Category: Class 9
Matter In Our Surroundings Class 9 Sample Paper With Answers
Matter In Our Surroundings Class 9 Sample Paper With Answers: “Matter in Our Surroundings” is a fundamental topic in the field of science that serves as a stepping stone for understanding the nature and properties of matter.
Addressed in the curriculum of Class 9, this subject delves into the intriguing world of materials that make up our environment – from the air we breathe to the substances that compose everyday objects.
Exploring the diverse states of matter, including solids, liquids, and gases, as well as the underlying principles of physical changes such as evaporation, condensation, and sublimation, this study is pivotal in comprehending the behavior and transformations of matter.

Through this exploration, students are introduced to the microscopic realm of particles, helping them grasp the essence of kinetic theory and its application to various real-world scenarios.
The study of “Matter in Our Surroundings” not only nurtures scientific curiosity but also forms the foundation for more advanced concepts in chemistry and physics, making it an integral and captivating component of the Class 9 curriculum.
In this article, we going to discuss Matter In Our Surroundings Class 9 Sample Paper With Answers. This will help you in your exam preparation.
Matter In Our Surroundings Class 9 Sample Paper With Answers (Q 1 – Q 10)
1. Why do we see water droplets collected on the outer surface of a glass container containing ice?
The water droplets form due to condensation. When the cold surface of the glass container comes into contact with the warmer air, the air’s moisture condenses into liquid water droplets on the glass’s outer surface.
2. Explain why solids have a fixed shape but liquids and gases do not have a fixed shape.
Solids have a fixed shape because their particles are tightly packed and held in a fixed arrangement by strong forces of attraction. In liquids and gases, particles have more freedom of movement and weaker intermolecular forces, allowing them to flow and take the shape of their container.
3. Why is it advisable to use a pressure cooker at higher altitudes?
At higher altitudes, atmospheric pressure is lower, causing water to boil at a lower temperature. Using a pressure cooker increases the cooking temperature and helps food cook faster despite the lower boiling point.
4. What are fluids?
Fluids are substances that can flow and do not have a fixed shape. They include both liquids and gases.
5. Why is water a liquid at room temperature?
Water is a liquid at room temperature due to its intermolecular forces. Although its molecules can move freely, they still have enough attraction to maintain a liquid state at typical room temperatures.
6. Cotton is solid but it floats on water. Why?
Cotton’s low density and structure with air pockets make it less dense than water, causing it to float. The air trapped within the fibers adds buoyancy.
7. Why are solids generally denser than liquids and gases?
Solids have particles closely packed in a regular arrangement, leading to higher density. In liquids and gases, particles have more space between them, resulting in lower density.
8. Name the factors that affect evaporation.
Factors affecting evaporation include temperature, surface area, humidity, and wind speed. Higher temperature, larger surface area, lower humidity, and higher wind speed accelerate evaporation.
9. How is the high compressibility property of gas useful to us?
The high compressibility of gases allows them to be easily compressed into smaller volumes. This property is utilized in applications like inflating tires, airbags, and gas storage tanks.
10. With the help of an example, explain how the diffusion of gases in water is essential?
Oxygen dissolves in water, allowing aquatic organisms to respire. For instance, fish extract dissolved oxygen from water through their gills for respiration.
Matter In Our Surroundings Class 9 Sample Paper With Answers (Q 11 – Q 20)
11. On a hot sunny day, why do people sprinkle water on the roof or open ground?
Sprinkling water increases humidity and cools the surroundings through the process of evaporation, providing relief from the heat.
12. Why do people perspire a lot on a hot humid day?
Humid conditions slow down evaporation, causing the body’s sweat to linger on the skin. This leads to a feeling of increased perspiration, even if the body is not actually producing more sweat.
13. A balloon when kept in the sun bursts after some time. Why?
The sun’s heat causes the air inside the balloon to expand, increasing the pressure. If the pressure becomes too high, it can exceed the balloon’s strength, causing it to burst.
14. Pressure and temperature determine the state of a substance. Explain this in detail.
Pressure and temperature affect the intermolecular forces and motion of particles. At higher temperatures, particles move more and can overcome forces to change states. Higher pressure can compress gases into liquids or solids.
15. Explain, giving examples, the various factors on which the rate of evaporation depends.
The rate of evaporation depends on temperature (higher temperature leads to faster evaporation), surface area (larger area increases the rate), humidity (lower humidity increases the rate), and air movement (wind enhances evaporation). For example, wet clothes dry faster on a hot, windy day than on a cool, humid day.
