Category: Class 9
Difference Between Light Microscope And Electron Microscope
Difference Between Light Microscope And Electron Microscope: Microscopes are indispensable tools in the world of science and research, enabling us to explore the microscopic world and uncover intricate details of specimens.
Two primary types of microscopes used for this purpose are the light microscope (LM) and the electron microscope (EM).
Despite their common goal of magnifying objects, these microscopes differ significantly in their principles of operation, capabilities, and the types of specimens they can examine. Here, we highlight the key differences between light microscopes and electron microscopes.

Difference Between Light Microscope And Electron Microscope
1. Principle of Operation:
- Light Microscope (LM): LMs use visible light to illuminate and magnify specimens. They employ glass lenses to bend and focus light, revealing the specimen’s details. LMs are also known as optical or compound microscopes.
- Electron Microscope (EM): EMs use a beam of electrons instead of light to magnify specimens. Electrons have much shorter wavelengths than visible light, allowing for much higher magnification and resolution.
2. Magnification:
- Light Microscope (LM): LMs typically offer magnifications of up to around 1,000 times the specimen’s actual size. They have limitations in achieving higher magnification due to the wavelength of visible light.
- Electron Microscope (EM): EMs can achieve magnifications exceeding 50,000 times, and some advanced EMs can go beyond 2,000,000 times. This exceptional magnification is due to the short wavelength of electrons.
3. Resolution:
- Light Microscope (LM): LMs have limited resolution, typically around 200 nanometers (nm). This means that two points closer than 200 nm will appear as a single point.
- Electron Microscope (EM): EMs offer much higher resolution, often reaching below 0.1 nm. This allows them to reveal fine details of specimens at the atomic and molecular level.
4. Specimen Type:
- Light Microscope (LM): LMs are suitable for viewing living and non-living specimens such as cells, tissues, bacteria, and larger structures. They can observe specimens in their natural, hydrated state.
- Electron Microscope (EM): EMs are used to examine non-living specimens, as the process requires a vacuum. They are ideal for studying ultra-thin sections of cells, viruses, nanomaterials, and inorganic structures.
5. Sample Preparation:
- Light Microscope (LM): Sample preparation for LMs is relatively simple. Specimens are typically mounted on glass slides, often stained to enhance contrast.
- Electron Microscope (EM): EMs require more complex sample preparation, including fixation, dehydration, embedding in resin, and ultra-thin sectioning. Samples must withstand the vacuum and electron beam.
6. Color:
- Light Microscope (LM): LMs provide color images as they use visible light. Stains and dyes can enhance contrast and highlight specific structures.
- Electron Microscope (EM): EMs produce grayscale images since they use electrons. Different shades of gray represent variations in electron density within the specimen.
7. Cost and Accessibility:
- Light Microscope (LM): LMs are generally more affordable and widely accessible in educational and research settings.
- Electron Microscope (EM): EMs are complex and expensive instruments, often found in specialized research institutions and laboratories.

In summary, light microscopes are versatile tools suitable for studying a wide range of biological and non-biological specimens in their natural state, whereas electron microscopes provide unparalleled resolution and are indispensable for detailed examinations of nanoscale structures but require more extensive sample preparation and are primarily used for non-living specimens. The choice between the two depends on the specific research objectives and the nature of the specimens being studied.
Read More
- Molar Mass Of Aluminium
- Molecular Mass Of Glucose
- Latent Heat Of Water
- Difference Between Work And Power
- Difference Between Gravitation And Gravity
Frequently Asked Questions (FAQs) Difference Between Light Microscope And Electron Microscope
1. What is the main difference between a light microscope and an electron microscope?
The primary difference is in the type of radiation used for imaging. Light microscopes use visible light, while electron microscopes use a beam of electrons.
2. How do light microscopes and electron microscopes differ in terms of magnification
Light microscopes typically offer magnifications of up to around 1,000 times the specimen’s actual size, while electron microscopes can achieve magnifications exceeding 50,000 times and even up to 2,000,000 times.
3. What is the resolution difference between these microscopes?
Light microscopes have limited resolution, typically around 200 nanometers (nm), whereas electron microscopes offer much higher resolution, often below 0.1 nm.
4. Can light microscopes be used for studying living specimens?
Yes, light microscopes are suitable for observing living specimens such as cells, tissues, and bacteria in their natural, hydrated state.
5. Are electron microscopes suitable for studying living specimens?
No, electron microscopes require a vacuum environment, making them unsuitable for living specimens. They are primarily used for non-living specimens.
Molar Mass Of Aluminium
Molar Mass Of Aluminium: Aluminium, with its remarkable combination of strength, lightness, and versatility, holds a prominent place in various industrial, technological, and everyday applications.
Understanding the molar mass of Al is essential in chemistry and materials science as it serves as a fundamental parameter for numerous calculations and applications. In this article, we delve into the concept of the molar mass of Al, its significance, and its diverse uses.

Molar Mass Of Aluminium
The Elemental Beauty of Aluminium
Before we explore the molar mass of aluminium, let’s appreciate its elemental properties. Aluminium, represented by the chemical symbol Al, is a silvery-white, non-ferrous metal. It is the third most abundant element in Earth’s crust, making up approximately 8.23% of the Earth’s mass. Despite its prevalence, extracting pure Al from its ores was once a complex and energy-intensive process.
Calculating the Molar Mass of Aluminium
The molar mass of Al is the mass of one mole of Al atoms. To calculate it, we consider the atomic mass of Al, which is approximately 26.98 atomic mass units (amu). One mole of any element contains Avogadro’s number of atoms, which is approximately 6.022 x 10^23 atoms.
Molar Mass of Aluminium = Atomic Mass of Aluminium x Avogadro’s Number
Molar Mass of Al ≈ 26.98 amu x 6.022 x 10^23 atoms/mol
Molar Mass of Al ≈ 26.98 grams per mole (g/mol)
Thus, the molar mass of aluminium is approximately 26.98 g/mol, which means that one mole of Al atoms has a mass of 26.98 grams.
