Category: Class 12
Molar Mass Of Oxalic Acid
Molar Mass Of Oxalic Acid: The molar mass of oxalic acids, which has the chemical formula C2H2O4, can be calculated by summing the atomic masses of all the atoms in one mole of the compound. Here’s how you can calculate it:

Molar Mass Of Oxalic Acid
Calculating the Molar Mass of Oxalic Acid
To calculate the molar mass of oxalic acids (C2H2O4), we sum the atomic masses of its constituent elements:
- Carbon (C) has an atomic mass of approximately 12.01 g/mol.
- Hydrogen (H) has an atomic mass of approximately 1.01 g/mol.
- Oxygen (O) has an atomic mass of approximately 16.00 g/mol.
Now, let’s compute the molar mass of oxalic acid:
Molar Mass of Oxalic Acids (C2H2O4) = (2 × Atomic Mass of Carbon) + (2 × Atomic Mass of Hydrogen) + (4 × Atomic Mass of Oxygen)
Molar Mass of Oxalic Acids (C2H2O4) = (2 × 12.01 g/mol) + (2 × 1.01 g/mol) + (4 × 16.00 g/mol)
The Molar Mass of Oxalic Acids (C2H2O4) = 24.02 g/mol + 2.02 g/mol + 64.00 g/mol
Molar Mass of Oxalic Acids (C2H2O4) ≈ 90.04 g/mol
So, the molar mass of oxalic acids (C2H2O4) is approximately 90.04 grams per mole.
Significance of Oxalic Acid’s Molar Mass
Understanding the molar mass of oxalic acids is essential for various reasons:
- Chemical Reactions: Molar mass plays a pivotal role in stoichiometry, helping chemists determine the quantity of substances needed and produced in chemical reactions involving oxalic acids.
- Laboratory Procedures: In laboratories, chemists use molar mass to measure and prepare precise amounts of substances, ensuring accurate experiments and analyses.
- Solution Concentrations: Molar mass aids in calculating the concentration of oxalic acids solutions, which is crucial in fields like analytical chemistry and titration experiments.
- Industrial Applications: Oxalic acids finds application in various industries, including textile, metal polishing, and pharmaceuticals. Knowing its molar mass is essential for quality control and production processes.
- Safety Precautions: Understanding the molar mass of oxalic acids is vital for safety considerations in handling, storage, and transportation, as it helps assess potential hazards.
Conclusion
The molar mass of oxalic acids, approximately 90.04 g/mol, may seem like a mere numerical value, but it holds great importance in the realm of chemistry and scientific endeavors.
Read More
- ypes Of Chemical Bonding
- Chemistry In Everyday Life
- Energy Stored In A Capacitor
- Compound Lenses Thin Lenses In Contact
- 12th Class English Book Question Answer Pdf Download
Frequently Asked Questions (FAQs) Molar Mass Of Oxalic Acid
-
What is the molar mass of oxalic acid (C2H2O4)?
The molar mass of oxalic acids is approximately 90.04 grams per mole (g/mol).
-
Why is knowing the molar mass of oxalic acid important?
Knowing the molar mass of oxalic acids is crucial for various chemical calculations, including stoichiometry, determining solution concentrations, and conducting laboratory experiment. It is also essential for quality control in industrial applications involving oxalic acids.
-
How is the molar mass of oxalic acid calculated?
To calculate the molar mass of oxalic acids, sum the atomic masses of its constituent elements: carbon (C), hydrogen (H), and oxygen (O). Use the atomic masses of these elements and multiply them by the number of atoms of each element in the compound (as indicated by the chemical formula).
-
What is oxalic acid used for in industry?
Oxalic acids has various industrial applications. It is used in metal cleaning and polishing, textile processing, and as a reducing agent in chemical processes. It is also employed in pharmaceutical and research applications.
-
Is oxalic acid safe to handle?
Oxalic acids should be handled with care as it is toxic. Proper safety precautions, including the use of protective equipment and handling procedures, should be followed when working with oxalic acids to minimize health risks.
Types Of Chemical Bonding
Types Of Chemical Bonding: Chemical bonding is a fundamental concept in chemistry that helps us understand how atoms combine to form molecules and compounds.
It governs the physical and chemical properties of matter and is the basis for the diversity of substances we encounter in our daily lives. In this article, we will delve into the different types of chemical bonding and their significance in the world of chemistry.