16. What is matter?
Matter is anything that occupies space and has mass. It is composed of tiny particles called atoms and molecules.
17. What are the three states of matter?
The three states of matter are solids, liquids, and gases.
18. Define evaporation and give an example.
Evaporation is the process by which a liquid changes into a vapor or gas at temperatures below its boiling point. An example is water evaporating from a wet clothesline.
19. What is sublimation?
Sublimation is the process in which a solid directly changes into a gas without passing through the liquid state. Dry ice (solid carbon dioxide) is an example of sublimation.
20. Explain the concept of the boiling point.
The boiling point is the temperature at which a liquid changes into a gas throughout the bulk of the liquid. At this temperature, the vapor pressure of the liquid equals the atmospheric pressure.
Matter In Our Surroundings Class 9 Sample Paper With Answers (Q 21 – Q 27)
21. What is the difference between a physical change and a chemical change?
In a physical change, the substance’s identity remains the same even though its appearance may change. In a chemical change, new substances with different properties are formed.
22. Define diffusion. Answer:
Diffusion is the process of movement of particles from an area of higher concentration to an area of lower concentration, resulting in their even distribution.
23. Explain the concept of the kinetic theory of matter.
The kinetic theory states that all matter consists of tiny particles (atoms or molecules) in constant motion. The temperature of a substance is related to the average kinetic energy of its particles.
24. Why do gases exert pressure on their containers?
Gas particles are in constant random motion. When they collide with the walls of their container, they exert pressure.
25. How does increasing the temperature affect the rate of evaporation?
Increasing the temperature increases the kinetic energy of particles, leading to more rapid movement and more frequent escape from the liquid’s surface, thus increasing the rate of evaporation.
26. What is humidity?
Humidity refers to the amount of water vapor present in the air.
27. Why do solids have a definite shape and volume?
In solids, the particles are closely packed and held in a fixed position by strong intermolecular forces, which is why they have a definite shape and volume.
Long Type of Questions on Matter In Our Surroundings Class 9 Sample Paper With Answers
Q 1. Explain the difference between physical change and chemical change.
Certainly, here’s a concise explanation of the difference between physical change and chemical change:
Physical Change:
A physical change is a transformation in which the substance’s state, appearance, or form alters without changing its chemical composition. During a physical change, the substance’s molecules or atoms reorganize themselves, but the fundamental nature of the substance remains the same.
Examples:
– Melting ice to form water: The molecules of water remain the same; only the arrangement changes from a solid to a liquid state.
– Cutting a piece of paper: The paper’s composition remains unchanged; it’s just broken into smaller pieces.
Chemical Change:
A chemical change, also called a chemical reaction, results in the formation of new substances with different chemical compositions and properties. During a chemical change, the bonds between atoms or molecules are broken and rearranged to create entirely new substances.
Examples:
– Burning wood: Wood reacts with oxygen to produce carbon dioxide, water, and ash. The original wood is chemically transformed into these new substances.
– Rusting of iron: Iron reacts with oxygen and moisture to form iron oxide (rust), altering the chemical composition of the iron.
In summary, physical changes involve alterations in the physical properties of a substance, such as state or appearance, while the substance’s chemical composition remains unchanged. Chemical changes involve the creation of new substances with different properties due to the rearrangement of atoms or molecules, resulting in a change in chemical composition.
Q 2. Discuss the process of sublimation with relevant examples.
Sublimation is the process in which a substance transitions directly from its solid state to its gaseous state without passing through the liquid state. This occurs when the vapor pressure of the solid becomes equal to the atmospheric pressure. Sublimation is less common than other phase changes like melting, freezing, evaporation, or condensation, but it’s still an important phenomenon with various practical applications.
Examples of Sublimation:
1. Dry Ice (Solid Carbon Dioxide): Dry ice is a well-known example of sublimation. At normal atmospheric pressure, dry ice sublimes directly from a solid to a gas at around -78.5°C (-109.3°F). It’s often used in theatrical fog effects and to preserve frozen foods.
2. Naphthalene Balls: Naphthalene balls, often used as moth repellents, exhibit sublimation. Over time, they gradually evaporate without leaving a liquid residue, as they undergo sublimation from solid to gas.