The Significance of Molar Mass
The molar mass of Al is a vital concept in chemistry and materials science, carrying significant implications:
1. Chemical Reactions: Molar mass is used to calculate the amount of Al required or produced in chemical reactions, allowing for precise stoichiometric calculations.
2. Materials Science: Understanding the molar mass helps engineers and materials scientists design and analyze Al alloys and composites for various applications, such as aerospace, automotive, and construction.
3. Aluminum Production: In the Al industry, the determination of molar mass is crucial for process optimization and quality control during smelting and refining.
4. Everyday Life: Aluminium’s lightness and resistance to corrosion make it a popular choice for a wide range of products, from beverage cans to window frames and aircraft parts.
5. Energy Efficiency: The use of aluminium in transportation and construction contributes to energy-efficient designs due to its lightweight properties.
6. Environmental Impact: Recycling aluminium saves energy and resources, reducing the environmental footprint associated with its production.
Conclusion
The molar mass of aluminium, approximately 26.98 g/mol, is a foundational concept in chemistry and materials science. It plays a pivotal role in chemical calculations, materials engineering, and the practical applications of aluminium in modern life.
Whether you’re studying chemical reactions, designing advanced materials, or appreciating the lightweight strength of aluminium in everyday products, a grasp of its molar mass is key to unlocking the full potential of this versatile metal.
From the aerospace industry to your household, aluminium continues to shape our world with its unique combination of properties, making it an indispensable element in the realm of materials science and technology.
Read More
- Molecular Mass Of Glucose
- Latent Heat Of Water
- Difference Between Work And Power
- Difference Between Gravitation And Gravity
- Molecular Weight Of H2SO4
Frequently Asked Questions (FAQs) Molar Mass Of Aluminium
What is the molar mass of aluminium?
The molar mass of Al is approximately 26.98 grams per mole (g/mol). This value represents the mass of one mole of aluminium atoms.
Why is the molar mass of aluminium important in chemistry?
The molar mass of Al is crucial for various chemical calculations, including stoichiometry, determining the amount of Al in compounds, and balancing chemical equations.
How is the molar mass of aluminium calculated?
The molar mass of Al is calculated by considering the atomic mass of Al (approximately 26.98 atomic mass units) and multiplying it by Avogadro’s number, which is approximately 6.022 x 10^23.
What is the significance of aluminium in materials science?
Al is a versatile metal widely used in materials science and engineering due to its lightweight, corrosion resistance, and strength. Its molar mass is crucial for designing and analyzing aluminium alloys and composites for various applications.
How is aluminium produced and refined in the aluminium industry?
The aluminium production process involves extracting Al from bauxite ore through a series of steps, including refining and smelting. The determination of molar mass is essential for process optimization and quality control in Al production.
Molecular Mass Of Glucose
Molecular Mass Of Glucose: Glucose, often referred to as the “fuel” of life, is a fundamental molecule in biology and biochemistry.
Its molecular mass plays a pivotal role in various scientific and practical applications, ranging from nutrition and energy metabolism to pharmaceuticals and clinical diagnostics. In this article, we delve into the molecular mass of C6H12O6, its significance, and its diverse applications.

Molecular Mass Of Glucose
Molecular Formula of Glucose
Before we explore the molecular mass of C6H12O6, it’s essential to understand its molecular formula. Glucose has the chemical formula C6H12O6. This formula represents the number of carbon (C), hydrogen (H), and oxygen (O) atoms in a single glucose molecule.
Calculating Molecular Mass
The molecular mass of a compound is the sum of the atomic masses of all the constituent atoms in its molecular formula. To calculate the molecular mass of C6H12O6, we add the atomic masses of the individual elements:
- Carbon (C) has an atomic mass of approximately 12.01 atomic mass units (amu).
- Hydrogen (H) has an atomic mass of approximately 1.01 amu.
- Oxygen (O) has an atomic mass of approximately 16.00 amu.
Now, let’s calculate the molecular mass of C6H12O6:
Molecular mass of glucose = (6 × C) + (12 × H) + (6 × O) Molecular mass of C6H12O6 ≈ (6 × 12.01 amu) + (12 × 1.01 amu) + (6 × 16.00 amu) Molecular mass of glucose ≈ 72.06 amu + 12.12 amu + 96.00 amu Molecular mass of glucose ≈ 180.18 amu
Rounded to two decimal places, the molecular mass of C6H12O6 is approximately 180.18 amu or 180.18 g/mol (grams per mole).
Significance of Molecular Mass
The molecular mass of glucose holds immense significance in various fields:
1. Nutrition: Glucose is a primary source of energy for the human body. Its molecular mass is used to calculate the calorie content in foods and beverages, providing vital nutritional information.
2. Biochemistry: In cellular metabolism, glucose undergoes glycolysis and other biochemical processes. Understanding its molecular mass is crucial for studying these metabolic pathways.
3. Pharmaceutical and Medical Applications: Glucose serves as a key component in various medications, intravenous solutions, and diagnostic tests. Knowledge of its molecular mass is essential for formulation and dosage calculations.
4. Chemical Reactions: In chemistry, the molecular mass of glucose is used in stoichiometric calculations, chemical reactions, and the determination of molar concentrations in solutions.
5. Industrial Processes: Glucose is utilized in the food industry for sweetening and in fermentation processes for biofuel and alcohol production. Its molecular mass is relevant in industrial calculations.
Glucose in the Human Body
In the human body, glucose is transported through the bloodstream and taken up by cells. It is a primary substrate for cellular respiration, where it is oxidized to produce adenosine triphosphate (ATP), the energy currency of cells. Proper regulation of blood glucose levels is essential for maintaining health.
Conclusion
The molecular mass of glucose, approximately 180.18 g/mol, is a fundamental parameter in nutrition, biochemistry, pharmaceuticals, and numerous scientific disciplines. It underscores the essential role glucose plays as an energy source and its widespread applications in various industries.