Types Of Chemical Bonding
1. Ionic Bonding
Ionic bonding occurs when one or more electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges. This type of bonding typically occurs between metals and nonmetals. The metal loses electrons to become a positively charged cation, while the nonmetal gains those electrons to become a negatively charged anion. The strong electrostatic attraction between these oppositely charged ions holds them together in ionic compounds.
Significance: Ionic compounds, such as table salt (sodium chloride, NaCl) and calcium carbonate (CaCO3), are vital in various applications, including in the food industry, medicine, and the production of ceramics and glass.
2. Covalent Bonding
Covalent bonding results from the sharing of electrons between atoms, typically between nonmetals. In a covalent bond, atoms share one or more pairs of electrons, resulting in the formation of molecules. This type of bonding is characterized by the strong attraction between the shared electrons and the positively charged nuclei of the atoms involved.
Significance: Covalent compounds, like water (H2O) and methane (CH4), are widespread in nature and have diverse uses, from serving as essential compounds for life to forming the basis of many organic chemicals.
3. Metallic Bonding
Metallic bonding occurs in metals, where atoms within a solid lattice structure share their valence electrons freely. This sharing of electrons creates a “sea of electrons” that moves throughout the structure, giving metals their unique properties such as electrical conductivity and malleability.
Significance: Metallic bonds are crucial in the construction of electrical wires, the production of structural materials, and the formation of alloys, which have applications in aerospace, construction, and manufacturing.
4. Hydrogen Bonding
Hydrogen bonding is a special type of dipole-dipole interaction that occurs when a hydrogen atom is covalently bonded to a highly electronegative atom (usually oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom in a nearby molecule. This bond is responsible for the unique properties of water, such as its high boiling point and surface tension.
Significance: Hydrogen bonding is essential in biology, contributing to the structure of DNA, the folding of proteins, and the properties of water that support life.
5. Van der Waals Forces
Van der Waals forces, also known as London dispersion forces and dipole-dipole interactions, are relatively weak attractions that exist between all molecules, regardless of their polarity. These forces are caused by temporary fluctuations in electron distribution within molecules, creating temporary positive and negative charges that attract nearby molecules.
Significance: Van der Waals forces play a significant role in the behavior of gases, the condensation of liquids, and the adsorption of molecules onto surfaces, influencing phenomena like adhesion and cohesion.
6. Coordinate Covalent Bonding
In coordinate covalent bonding, also known as dative bonding, one atom donates a pair of electrons to be shared in a covalent bond with another atom. This type of bonding is commonly found in complex ions and coordination compounds.
Significance: Coordinate covalent bonds are essential in the formation of metal complexes, which are widely used in catalysis, as pigments, and in the study of coordination chemistry.
Understanding the various types of chemical bonding is fundamental to comprehending the behavior of matter, the properties of substances, and the countless applications of chemistry in our daily lives.
Read More
- Chemistry In Everyday Life
- Energy Stored In A Capacitor
- Compound Lenses Thin Lenses In Contact
- 12th Class English Book Question Answer Pdf Download
Frequently Asked Question (FAQs) Types Of Chemical Bonding
1. What is chemical bonding?
Chemical bonding is the process by which atoms combine to form molecules or compounds. It involves the sharing or transfer of electrons between atoms to achieve a stable electronic configuration.
2. What are the main types of chemical bonding?
The main types of chemical bonding are ionic bonding, covalent bonding, metallic bonding, hydrogen bonding, Van der Waals forces, and coordinate covalent bonding.
3. What is ionic bonding, and how does it work?
Ionic bonding occurs when one atom transfers electrons to another, resulting in the formation of ions with opposite charges. The electrostatic attraction between these oppositely charged ions holds them together in an ionic compound.
4. Can you provide examples of substances with ionic bonding?
Examples of substances with ionic bonding include table salt (sodium chloride, NaCl), calcium carbonate (CaCO3), and magnesium oxide (MgO).
5. What is covalent bonding, and how does it differ from ionic bonding?
Covalent bonding involves the sharing of electrons between atoms, typically between nonmetals. In contrast to ionic bonding, where electrons are transferred, covalent bonds result from the mutual attraction between shared electrons and the positively charged nuclei of the atoms involved.
Chemistry In Everyday Life
Chemistry in everyday life: Chemistry is not confined to laboratory experiments and chemical equations; it is an integral part of our everyday lives.

Chemistry In Everyday Life
Medicine and Healthcare: Chemistry plays a pivotal role in the development of pharmaceuticals and medical treatments. From pain relievers to antibiotics, chemistry enables the creation of life-saving drugs that combat diseases and alleviate suffering.