3. Iodine: Iodine crystals can sublime at room temperature. When exposed to air, solid iodine slowly changes into a purple vapor, bypassing the liquid state. This property is used to demonstrate sublimation in laboratory experiments.
4. Camphor: Camphor is another substance that sublimes. Solid camphor gradually transforms into a vapor, releasing its characteristic odor. This property is utilized in traditional practices and rituals.
5. Freeze-Drying: In the food industry, freeze-drying is a process that involves freezing a substance and then sublimating the frozen water content, leaving behind a dry product. This method is used to preserve the flavor and nutritional value of various foods.
6. High-Altitude Snow and Ice: In regions with high altitudes and low atmospheric pressure, snow and ice can undergo sublimation directly into water vapor without melting into liquid water first.
In summary, sublimation is the phase transition from a solid directly to a gas without passing through the liquid state. This process is exhibited by various substances, including dry ice, naphthalene, iodine, and camphor, and it has practical applications in industries such as food preservation and laboratory techniques.
Q 3. What is the critical temperature and critical pressure of a substance?
The critical temperature and critical pressure are specific thermodynamic properties of a substance that mark the conditions at which the substance transitions from its gas phase to its liquid phase without any distinction between the two phases. These properties are important in understanding a substance’s behavior under extreme conditions.
- Critical Temperature (Tc): The critical temperature is the highest temperature at which a substance can exist in its liquid state, regardless of the pressure applied. Above this temperature, no amount of pressure will cause the substance to liquefy; it will remain in the gaseous state. It is essentially the temperature beyond which the distinction between gas and liquid phases disappears.
- Critical Pressure (Pc): The critical pressure is the minimum pressure required to liquefy a substance at its critical temperature. Below the critical pressure, the substance cannot be liquefied, no matter how low the temperature is.
These critical properties are often depicted on a phase diagram, which is a graph that shows the relationships between temperature, pressure, and the different phases (solid, liquid, and gas) of a substance. The critical point is the specific point on the phase diagram where the critical temperature and critical pressure intersect.
For example, water’s critical temperature is approximately 374°C (647.1°F), and its critical pressure is about 218 atmospheres. This means that if you were to try to liquefy water at temperatures above 374°C (no matter how high the pressure), or if you were to try to pressurize water at pressures below 218 atmospheres (no matter how low the temperature), it would remain in the gas phase.
Understanding the critical temperature and critical pressure of a substance is important in various scientific and industrial applications, such as in the design of supercritical fluid extraction processes and in predicting the behavior of substances at extreme conditions.
Read Also
- Class 9 Science Chapter 1 Matter in Our Surroundings Notes
- CBSE Previous Year Question Papers Class 9 Maths with Solutions
- Sample Question Paper for Class 9 CBSE Hindi Course B
- MCQ for Class 9 Science Chapter 1 Matter in Our Surroundings
Importance of Matter In Our Surroundings Class 9 Sample Paper With Answers
Following are the benefits of Matter In Our Surroundings Class 9 Sample Paper With Answers
- Familiarity with Exam Format: Sample papers expose students to the format and types of questions they might encounter in exams, reducing anxiety and improving confidence.
- Concept Clarity: By attempting sample papers and comparing their answers with the provided solutions, students can identify areas where their understanding might be lacking and seek further clarification.
- Application of Knowledge: Sample papers require students to apply their knowledge to specific scenarios and problems, helping them practice critical thinking and problem-solving skills.
- Time Management: Sample papers help students practice time management during exams by providing a simulated exam environment where they need to allocate time for each question.
- Self-Assessment: After attempting sample papers, students can assess their own performance by comparing their answers with the provided solutions. This self-assessment helps them understand their strengths and weaknesses.
- Targeted Revision: Based on their performance on sample papers, students can focus their revision on areas where they need improvement.
- Confidence Building: Successfully completing sample papers can boost students’ confidence and reduce exam-related stress, as they have already practiced similar questions.
- Holistic Learning: Sample papers cover various aspects of the topic, promoting a well-rounded understanding of “Matter in Our Surroundings.”
Frequently Asked Questions – FAQs
What are the three states of matter, and how do they differ from each other?
The three states of matter are solids, liquids, and gases. Solids have a definite shape and volume, liquids have a definite volume but take the shape of their container, and gases have neither a definite shape nor volume.
How does evaporation occur, and what factors influence the rate of evaporation?