Understanding the molecular mass of glucose allows scientists, healthcare professionals, and researchers to make precise calculations and advances in their respective fields, ultimately contributing to our knowledge of life processes and the practical applications of this vital molecule.
Read More
- Latent Heat Of Water
- Difference Between Work And Power
- Difference Between Gravitation And Gravity
- Molecular Weight Of H2SO4
- Distance Time Velocity Time Graph
Frequently Asked Questions (FAQs) Molecular Mass Of Glucose
What is the molecular mass of glucose?
The molecular mass of C6H12O6, also known as the molar mass, is approximately 180.16 grams per mole (g/mol).
What is the molecular formula of glucose?
The molecular formula of glucose is C6H12O6. This formula represents the number of carbon (C), hydrogen (H), and oxygen (O) atoms in a glucose molecule.
How is the molecular mass of glucose calculated?
The molecular mass of C6H12O6 is calculated by summing the atomic masses of all the constituent atoms in its molecular formula. You add the atomic masses of 6 carbon atoms (C), 12 hydrogen atoms (H), and 6 oxygen atoms (O).
Why is the molecular mass of glucose important?
The molecular mass of C6H12O6 is important in chemistry and biology for various calculations, including stoichiometry, chemical reactions, and determining the concentration of C6H12O6 solutions.
How is the molecular mass of a compound used in chemistry?
The molecular mass is used to calculate the number of moles of a substance, which is important in stoichiometric calculations, balancing chemical equations, and understanding the relationships between reactants and products in chemical reactions.
Latent Heat Of Water
Latent Heat Of Water: The latent heat of water is a fundamental concept in thermodynamics and describes the amount of heat energy required to change the phase of water from one state to another while keeping the temperature constant.

Latent Heat Of Water
Phases of Water
- Solid (Ice): In the solid phase, water molecules are arranged in a regular, crystalline structure. Heat must be added to convert ice into liquid water.
- Liquid (Water): In the liquid phase, water molecules are loosely arranged, allowing them to flow. Heat is absorbed when ice melts to become liquid water.
- Gas (Water Vapor): In the gas phase, water molecules are highly disordered and move freely. Heat is required to change liquid water into water vapor (evaporation), and heat is released when water vapor condenses back into liquid water.
Latent Heat of Fusion
The latent heat of fusion () is the amount of heat energy required to change one unit mass of a substance from a solid to a liquid phase (or vice versa) at a constant temperature. For water, the latent heat of fusion is approximately 334,000 joules per kilogram (J/kg). This means that to melt 1 kilogram of ice at 0°C into liquid water at 0°C, approximately 334,000 joules of heat energy must be added.
Latent Heat of Vaporization
The latent heat of vaporization () is the amount of heat energy required to change one unit mass of a substance from a liquid to a gas phase (or vice versa) at a constant temperature. For water, the latent heat of vaporization is approximately 2,257,000 joules per kilogram (J/kg). This means that to vaporize 1 kilogram of liquid water at 100°C into water vapor at 100°C, approximately 2,257,000 joules of heat energy must be added.
Significance and Applications
Understanding the latent heat of water is crucial in various natural and practical scenarios:
- Weather and Climate: Latent heat plays a significant role in weather phenomena. The release of latent heat during the condensation of water vapor in the atmosphere is responsible for cloud formation, precipitation, and the release of energy in thunderstorms.
- Cooking: Latent heat is involved in cooking processes, such as boiling and steaming. Heat is continuously supplied to convert liquid water into water vapor, which cooks food.
- Heating and Cooling Systems: In heating and cooling systems, heat pumps utilize the latent heat of vaporization and fusion to transfer heat efficiently.
- Thermal Comfort: Sweating is a cooling mechanism in humans. When sweat evaporates from the skin, it absorbs latent heat from the body, providing a cooling effect.
- Energy Storage: Latent heat storage systems are used in some renewable energy applications to store excess energy for later use. For example, phase-change materials (PCMs) store and release energy during phase transitions.
- Cryogenics: In cryogenic applications, understanding the latent heat of vaporization is crucial for the liquefaction and storage of gases like nitrogen and oxygen.
In summary, the latent heat of water is a fundamental property that governs phase changes in water and plays a pivotal role in various natural phenomena and practical applications, from weather patterns to everyday cooking and energy storage. It is a key concept in thermodynamics and heat transfer.
Read More
- Difference Between Work And Power
- Difference Between Gravitation And Gravity
- Molecular Weight Of H2SO4
- Distance Time Velocity Time Graph
- Difference Between Kinetics And Kinematics
Frequently Asked Questions (FAQs) Latent Heat Of Water
What is latent heat?
Latent heat is the amount of heat energy absorbed or released during a phase change of a substance (solid to liquid, liquid to gas, etc.) at a constant temperature, without a change in temperature.
What is the latent heat of water?
The latent heats of water refers to the amount of heat energy required or released during phase changes involving water. Specifically, it includes the latent heat of fusion (melting or freezing) and the latent heat of vaporization (evaporation or condensation).
What is the latent heat of fusion for water, and why is it important?
The latent heats of fusion for water is approximately 334,000 joules per kilogram (J/kg). It’s the energy required to change 1 kilogram of ice at 0°C into 1 kilogram of liquid water at 0°C. This process is fundamental in weather patterns, ice melting, and heat transfer in cooling systems.
What is the latent heat of vaporization for water, and why is it significant?
The latent heats of vaporization for water is approximately 2,257,000 joules per kilogram (J/kg). It’s the energy required to change 1 kilogram of liquid water at 100°C into 1 kilogram of water vapor at 100°C. This process is crucial in weather phenomena, cooking, and many industrial processes.
How does latent heat affect weather and climate?
