phenols
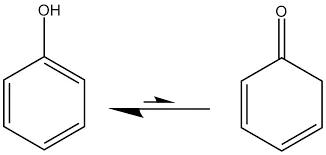
Structure and Nomenclature: Phenols are organic compounds with a hydroxyl group (-OH) attached directly to an aromatic benzene ring. The general formula for phenols is Ar-OH, where Ar represents an aromatic group. Phenols are typically named by adding the word “phenol” in front of the substituents on the benzene ring.
Properties:
- Phenols are slightly acidic due to the presence of the hydroxyl group, which can release a proton (H+) into solution.
- They have a distinct odor and are often used in perfumes and antiseptic products.
- Many phenolic compounds exhibit antioxidant properties.
Applications:
- Phenol itself is used in the production of resins, plastics, and pharmaceuticals.
- Compounds like cresol and xylenol are used as disinfectants.
- Natural phenols, such as those found in green tea and fruits, are known for their health benefits.
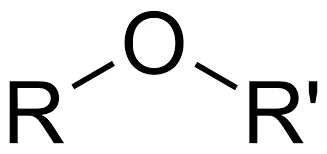
III. Ethers
Structure and Nomenclature: Ethers consist of two organic groups (alkyl or aryl) bonded to an oxygen atom, which serves as the bridge between them. The general formula for ethers is R-O-R’, where R and R’ represent alkyl or aryl groups. Ethers are named by listing the two organic groups on either side of the oxygen atom, followed by the word “ether.”
Properties:
- Ethers have relatively low boiling points and are often volatile compounds.
- They are less polar than alcohols and less soluble in water but highly flammable.
- Ethers are relatively stable compounds.
Applications:
- Diethyl ether, historically known as “ether,” was once widely used as a general anesthetic.
- Ethers are used as solvents in laboratories and industries.
- Dimethyl ether (DME) is gaining attention as a clean-burning alternative fuel.
Conclusion
Alcohols, phenols, and ethers are essential organic compounds with diverse structures and properties, making them invaluable in various fields, including chemistry, industry, medicine, and everyday life. Their versatility in chemical reactions and their role as intermediates in the synthesis of various products underscore their importance in the world of organic chemistry. Understanding these compounds is essential for scientists and chemists working in a wide range of applications.
Read More
- Alcohols Phenols And Ethers
- Effect Of Magnet On Current Carying Wire
- Simple Harmonic Motion Formulas
- Dipole Uniform Magnetic Field
- Light Filtering Polaroid Films Polarization
Frequently Asked Questions (FAQs) Alcohols Phenols And Ethers
1. What are alcohols, phenols, and ethers?
Alcohols are organic compounds characterized by the presence of a hydroxyl (-OH) group attached to a carbon atom. Phenols are similar but have the -OH group directly attached to an aromatic benzene ring. Ethers consist of two organic groups bonded to an oxygen atom.
2. How are alcohols named?
Alcohols are typically named by replacing the -e at the end of the alkane name with -ol. For example, methane becomes methanol when a hydroxyl group is attached.
3. Are alcohols soluble in water?
Yes, alcohols are often highly soluble in water due to hydrogen bonding between the hydroxyl groups and water molecules.
4. What is the acidity of phenols?
Phenols are slightly acidic because they can release a proton (H+) into solution due to the presence of the hydroxyl group.
5. What is the main characteristic of ethers?
Ethers are characterized by the presence of an oxygen atom that serves as a bridge between two organic groups.
Alcohols Phenols And Ethers
Alcohols Phenols And Ethers: Organic chemistry is a branch of chemistry that deals with the study of carbon-containing compounds, and within this realm, alcohols, phenols, and ethers are fundamental functional groups.
These compounds play pivotal roles in both the natural world and the realm of synthetic chemistry, impacting fields ranging from pharmaceuticals to agriculture, and from materials science to biochemistry.
In this extensive 2000-word article, we will delve deep into the fascinating world of alcohols, phenols, and ethers, exploring their structures, nomenclature, properties, reactions, and significant applications.