Evaporation occurs when the particles at the surface of a liquid gain enough energy to escape into the gas phase. Factors affecting the rate of evaporation include temperature, surface area, humidity, and wind speed.
Explain sublimation with examples. How is sublimation different from evaporation?
Sublimation is the direct transformation of a solid into a gas without passing through the liquid phase. Examples include dry ice and naphthalene balls. Unlike evaporation, which occurs only at the surface, sublimation involves the entire solid transforming into a gas.
Class 9 Science Chapter 1 Matter in Our Surroundings Notes
If you are class 9th student then this article “Class 9 Science Chapter 1 Matter in Our Surroundings Notes” is very important for you.
Matter encompasses anything possessing mass and occupying space. This includes substances like hydrogen and oxygen, sugar and sand, as well as air and water.
Matter is composed of tiny, diminutive particles. These particles are drawn together due to the attractive forces between them, despite the gaps that exist between them.

Class 9 Science Chapter 1 Matter in Our Surroundings Notes
NCERT Class 9 Science Chapter 1 Matter in Our Surroundings Notes
States of Matter
As per Class 9 Science Chapter 1 Matter in Our Surroundings Notes matter finds its classification as solid, liquid, or gas dependent on the interactions between particles and the manner in which they are arranged.
The transformation between these three states of matter can be achieved by adjusting pressure and temperature. For instance, by elevating the temperature, solid ice can transition into a liquid state.
| Property | Solid | Liquid | Gas |
|---|---|---|---|
| Shape and volume | Fixed shape and volume | No fixed shape but has volume | Neither definite shape nor volume |
| Energy | Lowest | Medium | Highest |
| Compressibility | Difficult | Nearly difficult | Easy |
| Arrangement of molecules | Regular and closely arranged | Random and little sparsely arranged | Random and more sparsely arranged |
| Fluidity | Cannot flow | Flows from higher to lower level | Flows in all directions |
| Movement | Negligible | Depends on interparticle attraction | Free, constant and random |
| Interparticle space | Very less | More | Large |
| Interparticle attraction | Maximum | Medium | Minimum |
| Density | Maximum | Medium | Minimum |
| Rate of diffusion | Negligible | It depends on interparticle attraction. | Maximum |
Atomic View of the Three States of Matter
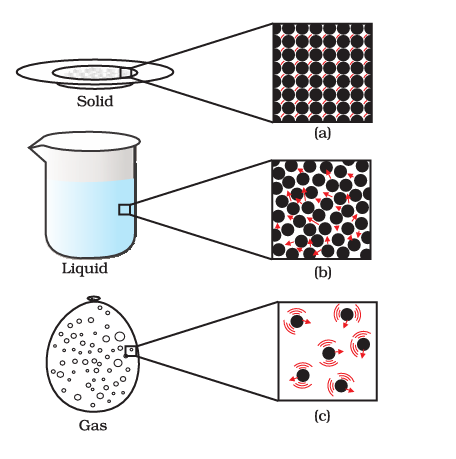
Physical Nature of Matter
A physical property denotes a facet of matter that is observable or quantifiable without altering its inherent character or composition.
Such properties remain unaffected by the quantity of matter in question. Examples of physical properties encompass visual attributes, coloration, scent, density, texture, melting and boiling points, solubility, and more.
Characteristics of Particles of Matter
Matter
Matter constitutes entities possessing mass and taking up space.
All that we can physically sense – touch, sight, hearing, taste, and even smell – falls under the category of matter.
Matter is comprised of incredibly minute particles that remain invisible to the naked eye.
The constitution of matter’s particles shapes its state and attributes, encompassing both physical and chemical aspects.
1. Particles within matter exhibit gaps between them.
This property underlies the concept of substances dissolving in others. For instance, when sugar dissolves in water, the water level remains unchanged because the sugar particles fit into the spaces between the water particles.
2. Matter’s particles are in constant motion.
Continuous and random movements result from the particles’ kinetic energy. Elevating the temperature increases their kinetic energy, intensifying their motion.
3. Particles of matter mutually attract each other.
Interparticle forces of attraction operate within every substance. These forces must be overcome to break a substance apart. The strength of this force varies among different substances.
Diffusion
The occurrence where matter’s particles spontaneously blend with one another is termed diffusion. An instance of this is the dispersion of ink in water.
Throughout diffusion, particles take up the interparticle gaps.