Latent heat is involved in the formation of clouds, precipitation (rain, snow, etc.), and the release of energy during thunderstorms. It plays a vital role in Earth’s climate system by redistributing heat in the atmosphere and oceans.
Difference Between Work And Power
Difference Between Work And Power: Works and power are fundamental concepts in physics, describing how forces and energy are related to the motion and transformation of objects.
While they are related, they represent different aspects of physical processes. Here’s a breakdown of the key differences between works and power:

Difference Between Work And Power
1. Definition:
- Work: Works is a measure of the energy transferred when a force is applied to an object, causing it to move a certain distance in the direction of the force. Mathematically, work (W) is calculated as the product of the force (F) applied to an object and the distance (d) over which the force is applied in the direction of the force’s displacement: W = F × d.
- Power: Power is the rate at which work is done or the rate at which energy is transferred or transformed. It measures how quickly works is performed. Mathematically, power (P) is calculated as the amount of work (W) done divided by the time (t) it takes to do that work: P = W / t.
2. Quantity and Unit:
- Work: Works is a scalar quantity, meaning it has magnitude but no direction. Its standard unit in the International System of Units (SI) is the joule (J), which is equal to one newton-meter (1 N·m).
- Power: Power is a scalar quantity as well, and it measures how fast works is done. Its standard unit in the SI system is the watt (W), which is equal to one joule per second (1 J/s).
3. Time Dependency:
- Work: Works is not explicitly dependent on time. It represents the total energy transfer or transformation that occurs as a result of applying a force over a certain distance.
- Power: Power explicitly involves time. It quantifies the rate at which work is done or energy is transferred, indicating how quickly a task is accomplished.
4. Example:
- Imagine lifting a heavy box vertically upward. The works done would be equal to the force applied (lifting force) multiplied by the distance the box is lifted.
- Power, in this context, would express how quickly you lifted the box. If you lifted it quickly, you exerted more power. If you lifted it slowly, you exerted less power, even though the work done remains the same.
5. Relationship:
- Works and power are related mathematically. If you know the amount of work done and the time it took to do that work, you can calculate the power using the formula: P = W / t.
6. Practical Applications:
- Work: Work is often used to describe tasks or processes that involve the transfer or transformation of energy, such as lifting objects, moving machinery, or performing physical exercises.
- Power: Power is useful in contexts where the rate of energy transfer or the speed of performing a task is important. It is applied in fields like engineering, mechanics, and electricity, especially when measuring the performance of machines or engines.
In summary, work and power are closely related concepts in physics, but they focus on different aspects of physical processes.
Work measures the total energy transfer or transformation, while power quantifies how quickly that work is accomplished. Understanding these concepts is crucial in various scientific and engineering applications.
Read More
- Difference Between Gravitation And Gravity
- Molecular Weight Of H2SO4
- Distance Time Velocity Time Graph
- Difference Between Kinetics And Kinematics
- Changing States Of Matter Class 9
Frequently Asked Question (FAQs) Difference Between Work And Power
What is the fundamental difference between work and power?
The fundamental difference between work and power is that work measures the total energy transferred or transformed when a force is applied to an object over a certain distance, while power measures how quickly that works is done or the rate at which energy is transferred or transformed.
Can you provide an example to illustrate the difference between work and power?
Sure, let’s consider lifting a heavy box. The work done would be the product of the force applied (lifting force) and the distance the box is lifted. However, power would express how quickly you lifted the box. If you lifted it rapidly, you exerted more power, but the work done remained the same. If you lifted it slowly, you exerted less power.
Is work dependent on time?
No, work is not explicitly dependent on time. It represents the total energy transfer or transformation that occurs as a result of applying a force over a certain distance. Time is not a direct factor in calculating work.
How is power related to work mathematically?
Power is related to works through a mathematical formula: Power (P) = Work (W) / Time (t). If you know the amount of works done and the time it took to do that works, you can calculate the power.
What are the units for work and power in the International System of Units (SI)?
In the SI system, the unit for work is the joule (J), which is equivalent to one newton-meter (1 N·m). The unit for power is the watt (W), which is equivalent to one joule per second (1 J/s).
Difference Between Gravitation And Gravity
Difference Between Gravitation And Gravity: Gravitation and gravity are two terms often used interchangeably, but they have distinct meanings and implications in the realm of physics and science.
Understanding the difference between them can help clarify their roles in the universe:

Difference Between Gravitation And Gravity
1. Definition:
- Gravitation: Gravitation is a fundamental force of nature that describes the attraction between all objects with mass or energy. It is a universal force that governs the motion of celestial bodies and objects in the cosmos. Gravitation is responsible for phenomena such as planetary orbits, the falling of objects, and the behavior of galaxies.
- Gravity: Gravity is a specific manifestation of the gravitational force on Earth. It is the force that pulls objects with mass toward the center of the Earth and gives them weight. Gravity is a subset of gravitation, applying specifically to objects near the Earth’s surface.
2. Scope:
- Gravitation: Gravitation applies universally to all objects with mass or energy in the universe. It is responsible for the motion of planets, moons, stars, and galaxies, as well as the behavior of celestial bodies on a cosmic scale.
- Gravity: Gravity specifically applies to objects on or near the Earth. It accounts for the force of attraction that objects experience due to the Earth’s mass. Gravity’s influence extends to the Earth’s immediate vicinity and is responsible for phenomena like falling objects.
3. Examples:
- Gravitation: Gravitation explains the orbits of planets around the Sun, the gravitational interactions between celestial objects, and the formation and behavior of galaxies. It encompasses the entire spectrum of gravitational forces in the universe.
- Gravity: Gravity explains why objects fall when dropped, why we feel weight when standing on the Earth’s surface, and how objects interact with the Earth’s gravitational field. It is the specific force that keeps us grounded.
4. Application Beyond Earth:
- Gravitation: Gravitation operates universally throughout the cosmos. It applies to all celestial bodies and objects in the universe, influencing their behavior and motion.