Food and Nutrition: Chemistry helps us understand the composition of the food we consume. It involves processes like cooking, baking, and fermentation, which transform raw ingredients into flavorful and nutritious meals.
Cleaning and Hygiene: Household cleaning products, detergents, and disinfectants are formulated using chemical principles. They aid in maintaining cleanliness and preventing the spread of infections.
Cosmetics and Personal Care: Chemistry is behind the creation of cosmetics, shampoos, soaps, and skincare products. These items enhance our appearance and personal hygiene.
Environmental Protection: Understanding chemical processes in the environment is essential for addressing pollution, climate change, and conservation. Chemistry contributes to cleaner energy sources and sustainable technologies.
Water Purification: Chemical processes purify water, making it safe for drinking and everyday use. This is a critical aspect of public health.
Textiles and Apparel: Chemistry plays a role in fabric dyeing, textile manufacturing, and the creation of wrinkle-resistant and stain-repellent clothing.
Transportation: Chemistry is crucial in the development of fuels, lubricants, and materials used in transportation, making vehicles more efficient and eco-friendly.
Plastics and Packaging: Chemistry is responsible for producing versatile and lightweight materials like plastics, which are used in countless everyday items and packaging.
Electronics: Advances in materials science and semiconductor chemistry drive the development of electronic devices, making them smaller, faster, and more energy-efficient.
Agriculture and Food Production: Chemical fertilizers, pesticides, and herbicides boost crop yields and safeguard crops from pests and diseases. Soil chemistry and nutrient uptake are also areas where chemistry is vital.
Cooking and Baking: Everyday culinary activities involve chemical reactions like caramelization, Maillard reactions, and leavening, contributing to the delicious flavors and textures of our meals.
Energy Generation: Chemistry is central to energy production through processes such as combustion, nuclear reactions, and renewable energy technologies.
Aesthetics and Fragrances: Chemistry plays a significant role in creating fragrances, scents, and colors used in perfumes, cosmetics, and art.
Healthcare Diagnostics: Chemistry-based diagnostic tests, including blood tests and urinalysis, help healthcare professionals diagnose and monitor various medical conditions.
In summary, chemistry surrounds us and enriches our daily existence in countless ways. Its contributions to medicine, food, hygiene, the environment, and technology are evident throughout our lives, making it a vital and fascinating field of study and application.
Read More
- Energy Stored In A Capacitor
- Compound Lenses Thin Lenses In Contact
- 12th Class English Book Question Answer Pdf Download
Frequently Asked Question (FAQs) Chemistry In Everyday Life
1. What is the significance of chemistry in our daily lives?
Chemistry is essential in our daily lives because it helps us understand and improve various aspects of life, including health, nutrition, hygiene, energy production, and environmental conservation.
2. How does chemistry impact our health?
Chemistry plays a crucial role in healthcare by enabling the development of pharmaceuticals, diagnostics, and medical treatments that improve our well-being and combat diseases.
3. What are some common examples of chemical reactions in cooking and baking?
Common chemical reactions in cooking and baking include caramelization, the Maillard reaction (responsible for browning), and the leavening of dough in baking.
4. How does chemistry contribute to the food we eat?
Chemistry helps us understand food composition, preservation techniques, and flavor development. It’s involved in processes like fermentation, pasteurization, and food additives.
5. What are some environmentally significant applications of chemistry?
Chemistry helps address environmental challenges by contributing to clean energy technologies, waste management, and pollution control. It also aids in understanding climate change.
Energy Stored In A Capacitor
Energy Stored In A Capacitor. A capacitor is an essential component in electronics that stores electrical energy in an electric field.
The energy stored in a capacitor is a direct result of the voltage applied across its terminals and the amount of charge it can hold. The formula to calculate the energy stored in a capacitor is given by:

Energy (E) = 0.5 × Capacitance (C) × Voltage (V)^2
Where:
- Energy (E) is the energy stored in the capacitor, measured in joules.
- Capacitance (C) is the measure of a capacitor’s ability to store charge, measured in farads (F).
- Voltage (V) is the potential difference across the capacitor’s terminals, measured in volts.
This formula demonstrates that the energy stored in a capacitor is proportional to the square of the voltage and the capacitance. Capacitors with higher capacitance or higher voltage can store more energy. When a capacitor is charged, it accumulates energy in the electric field between its plates. This stored energy can later be released when the capacitor is discharged.