Alcohols Phenols And Ethers
Table of Contents
1. Alcohols
- 1.1 Introduction to Alcohols
- 1.2 Nomenclature of Alcohols
- 1.3 Classification of Alcohols
- 1.4 Physical Properties of Alcohols
- 1.5 Chemical Properties of Alcohols
- 1.6 Applications of Alcohols
2. Phenols
- 2.1 Introduction to Phenols
- 2.2 Nomenclature of Phenols
- 2.3 Physical Properties of Phenols
- 2.4 Chemical Properties of Phenols
- 2.5 Acidity of Phenols
- 2.6 Applications of Phenols
3. Ethers
- 3.1 Introduction to Ethers
- 3.2 Nomenclature of Ethers
- 3.3 Physical Properties of Ethers
- 3.4 Chemical Properties of Ethers
- 3.5 Applications of Ethers
1. Alcohols
1.1 Introduction to Alcohols
Alcohols are organic compounds characterized by the presence of one or more hydroxyl (-OH) functional groups attached to carbon atoms. These compounds are ubiquitous in nature and have a significant impact on human life, ranging from the ethanol in alcoholic beverages to the glycerol in skincare products.
1.2 Nomenclature of Alcohols
The International Union of Pure and Applied Chemistry (IUPAC) provides a systematic method for naming alcohols. The general rule is to replace the -e ending of the corresponding alkane with -ol. Here are some examples:
- Methane becomes methanol
- Ethane becomes ethanol
- Propane becomes propanol
For more complex molecules, a number is used to indicate the position of the hydroxyl group, and prefixes such as “iso-” or “tert-” are added to denote branching or multiple hydroxyl groups.
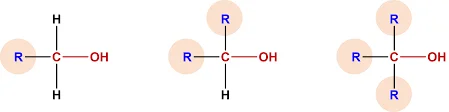
1.3 Classification of Alcohols
Alcohols can be classified into three main categories based on the number of alkyl or aryl groups bonded to the carbon atom bearing the hydroxyl group:
- Primary (1°) alcohols: The carbon atom bonded to the hydroxyl group is only attached to one other carbon atom.
- Secondary (2°) alcohols: The carbon atom bonded to the hydroxyl group is attached to two other carbon atoms.
- Tertiary (3°) alcohols: The carbon atom bonded to the hydroxyl group is attached to three other carbon atoms.
- This classification is essential because it influences the reactivity of the alcohol in various chemical reactions.
1.4 Physical Properties of Alcohols
Alcohols exhibit several notable physical properties:
Boiling and Melting Points: Alcohols generally have higher boiling and melting points compared to hydrocarbons of similar molecular weight. This is due to the presence of hydrogen bonding between alcohol molecules, which requires more energy to break.
- Solubility: Lower-molecular-weight alcohols (methanol, ethanol) are highly soluble in water due to their ability to form hydrogen bonds with water molecules. As the alkyl chain length increases, solubility in water decreases.
- Odor and Taste: Some alcohols, such as ethanol, have distinctive odors and flavors, which contribute to their use in beverages and perfumes.
1.5 Chemical Properties of Alcohols
Alcohols exhibit a wide range of chemical properties:
- Oxidation: Alcohols can be oxidized to form aldehydes or ketones, depending on their classification. Primary alcohols are oxidized to aldehydes, which can further be oxidized to carboxylic acids. Secondary alcohols are oxidized to ketones. Tertiary alcohols do not undergo oxidation under normal conditions.
- Esterification: Alcohols can react with carboxylic acids to form esters, a reaction commonly used in the fragrance and flavor industry.
- Dehydration: Alcohols can undergo dehydration reactions to form alkenes. This is often achieved by heating the alcohol in the presence of a strong acid catalyst.
- Substitution Reactions: Alcohols can undergo substitution reactions, where the -OH group is replaced with another functional group. Common substitutions include the conversion of alcohols to alkyl halides.