- Gravity: Gravity is specific to Earth and its immediate surroundings. While other celestial bodies have their own gravitational fields, “gravity” typically refers to the force experienced on Earth.
5. Scientific Study:
- Gravitation: Scientists study gravitation to understand the behavior of celestial bodies, cosmological phenomena, and the structure of the universe. It encompasses a wide range of gravitational interactions on cosmic scales.
- Gravity: Gravity is studied primarily in the context of Earth and its gravitational field. Research on gravity includes understanding its effects on objects and the principles of gravitational acceleration.
In summary, gravitation is the overarching concept of the gravitational force that exists between all objects with mass or energy in the universe. Gravity is the specific manifestation of this force on Earth, responsible for the weight we feel and the motion of objects on our planet. While gravitation is a universal and cosmic force, gravity is the Earth-specific application of that force.
Read More
- Molecular Weight Of H2SO4
- Distance Time Velocity Time Graph
- Difference Between Kinetics And Kinematics
- Changing States Of Matter Class 9
- Average Speed And Average Velocity Class 9
Frequently Asked Question (FAQs) Difference Between Gravitation And Gravity
What is gravitation, and what is gravity?
- Gravitation: Gravitation is a fundamental force of nature that describes the attraction between all objects with mass or energy. It operates universally in the cosmos and governs the behavior of celestial bodies.
- Gravity: Gravity is a specific instance of gravitation that refers to the force of attraction between objects on or near the Earth’s surface due to Earth’s mass. It is a subset of gravitation, applying to Earth-bound scenarios.
Are gravitation and gravity the same thing?
No, they are related but not the same. Gravitation is the general concept of the force of attraction between objects with mass, and it applies universally. Gravity, on the other hand, specifically refers to the gravitational force experienced on or near Earth.
Can you provide an example of how gravitation operates beyond Earth?
Gravitation explains the orbits of planets around the Sun. It also governs the gravitational interactions between celestial objects, such as stars in galaxies, and shapes the structure of the entire universe.
How does gravity work on Earth?
Gravity is the force that pulls objects with mass toward the center of the Earth. It is responsible for objects falling when dropped, and it gives weight to objects based on their mass and the strength of Earth’s gravitational field.
Is there a difference in the scope of gravitation and gravity?
Yes, gravitation has a universal scope and applies to all objects with mass or energy in the universe. Gravity, on the other hand, applies specifically to objects on or near Earth.
Molecular Weight Of H2SO4
Molecular Weight Of H2SO4: The molecular weight of sulfuric acid (H2SO4) can be calculated by adding the atomic masses of its constituent elements:

Molecular Weight Of H2SO4
Calculating the Molecular Weight of H2SO4:
Sulfuric acid (H2SO4) consists of three elements: hydrogen (H), sulfur (S), and oxygen (O). To calculate its molecular weight, we add the atomic masses of these elements based on the chemical formula:
- Hydrogen (H) has an atomic mass of approximately 1.01 g/mol.
- Sulfur (S) has an atomic mass of approximately 32.07 g/mol.
- Oxygen (O) has an atomic mass of approximately 16.00 g/mol.
Now, let’s compute the molecular weight of Sulphuric acid:
Molecular Weight of Sulphuric acid = (2 × Atomic Mass of Hydrogen) + Atomic Mass of Sulfur + (4 × Atomic Mass of Oxygen)
Molecular Weight of Sulphuric acid = (2 × 1.01 g/mol) + 32.07 g/mol + (4 × 16.00 g/mol)
Molecular Weight of Sulphuric acid = 2.02 g/mol + 32.07 g/mol + 64.00 g/mol
Molecular Weight of Sulphuric acid = 98.09 g/mol
So, the molecular weight of sulfuric acid (H2SO4) is approximately 98.09 grams per mole.
Significance of H2SO4’s Molecular Weight:
Understanding the molecular weight of sulfuric acid is of paramount importance for various reasons:
- Stoichiometry: Molecular weight is crucial in chemical calculations, allowing chemists to determine the amount of Sulphuric acid required or produced in chemical reactions. It provides the basis for balancing chemical equations.
- Concentration: In analytical chemistry and laboratory work, the molecular weight of Sulphuric acid is used to calculate the concentration of sulfuric acid solutions accurately. This is vital for preparing solutions of known strength for experiments.
- Industrial Applications: Sulfuric acid is widely used in industries such as manufacturing, metallurgy, and chemical processing. Knowledge of its molecular weight is essential for quality control, production processes, and safety considerations.
- Environmental Impact: Sulfuric acid is a component of acid rain, which can have harmful effects on the environment. Understanding its molecular weight and behavior is crucial for environmental monitoring and mitigation efforts.
- Acid-Base Chemistry: Sulfuric acid is a strong diprotic acid, meaning it can donate two hydrogen ions (H+) per molecule in aqueous solutions. This property is central to acid-base reactions and pH regulation.
Conclusion:
The molecular weight of sulfuric acid (Sulphuric acid), approximately 98.09 g/mol, is a fundamental parameter in the realm of chemistry.
Read More
- Distance Time Velocity Time Graph
- Difference Between Kinetics And Kinematics
- Changing States Of Matter Class 9
- Average Speed And Average Velocity Class 9
- Differences Between Acceleration And Velocity
Frequently Asked Question (FAQs) Molecular Weight Of H2SO4
What is the molecular weight of sulfuric acid (H2SO4)?
The molecular weight of sulfuric acid (H2SO4) is approximately 98.09 grams per mole (g/mol).
Why is knowing the molecular weight of H2SO4 important in chemistry?
Understanding the molecular weight of Sulphuric acid is crucial for various chemical calculations, such as stoichiometry, concentration determination, and balancing chemical equations. It forms the foundation for accurate chemical analysis and synthesis.
How is the molecular weight of H2SO4 calculated?