The energy stored in a capacitor plays a crucial role in various electronic applications, including smoothing voltage fluctuations, filtering signals, and providing bursts of energy in circuits. Understanding how capacitors store and release energy is fundamental in designing efficient and reliable electronic systems.
Energy Stored in a Capacitor: Explained Further
A capacitor, a fundamental component in electronics, is not just about storing charge; it’s also a reservoir of energy in the form of an electric field.
This stored energy becomes vital in various electronic applications, and comprehending its calculation sheds light on the capacitor’s significance.
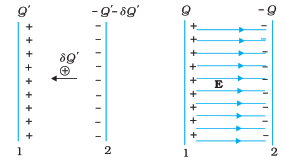
Formula and Components:
The energy stored in a capacitor is directly linked to the voltage across it and the amount of charge it can accumulate. The formula to calculate this energy is:
Energy (E) = 0.5 × Capacitance (C) × Voltage (V)^2
Capacitance (C): This property measures how much charge a capacitor can store for a given voltage. It is measured in farads (F).
Voltage (V): The potential difference between the capacitor’s plates, measured in volts. It’s the driving force behind the energy storage process.
Understanding the Equation:
The formula highlights that energy stored is proportional to both the capacitance and the square of the voltage. This means that doubling the voltage will result in a fourfold increase in energy, while doubling the capacitance will result in a twofold increase.
Energy Storage Process:
When a capacitor is connected to a power source, it charges as it accumulates electric potential energy. The energy is stored as an electric field between the capacitor’s plates.
This field exerts a force on the charges, preventing further accumulation of charge. Once fully charged, the capacitor contains energy that can be released when needed.
Applications:
Filtering and Smoothing: Capacitors are often used to filter out noise and voltage fluctuations from electrical signals, ensuring a stable output.
Energy Storage: In circuits like camera flashes, capacitors can quickly store energy and release it in a burst when needed.
Backup Power: Capacitors can provide temporary power during brief interruptions, allowing systems to shut down safely.
Timing Circuits: In combination with resistors, capacitors are crucial in creating time delays and oscillations in electronic circuits.
Conclusion:
The energy stored in a capacitors exemplifies the interplay between voltage, capacitance, and electric fields. This energy reservoir is pivotal in electronics, contributing to diverse applications that range from signal processing to energy bursts.
Understanding the nuances of energy storage enhances our ability to design efficient and responsive electronic systems.
Read More
Frequently Asked Question (FAQs)
What is the energy stored in a capacitor?
The energy stored in a capacitor refers to the electric potential energy stored in the electric field between its plates when it’s charged.
How is the energy stored in a capacitor calculated?
The energy stored in a capacitors can be calculated using the formula: (E) = 0.5 × Capacitance (C) × Voltage (V)^2.
What is capacitance?
Capacitance is a measure of a capacitor’s ability to store charge for a given voltage. It is measured in farads (F).
How does voltage affect the energy stored in a capacitor?
The energy stored in a capacitors is directly proportional to the square of the voltage applied across its terminals. Doubling the voltage results in a fourfold increase in stored energy.
Can a capacitor store an infinite amount of energy?
No, a capacitor’s energy storage is limited by its capacitance and voltage. It cannot store an infinite amount of energy.
Compound Lenses Thin Lenses In Contact
Compound Lenses Thin Lenses In Contact: In a basic lens system, a solitary lens is employed, in contrast to the compound lens system where the utilization of multiple lenses aligned along a shared axis is possible. The most straightforward form of such a compound lens setup involves the alignment of two thin lenses in direct contact. This article aims to delve into the realm of compound lenses, examining their distinctive properties.

The frequently utilized types of lenses encompass:
1. Convex Lenses (Converging Lenses)
2. Concave Lenses (Diverging Lenses)
As you may recall, we have extensively covered the characteristics of convex and concave lenses in previous sessions. Now, our focus shifts towards comprehending the intricacies of compound lenses.
Compound Lenses
The illustration below demonstrates a compound lens arrangement. Compound lenses are characterized by the presence of two thin lenses positioned on a shared axis, often in close proximity or bonded together.
Compound Lens Configuration
In cases where two thin lenses sharing an axis are positioned in contact with each other, the common focal length for the system can be determined using the following formula:
Where:
- is the combined focal length
- is the focal length of the first lens
- is the focal length of the second lens
Given that power is the reciprocal of focal length, a noticeable observation arises in this scenario. For thin lenses in contact, it becomes apparent that the combined power of the system results from the summation of the powers of the individual lenses.