1.6 Applications of Alcohols
Alcohols find applications in various industries and everyday life:
- Ethanol (Ethyl Alcohol): Perhaps the most well-known alcohol, ethanol, is used as a recreational beverage, industrial solvent, and biofuel. It’s also employed as an antiseptic and disinfectant.
- Methanol (Methyl Alcohol): Methanol is used as an industrial solvent, antifreeze, and fuel. It’s also a precursor in the production of formaldehyde and other chemicals.
- Glycerol: Glycerol is used extensively in the cosmetics and pharmaceutical industries due to its moisturizing properties. It’s also utilized in the production of nitroglycerin, a potent explosive.
- Butanol: Butanol is used as a solvent, in the manufacture of plastics, and as a fuel additive. It’s also a potential biofuel.
2. Phenols
2.1 Introduction to Phenols
- Phenols are organic compounds characterized by the presence of a hydroxyl (-OH) group attached to a benzene ring. These compounds have unique properties due to the combination of the aromatic benzene ring and the hydroxyl group.
2.2 Nomenclature of Phenols
- Nomenclature of phenols is relatively straightforward. The hydroxyl group is considered the functional group, and the compound is named by adding the word “phenol” to the name of the substituent(s) on the benzene ring. For example, if a hydroxyl group is attached to a benzene ring with a methyl group, it is called “methylphenol.”
2.3 Physical Properties of Phenols
Phenols exhibit several distinctive physical properties:
- Higher Melting Points: Phenols generally have higher melting points compared to their hydrocarbon counterparts due to hydrogen bonding between phenol molecules. This property makes them solid at room temperature.
- Acidity: Phenols are more acidic than alcohols because the hydroxyl group in phenols is directly attached to the benzene ring, making it more stable. The acidity of phenols allows them to react with strong bases to form phenoxide ions.
- Aroma and Taste: Some phenols contribute to the characteristic aroma and taste of various natural products. For example, phenol derivatives are found in essential oils and spices.
2.4 Chemical Properties of Phenols
Phenols exhibit a variety of chemical reactions:
- Electrophilic Aromatic Substitution: Phenols are highly susceptible to electrophilic aromatic substitution reactions due to the electron-rich nature of the benzene ring. This makes them valuable intermediates in the synthesis of numerous organic compounds.
- Esterification: Phenols can react with carboxylic acids to form phenolic esters, which have applications in the fragrance and flavor industry.
- Oxidation: Phenols can be oxidized to quinones, which are important in biological processes and as precursors in the synthesis of dyes and pigments.
2.5 Acidity of Phenols
- The increased acidity of phenols, compared to alcohols, is due to the resonance stabilization of the phenoxide ion formed when a proton is lost from the hydroxyl group. This makes phenols capable of reacting with strong bases, a property not shared by alcohols.
2.6 Applications of Phenols
Phenols have diverse applications in various industries:
- Antiseptics and Disinfectants: Phenol and its derivatives have been historically used as antiseptics and disinfectants. However, their use has diminished due to toxicity concerns.
- Phenolic Resins: Phenolic resins, produced by the condensation of phenol with formaldehyde, are widely used in the production of molded plastic objects, laminates, and coatings.
- Pharmaceuticals: Phenols are important intermediates in the synthesis of pharmaceuticals, agrochemicals, and dyes.
3. Ethers
3.1 Introduction to Ethers
- Ethers are organic compounds characterized by an oxygen atom (-O-) bonded to two alkyl or aryl groups. The general structure of ethers is R-O-R’, where R and R’ represent alkyl or aryl groups. Ethers are known for their unique chemical properties, especially their low reactivity compared to other functional groups.
3.2 Nomenclature of Ethers
- The nomenclature of ethers is based on the names of the alkyl or aryl groups bonded to the oxygen atom, followed by the word “ether.” For example, diethyl ether has two ethyl groups bonded to the oxygen atom.
3.3 Physical Properties of Ethers
Ethers possess several notable physical properties:
- Boiling Points: Ethers generally have lower boiling points compared to alcohols of similar molecular weight. This is because ethers do not form hydrogen bonds with one another, unlike alcohols.