The molecular weight of Sulphuric acid is calculated by adding the atomic masses of its constituent elements: hydrogen (H), sulfur (S), and oxygen (O). The atomic masses are multiplied by the number of atoms of each element present in one molecule of Sulphuric acid and then summed.
What are some practical applications of sulfuric acid (H2SO4) in industry?
Sulfuric acid is widely used in various industrial processes, including metal processing, fertilizer production, petroleum refining, and chemical manufacturing. It serves as a catalyst, reactant, and acid catalyst in numerous chemical reactions and processes.
How is the concentration of sulfuric acid solutions determined using its molecular weight?
The concentration of sulfuric acid solutions can be determined by calculating the number of moles of Sulphuric acid in a given volume of solution and then using its molecular weight. By dividing the mass of Sulphuric acid by its molecular weight, you can find the number of moles, which allows you to calculate the concentration, often expressed in units like molarity (M).
Distance Time Velocity Time Graph
Distance Time Velocity Time Graph: A “Distance-Time Velocity-Time Graph,” often referred to as a “velocity-time graph” or “speed-time graph,” is a graphical representation that illustrates how an object’s velocity or speed changes over time. Here’s how to understand and interpret such a graph:

Distance Time Velocity Time Graph
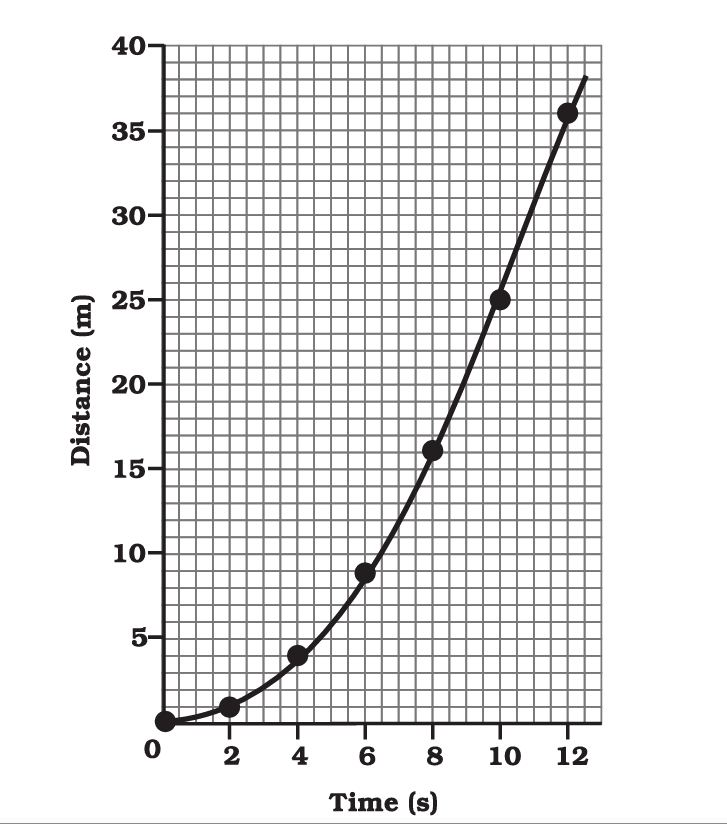
Distance-Time Graph:
- On the horizontal axis (x-axis), you’ll find time, typically measured in seconds (s).
- On the vertical axis (y-axis), you’ll find distance or displacement, typically measured in meters (m) or kilometers (km).
- A straight line on a distance-time graph represents constant velocity or speed. The slope of the line indicates the velocity.
- A horizontal line indicates that the object is at rest (not moving).
- An upward-sloping line indicates positive velocity (moving away from the starting point).
- A downward-sloping line indicates negative velocity (moving toward the starting point).
- Curved lines represent changing velocities or acceleration.
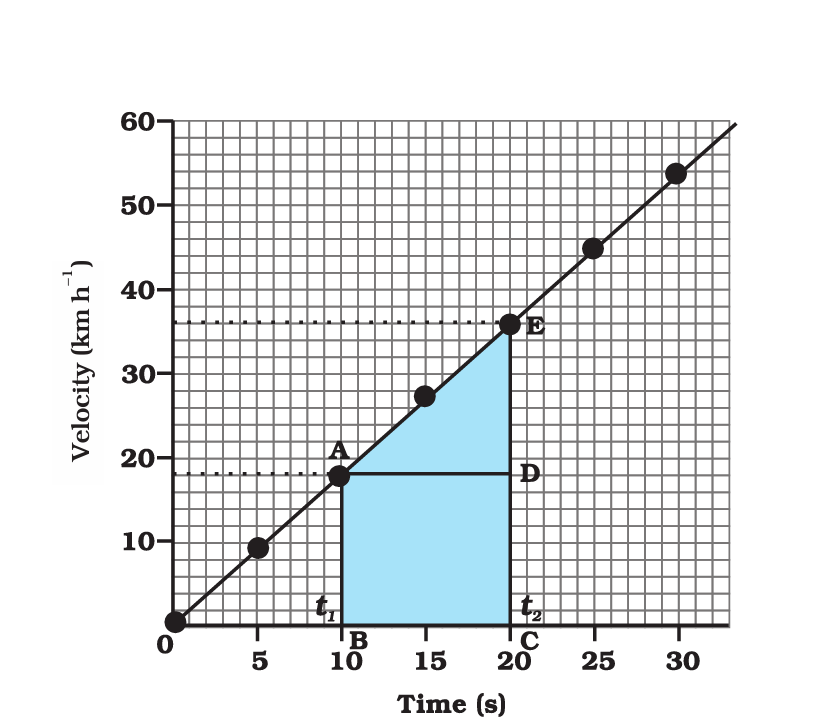
Velocity-Time Graph:
- On the horizontal axis (x-axis), you’ll find time, typically measured in seconds (s).
- On the vertical axis (y-axis), you’ll find velocity or speed, typically measured in meters per second (m/s) or kilometers per hour (km/h).