However, if the lenses are not in direct contact but separated by a distance , the combined focal length can be computed using the subsequent formula:
Now, let’s put your understanding to the test with a simple problem: A bi-convex lens with a focal length of 10 cm and a bi-concave lens with a focal length of 20 cm are positioned in contact. Both lenses share the same refractive index. Calculate the combined focal length.
When delving into the realm of lens combinations, you may encounter the following terminology.
A Focal System
When the separation distance (d) between the two lenses equals the sum of their individual focal lengths (f1 + f2), the resulting combined focal length becomes infinite. This configuration causes the refracted light rays to become parallel to one another.
Achromatic Doublet
The arrangement of the lenses is designed to align such that two specific wavelengths, usually the extremes of red and violet light, converge to a focused point. This utilization represents an instance of employing a compound lens system to rectify chromatic aberrations, a phenomenon that can occur with single lenses.
Application of Compound Lenses
Compounded lenses are employed in telescopes and microscopes, combining two or more lenses to achieve the following objectives:
1. Minimize aberrations and flaws resulting from single lens usage.
2. Obtain an upright image of the object.
3. Enhance image magnification.
I trust you now have a clear comprehension of compound lenses.
The Purpose and Benefits
The application of compound lenses, particularly with thin lenses in contact, is multifaceted and impactful. Let’s explore the primary reasons behind their adoption:
- Aberration Correction: Single lenses often suffer from optical aberrations, leading to distortion, blurring, and color fringing in images. Compound lenses tackle this issue by carefully aligning and combining thin lenses. This alignment aids in reducing aberrations, resulting in clearer, sharper images.
- Chromatic Aberration Mitigation: The dispersion of light into different colors, known as chromatic aberration, is a challenge that can affect the quality of images produced by single lenses. Compound lenses help alleviate this problem by ensuring that light of varying colors converges more effectively onto a single focal point.
- Erect Image Formation: Erecting images – producing images that are not inverted – is crucial in various optical devices, such as microscopes and telescopes. Compound lenses, when configured correctly, can generate upright images, enhancing the usability of such devices.
- Magnification Enhancement: The integration of thin lenses in contact can lead to increased magnification capabilities, which is vital for applications requiring close examination of intricate details.
Read More
Frequently Asked Questions – FAQs
What are compound lenses composed of?
Compound lenses are made up of two or more thin lenses positioned in contact with each other along a common axis.
What is the purpose of combining thin lenses in contact?
Combining thin lenses in contact helps to correct optical aberrations and improve the quality of images formed.
What advantage do compound lenses offer over single lenses?
Compound lenses help to correct chromatic aberrations that can occur with single lenses, leading to improved image quality.
Where are compound lenses commonly used?
Compound lenses are frequently used in telescopes and microscopes to achieve various optical benefits, including increased magnification and reduced defects.
Can compound lenses be used for erect image formation?
Yes, by properly arranging the thin lenses, compound lens systems can produce an erect image of an object.
What is the significance of correcting chromatic aberrations?
Correcting chromatic aberrations ensures that different colors of light converge to the same focal point, resulting in sharper and clearer images.
12th Class English Book Question Answer Pdf Download
Download 12th Class English Book Question Answer Pdf: English stands as a paramount and approachable subject for learning and achieving high scores in board examinations.
To facilitate a comprehensive grasp of both fundamental and advanced aspects of the English Flamingo curriculum today, we present an array of NCERT Solutions for Class 12 English Flamingo, encompassing all chapters.

It’s widely acknowledged that NCERT Solutions serve as an optimal preparatory resource, serving as the fundamental study material for all students.
The NCERT Class 12 English Flamingo Solutions Pdf furnishes crucial inquiries and their corresponding answers, presented in lucid language to foster facile comprehension of the underlying concepts.
These queries and solutions, encapsulated within the pages of NCERT English Flamingo Textbooks, are meticulously composed by subject matter experts in adherence to the guidelines set forth by the NCERT and CBSE boards.
Hence, you’re encouraged to procure chapter-specific NCERT English Solutions Pdf from this platform, thereby establishing a robust foundation in the subject, empowering you to confidently approach all queries posed in both board examinations and other competitive assessments.