- Solubility: Ethers are typically more soluble in organic solvents than in water. However, their solubility in water is still higher than that of most hydrocarbons due to their oxygen atom.
- Flammability: Some ethers are highly flammable, which can be a safety concern.
3.4 Chemical Properties of Ethers
- Ethers are known for their relative chemical stability compared to other functional groups. They do not readily undergo reactions with common reagents under normal conditions. However, they can be cleaved by strong acids to form alcohol and alkyl halide products, a reaction known as “acid-catalyzed cleavage of ethers.”
3.5 Applications of Ethers
Despite their low reactivity, ethers have several practical applications:
- Solvents: Ethers are valuable solvents in laboratories and industries. Diethyl ether, for instance, is commonly used as a solvent for various chemical reactions.
- Anesthetics: Diethyl ether was historically used as a general anesthetic before safer alternatives became available.
- Fuel Additives: Methyl tertiary-butyl ether (MTBE) and ethyl tertiary-butyl ether (ETBE) are ethers used as fuel additives to improve octane ratings and reduce emissions in gasoline.
- Crown Ethers: Crown ethers, a subclass of ethers, are widely used in chemistry for their ability to complex with metal ions, aiding in separations and ion transport.
4. Conclusion
In conclusion, alcohols, phenols, and ethers are essential functional groups in organic chemistry, each with its unique properties, nomenclature, and reactivity. These compounds play crucial roles in a wide range of applications, from the production of pharmaceuticals and plastics to the formulation of perfumes and the development of new materials. Understanding the chemistry of alcohols, phenols, and ethers is fundamental to both students embarking on a journey in chemistry and professionals working in fields where these compounds are integral. As our understanding of these compounds continues to grow, their applications and significance in our world will undoubtedly expand as well.
Read More
- Effect Of Magnet On Current Carying Wire
- Simple Harmonic Motion Formulas
- Dipole Uniform Magnetic Field
- Light Filtering Polaroid Films Polarization
- Animals Biology Science Lion Zoology
Frequently Asked Questions (FAQs) Alcohols Phenols And Ethers
What are alcohols, phenols, and ethers?
Alcohols are organic compounds characterized by the presence of one or more hydroxyl (-OH) groups attached to carbon atoms. Phenols have a hydroxyl group attached to a benzene ring, while ethers have an oxygen atom bonded to two alkyl or aryl groups.
How are alcohols named?
Alcohols are named by replacing the -e ending of the corresponding alkane with -ol. For example, methane becomes methanol, and ethane becomes ethanol.
What is the difference between primary, secondary, and tertiary alcohols?
Alcohols are classified into three categories based on the carbon atom bearing the hydroxyl group. Primary alcohols have one alkyl group attached, secondary alcohols have two, and tertiary alcohols have three.
Why do alcohols have higher boiling points compared to hydrocarbons of similar molecular weight?
Alcohols have higher boiling points due to hydrogen bonding between alcohol molecules, which requires more energy to break than the weaker van der Waals forces in hydrocarbons.
What are the main chemical reactions of alcohols?
Alcohols can undergo oxidation to form aldehydes or ketones, esterification to form esters, and dehydration to produce alkenes, among other reactions.
Effect Of Magnet On Current Carying Wire
Effect Of Magnet On Current Carying Wire: The interaction between magnets and electric currents is a fascinating aspect of electromagnetism, a fundamental branch of physics. This interaction has led to the development of various technological applications, from electric motors to generators.
In this article, we will explore the intriguing effect of magnets on current-carrying wires, shedding light on the principles behind it and its real-world applications.

Effect Of Magnet On Current Carying Wire
The Basics of Electromagnetism
Electromagnetism is a branch of physics that deals with the relationship between electric currents and magnetic fields. It was James Clerk Maxwell who, in the 19th century, formulated the fundamental equations that describe how electric charges and currents generate magnetic fields and how changing magnetic fields induce electric currents.