- A straight line on a velocity-time graph represents constant acceleration or deceleration.
- The slope of the line indicates acceleration (positive or negative) when the line is not horizontal.
- A horizontal line represents constant velocity (no acceleration).
- Areas under the curve represent displacement or distance traveled. The area between the graph and the time axis indicates the total distance covered.
- Points where the velocity-time graph crosses the time axis represent moments when the object is at rest (velocity = 0).
Interpreting the Graphs:
- On a distance-time graph, the steeper the slope, the greater the speed or velocity.
- On a velocity-time graph, the slope indicates acceleration. Positive slopes indicate acceleration in the positive direction, while negative slopes indicate acceleration in the negative direction.
- A horizontal line on a velocity-time graph implies constant speed.
- The area under a velocity-time graph curve represents the change in distance or displacement.
In summary, a distance-time graph shows how an object’s position changes with time, while a velocity-time graph reveals how an object’s velocity or speed changes with time.
Read More
- Average Speed And Average Velocity Class 9
- Differences Between Acceleration And Velocity
- Matter In Our Surroundings Class 9 Summary
- Class 9th Chapter 2 Science Question Answer of NCERT
- NCERT Physics Class 9 Chapter 1 Solutions PDF Download
Frequently Asked Question (FAQs) on Distance Time Velocity Time Graphs:
What is a distance-time graph, and how is it used?
A distance-time graph illustrates how an object’s position or distance changes over time. It’s used to visualize an object’s motion, calculate its speed, and understand whether it is moving, at rest, or changing its speed.
How do you interpret a straight line on a distance-time graph?
A straight line on a distance-time graph represents constant speed. The slope of the line indicates the speed, with a steeper slope representing a greater speed.
What does a horizontal line on a distance-time graph signify?
A horizontal line indicates that the object is at rest or not changing its position with time.
What is a velocity-time graph, and what does it show?
A velocity-time graph depicts how an object’s velocity (speed and direction) changes over time. It provides insights into an object’s acceleration, constant velocity, and moments of rest.
How is acceleration represented on a velocity-time graph?
Acceleration is represented by the slope of a line on a velocity-time graph. A positive slope indicates acceleration in the positive direction, while a negative slope represents acceleration in the negative direction.
Difference Between Kinetics And Kinematics
Difference Between Kinetics And Kinematics: Kinetics and kinematics are both subfields of physics that encompass the study of an object’s motion. While these terms may appear similar, they do exhibit distinct differences.

Difference Between Kinetics And Kinematics
Nature of Study:
Kinematics: Kinematics is the branch of physics that deals with the study of motion of objects without considering the forces causing that motion. It focuses on describing the position, velocity, and acceleration of objects as they move through space and time.
Kinetics: Kinetics, on the other hand, is the branch of physics that delves into the causes of motion. It is concerned with the analysis of the forces, torques, and energy transfers that lead to changes in an object’s motion.
Fundamental Concepts:
Kinematics: Kinematics primarily deals with concepts like displacement, velocity, acceleration, and time. It provides a framework for describing how objects move and change position relative to time.
Kinetics: Kinetics is centered on concepts like force, mass, inertia, work, energy, and momentum. It investigates how these factors interact to produce or change motion.
Representation:
Kinematics: Kinematics is often represented mathematically through equations and graphs that describe an object’s motion in terms of position, velocity, and acceleration as functions of time.
Kinetics: Kinetics involves the use of Newton’s laws of motion, equations of motion, and various mathematical principles to analyze and predict the forces acting on objects and their resulting motion.
Key Questions:
Kinematics: Kinematics answers questions like “What is the object’s position at a given time?” or “How fast is the object moving?” It focuses on the “what” and “how” of motion.
Kinetics: Kinetics addresses questions like “Why is the object accelerating?” or “What force is causing the object to move?” It investigates the underlying causes and the “why” of motion.
Example:
Kinematics: When describing the motion of a car on a highway, kinematics would provide information about the car’s speed, its change in position over time, and how its velocity is changing.
Kinetics: Kinetics, in the context of the same car, would analyze the forces acting on it, such as engine force, friction, and air resistance, to explain why the car is accelerating, decelerating, or maintaining a constant speed.
In summary, kinematics is concerned with describing the motion of objects and their geometric characteristics (position, velocity, acceleration) without considering the underlying forces.
Kinetics, on the other hand, focuses on understanding the forces and interactions that cause objects to move or change their state of motion. Both branches are essential in the study of mechanics and the analysis of physical systems.
Read More
- Changing States Of Matter Class 9
- Average Speed And Average Velocity Class 9
- Differences Between Acceleration And Velocity
- Matter In Our Surroundings Class 9 Summary
- Class 9th Chapter 2 Science Question Answer of NCERT
Frequently Asked Question (FAQs)
What is the primary focus of kinematics?
Kinematics primarily focuses on describing the motion of objects, including parameters such as position, velocity, acceleration, and time, without considering the forces causing that motion.
How does kinetics differ from kinematics in terms of focus?
Kinetics is concerned with understanding the causes of motion, particularly the forces, torques, and energy transfers that lead to changes in an object’s motion.
Can you provide an example illustrating the difference between kinetics and kinematics?
Certainly. Consider a car moving along a road. Kinematics would describe its speed, position changes, and acceleration patterns. Kinetics, on the other hand, would analyze the forces responsible for the car’s motion, such as engine force, friction, and air resistance.
What are some fundamental concepts in kinematics?
In kinematics, fundamental concepts include displacement, velocity, acceleration, and time. These concepts help describe how objects move without delving into the causes of motion.
What key concepts are involved in kinetics?
Kinetics involves concepts like force, mass, inertia, work, energy, and momentum. It investigates how these factors interact to produce or change motion and explores the underlying causes of motion.
Changing States Of Matter
Changing States Of Matter The concept of changing states of matter revolves around the fascinating transformations substances undergo when transitioning from one physical state to another.