12th Class English Book Question Answer Pdf Download
Class 12 English Flamingo NCERT Solutions PDF
- Chapter 1: The Last Lesson
- Chapter 2: Lost Spring
- Chapter 3: Deep Water
- Chapter 4: The Rattrap
- Chapter 5: Indigo
- Chapter 6: Going Places
- Chapter 7: My Mother At Sixty Six
- Chapter 8: An Elementary School Classroom In A Slum
- Chapter 9: Keeping Quiet
- Chapter 10: A Thing Of Beauty
- Chapter 11: Aunt Jennifers Tigers
Class 12 English Vistas NCERT Solutions PDF
- Chapter 1: Third Level
- Chapter 2: The Tiger King
- Chapter 3: The Enemy
- Chapter 4: Journey To The End Of The Earth
- Chapter 5: Should Wizard Hit Mommy
- Chapter 6: On The Face Of It
- Chapter 7: Evans Tries An O level
- Chapter 8: Memories Of Childhood
Convenience at Your Fingertips:
Gone are the days when students had to carry heavy textbooks or search through physical notes to find answers to their queries.
With 12th Class English Book Question-Answer PDFs, all the necessary content is a few clicks away. These PDFs can be downloaded and stored on various devices, enabling students to access them whenever and wherever they require.
This convenience ensures that learning isn’t bound by location or time constraints, facilitating effective self-paced studying.
Comprehensive Learning:
The 12th-grade English curriculum often entails intricate concepts, literary analysis, and language nuances.
Question-answer PDFs derived from the English textbook provide a structured and comprehensive approach to learning.
Each chapter’s set of questions and corresponding answers cater to a holistic understanding of the material. This methodical approach helps students delve deeper into the subject matter, clarifying doubts and building a strong foundation.
Enhanced Retention and Revision:
Retaining information and revising concepts is crucial for academic success. The question-answer format aids in this process.
By reviewing questions and answers periodically, students reinforce their memory of key points, literary elements, and analytical skills.
This active engagement with the content significantly contributes to long-term retention, making it easier to recall information during examinations.
Practice Makes Perfect:
Practice is the cornerstone of proficiency. With downloadable question-answer PDFs, students can engage in extensive practice sessions.
By attempting a diverse range of questions, they hone their critical thinking and problem-solving abilities.
Moreover, the answers provided in the PDFs act as benchmarks, allowing students to assess their performance and identify areas that require further attention.
Preparation for Examinations:
When the 12th-grade examinations approach, students often seek consolidated resources for revision.
The question-answer PDFs serve as invaluable tools during this phase. They condense the entire syllabus into manageable sections, enabling students to systematically review and revise each topic.
Additionally, practicing questions similar to those that might appear in exams fosters a sense of familiarity, reducing anxiety and enhancing confidence.
Inclusivity and Accessibility:
One of the greatest advantages of 12th Class English Book Question-Answer PDFs is their inclusivity.
They break down barriers to learning by being accessible to students of varying learning styles, abilities, and locations.
These resources promote equity in education by providing a level playing field for all students, regardless of their circumstances.
In conclusion, the availability of 12th Class English Book Question-Answer PDFs for download has ushered in a new era of convenience and efficiency in education.
These resources empower students to take charge of their learning, enabling them to navigate the complexities of the curriculum with confidence.
As digital learning continues to evolve, these PDFs stand as a testament to the positive impact technology can have on education.
Frequently Asked Questions – FAQs on 12th Class English Book Question Answer Pdf
1. What are 12th Class English Book Question Answer Pdf?
12th Class English Book Question-Answer PDFs are digital documents that contain a curated set of questions and their corresponding answers derived from the 12th-grade English textbook. These PDFs serve as supplementary study materials, aiding students in comprehending and mastering the curriculum.
2. How can I access and download the 12th Class English Book Question Answer Pdf?
You can access and download 12th Class English Book Question-Answer PDFs from various educational websites, online platforms, or educational forums. Search for the specific textbook and chapter you’re looking for, and you’ll likely find downloadable PDFs for practice and revision.
3. What benefits do 12th Class English Book Question Answer Pdf offer to students?
These PDFs offer several benefits, including convenience, comprehensive learning, enhanced retention, and ample practice opportunities. They provide an organized approach to studying, aiding students in understanding complex concepts, revising effectively, and preparing for exams.
4. Are the questions in these PDFs aligned with the exam pattern?
Yes, many of these question-answer PDFs are designed to align with the exam pattern and question types commonly found in 12th-grade English board examinations. They often include a mix of short answer questions, long answer questions, comprehension passages, and literary analysis questions.
5. Can these PDFs replace the official textbooks?
While 12th Class English Book Question-Answer PDFs are valuable study aids, they are not meant to replace the official textbooks. Textbooks provide the foundational content and context necessary for a comprehensive understanding of the subject. PDFs, on the other hand, offer focused practice and revision.