The Right-Hand Rule
One of the key principles in understanding the interaction between magnets and current-carrying wires is the right-hand rule. This rule states that if you point your thumb in the direction of the current flow (conventional current, from positive to negative), and you curl your fingers, the direction in which your fingers curl represents the direction of the magnetic field lines around the wire. The strength of the magnetic field depends on the current intensity and the distance from the wire.
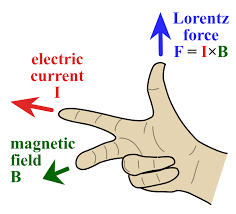
The Effect of Magnets on Current-Carrying Wires
When a magnetic field interacts with a current-carrying wire, several important phenomena occur:
- Magnetic Force: The magnetic field exerts a force on the wire. This force is perpendicular to both the current direction and the magnetic field direction and is described by the magnetic force formula: F = I * B * L * sin(θ), where F is the force, I is the current, B is the magnetic field strength, L is the length of the wire in the field, and θ is the angle between the current and magnetic field directions.
- Lorentz Force: The magnetic force on the wire is part of the larger Lorentz force, which includes the electric force on charged particles within the wire. This combined force can cause the wire to move or experience a torque.
Applications of the Effect
- Understanding the effect of magnets on current-carrying wires has a multitude of practical applications:
- Electric Motors: Electric motors utilize the interaction between magnetic fields and current-carrying wires to convert electrical energy into mechanical motion. This principle is at the core of various appliances and machines.
- Generators: In generators, mechanical motion is used to move wires through magnetic fields, inducing electric currents. This process is how electrical energy is generated in power plants.
- Magnetic Sensors: Magnetic sensors and compasses rely on the interaction between magnetic fields and current in coils to detect and measure magnetic fields, providing critical information in navigation and various industries.
- Transformers: Transformers use the principles of electromagnetic induction to change the voltage levels in electrical circuits, making power distribution and voltage conversion efficient.
Conclusion
The effect of magnets on current-carrying wires is a fundamental aspect of electromagnetism with profound implications in modern technology. It enables the creation of electric motors, generators, sensors, and transformers, all of which are integral to our daily lives. By harnessing the principles of electromagnetism, scientists and engineers have revolutionized the way we generate, distribute, and use electrical energy, making the world of technology and innovation possible. Understanding these principles is crucial for anyone interested in the fields of physics and electrical engineering.
Read More
- Simple Harmonic Motion Formulas
- Dipole Uniform Magnetic Field
- Light Filtering Polaroid Films Polarization
- Animals Biology Science Lion Zoology
- Difference Between Voltage And Current
Frequently Asked Questions (FAQs) Effect Of Magnet On Current Carying Wire
Q1: What is the basic principle behind the interaction between magnets and current-carrying wires?
A1: The basic principle is that a magnetic field exerts a force on a current-carrying wire. This force is perpendicular to both the current direction and the magnetic field direction and is described by the magnetic force formula.
Q2: What is the right-hand rule in electromagnetism?
A2: The right-hand rule is a convention used to determine the direction of magnetic field lines generated by a current-carrying wire. If you point your right thumb in the direction of the current, the curling of your fingers represents the direction of the magnetic field lines around the wire.
Q3: How does the strength of the magnetic field affect the force on a current-carrying wire?
A3: The strength of the magnetic field (denoted as B) directly affects the force (F) experienced by the wire. The greater the magnetic field strength, the stronger the force on the wire, assuming other factors like current and length remain constant.
Q4: What is the Lorentz force, and how does it relate to the effect of magnets on current-carrying wires?
A4: The Lorentz force is the combined force acting on a charged particle due to both an electric field and a magnetic field. In the context of current-carrying wires, it includes the magnetic force on the wire as well as the electric force on the charged particles (electrons) within the wire.
Q5: What are some practical applications of the effect of magnets on current-carrying wires?
A5: Some practical applications include electric motors, generators, magnetic sensors, compasses, and transformers. These devices rely on the interaction between magnetic fields and current-carrying wires to perform various functions, such as generating electricity, providing directional information, and converting electrical energy into mechanical motion.