Matter, which can exist as solids, liquids, and gases, can shift between these states due to alterations in temperature and pressure. Understanding these transitions requires delving into the behavior of particles at the molecular level.

States of Matter:
- Solid State: In the solid state, particles are tightly packed and maintain a fixed arrangement. They vibrate in place due to their low energy levels. Solids have a defined shape and volume.
- Liquid State: In liquids, particles have more freedom to move. They are still relatively close together, allowing them to slide past each other. Liquids take the shape of their container and have a definite volume.
- Gaseous State: Gases have the highest energy levels among the three states. Particles in gases are widely spaced and move freely. Gases lack a fixed shape or volume and expand to fill their container.
Energy and Transitions:
Changing states of matter primarily involve the transfer of thermal energy. Adding energy to a substance causes its particles to gain kinetic energy, increasing their motion and interactions. Conversely, removing energy results in decreased motion and interactions.
Transitions Explained:
- Melting: When a solid is heated, it gains energy. At a specific temperature known as the melting point, the particles’ kinetic energy becomes strong enough to break the bonds that hold them in a fixed structure. The substance transitions from a solid to a liquid.
- Freezing: Conversely, when a liquid is cooled, it loses energy. As the particles lose kinetic energy, their motion slows down, allowing attractive forces between them to bring them closer together. The substance transitions from a liquid to a solid through freezing.
- Evaporation and Boiling: In a liquid, particles have varying kinetic energies. Some particles at the surface with higher energy can overcome the attractive forces and enter the gas phase. This process, called evaporation, gradually occurs at any temperature. Boiling, however, is rapid vaporization that happens at a specific temperature (boiling point) throughout the liquid.
- Condensation: When a gas loses energy (often through cooling), its particles slow down and come closer together. This allows attractive forces to take over, and the gas transitions into a liquid through condensation.
- Sublimation and Deposition: Sublimation involves the direct transition of a solid into a gas without passing through the liquid state. Deposition is the reverse process, where gas particles lose energy and transition directly to a solid.
Changes in States of Matter: A Deeper Exploration
The world around us is in a constant state of flux, and one of the most intriguing aspects is the changing states of matter. These transitions, from solid to liquid, liquid to gas, and vice versa, are not mere random occurrences but intricate processes driven by the interactions between particles and the energy they possess.
Kinetic Theory and Particle Behavior:
At the heart of understanding changing states of matter lies the kinetic theory. This theory explains the behavior of particles in different states:
Solids: In solids, particles are tightly packed and exhibit minimal movement. They vibrate in place due to thermal energy, but their positions remain relatively fixed.
Liquids: Liquid particles have more energy than their solid counterparts. They can move around, sliding past each other. This mobility allows liquids to flow and take the shape of their containers.
Gases: Gas particles possess the most energy, leading to rapid and chaotic movement. They are widely spaced and have the freedom to move in all directions.
Energizing Transitions:
Melting and Freezing: When you heat a solid, you’re infusing energy into its particles. As they gain kinetic energy, they overcome their fixed positions, and the solid turns into a liquid in a process called melting. Conversely, cooling a liquid causes particles to lose energy. They slow down and arrange themselves in a fixed pattern, forming a solid through freezing.
Evaporation and Condensation: In a liquid, particles with higher energy are at the surface. Evaporation occurs when these energetic particles escape the attractive forces of the liquid and become a gas. Condensation, the reverse process, takes place when gas particles lose energy and revert to the liquid state.
Boiling: Boiling is an intense form of evaporation that happens throughout the liquid. It occurs when the vapor pressure of the liquid matches the atmospheric pressure.
Sublimation and Deposition: Some substances, like dry ice (solid carbon dioxide), can transition directly from a solid to a gas without passing through the liquid phase, a phenomenon known as sublimation. The reverse process, deposition, involves gas turning directly into a solid.
Energy Source: Thermal Energy:
In most cases, thermal energy in the form of heat drives these changes. The more thermal energy particles have, the faster they move and the farther they spread apart. This energy disrupts the attractive forces between particles, enabling them to change states.
Applications and Significance:
Understanding these state changes is essential in various fields. It’s the basis for weather phenomena like clouds and rain, industrial processes like distillation, and even our day-to-day experiences, like the feeling of warmth when wet clothes dry on a sunny day.
Read More
- Average Speed And Average Velocity Class 9
- Differences Between Acceleration And Velocity
- Matter In Our Surroundings Class 9 Summary
- Class 9th Chapter 2 Science Question Answer of NCERT
- NCERT Physics Class 9 Chapter 1 Solutions PDF Download
Conclusion:
Changing states of matter illustrate the dynamic nature of substances and the impact of energy on their behavior. These transitions play a pivotal role in our understanding of various natural phenomena and technological applications, from the water cycle to industrial processes.
Frequently Asked Question (FAQs)
What are the different states of matter?
Matter exists in three primary states: solid, liquid, and gas. These states are characterized by the arrangement and movement of particles within the substance.
How do substances change from one state to another?
Substances change from one state to another by gaining or losing energy. This energy exchange, often in the form of heat, affects the motion and interactions of particles, causing them to transition between states.
What happens during melting and freezing?
Melting is the process in which a solid substance gains enough energy to overcome the forces holding its particles together, resulting in the transition to a liquid state. Freezing, on the other hand, occurs when a liquid loses enough energy to slow down its particles, leading to a transition to a solid state.
What is evaporation, and how does it differ from boiling?
Evaporation is the gradual conversion of liquid particles into gas due to the high-energy particles at the surface escaping the liquid’s attractive forces. Boiling is a rapid form of evaporation that occurs throughout the liquid and requires the liquid’s vapor pressure to match the atmospheric pressure.
Can gases change directly into solids or vice versa?
Yes, this phenomenon is known as sublimation and deposition. Sublimation involves a solid changing directly into a gas without passing through the liquid state. Deposition is the reverse process, where gas particles lose energy and transition directly to a solid.