The pace of diffusion escalates as temperature rises, a result of heightened kinetic energy in the particles.
Can Matter Change Its State?
Effect of Change of Temperature on the State of Matter
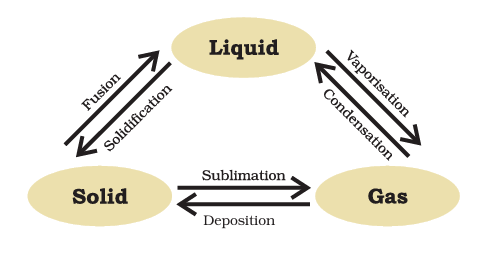
As temperature rises, the kinetic energy within matter’s particles escalates, leading to heightened vibrational energy. Consequently, the interparticle attractive force diminishes, causing particles to disengage from their positions and move with increased freedom.
- Consequently, the state of matter undergoes transformation.
- Solids transition to liquids through a phase change.
- Likewise, liquids experience phase changes to become gases.
Melting Point
The temperature at which a solid transforms into a liquid under atmospheric pressure is known as its melting point.
At the melting point, there exists an equilibrium between these two phases, namely solid and liquid. This means that both the solid and liquid states coexist simultaneously at this specific temperature.
Fusion
Nuclear fusion is a process where two atomic nuclei come together to form a heavier nucleus, releasing a significant amount of energy in the process.
This is the process that powers stars like our Sun, where hydrogen atoms combine to form helium through fusion, releasing a tremendous amount of energy in the form of light and heat.
In a broader context, fusion can also refer to the merging or joining of different elements or entities to create something new.
Class 9 Science Chapter 1 Matter in Our Surroundings Notes
Boiling Point
The boiling point of a substance is the temperature at which it transitions from its liquid phase to its gaseous phase under a specific pressure, typically atmospheric pressure.
At this temperature, the vapor pressure of the liquid equals the atmospheric pressure, allowing bubbles of vapor to form within the liquid and escape into the air, causing the liquid to boil.
The boiling point is a characteristic property of a substance and can vary depending on the atmospheric pressure.
Latent Heat of Fusion
The latent heat of fusion refers to the amount of heat energy required to change a substance from its solid state to its liquid state without a change in temperature.
This energy is used to overcome the forces of attraction between the particles within the solid, allowing them to transition into a more disordered arrangement in the liquid phase.
During the process of fusion, or melting, the substance absorbs energy without a temperature increase. Once the particles are in the liquid state, they have higher energy due to the additional heat energy provided.
The reverse process, where a liquid turns into a solid, also releases the same amount of latent heat of fusion. This phenomenon is crucial in understanding the energy exchange involved in phase transitions and is specific to each substance.
Latent Heat of Vaporisation
The latent heat of vaporization refers to the amount of heat energy needed to transform a substance from its liquid state to its gaseous state at a constant temperature and pressure.
Unlike the process of raising the temperature of a substance, during which its temperature increases, the latent heat of vaporization occurs at a consistent temperature while the substance changes its phase from liquid to gas.
When a substance vaporizes, such as water turning into steam, it absorbs energy to break the intermolecular forces holding the liquid together and allow its molecules to transition into the gas phase.
This energy is later released when the gas condenses back into a liquid. The latent heat of vaporization is a critical factor in processes like evaporation, boiling, and condensation and varies for different substances.
Sublimation
Sublimation is a phase transition process in which a substance directly transforms from its solid state to its gaseous state without passing through the intermediate liquid phase.
This occurs when the pressure and temperature conditions are such that the substance’s vapor pressure at its solid phase is greater than the atmospheric pressure.
During sublimation, the solid substance absorbs heat energy, causing its particles to gain enough energy to break the intermolecular forces holding them together.
These particles then transition into the gaseous state without first becoming a liquid. Dry ice (solid carbon dioxide) sublimating into carbon dioxide gas is a common example of this phenomenon.
Sublimation is distinct from other phase transitions like melting and boiling, which involve changes between solid and liquid or liquid and gas phases, respectively.
Effect of Change in Pressure on the State of Matter
A change in pressure can have a significant impact on the state of matter, particularly for gases. The behavior of solids and liquids is less affected by pressure changes compared to gases due to their relatively higher intermolecular forces and closely packed structures.
For gases:
1. Increase in Pressure: When the pressure on a gas is increased, the gas molecules are forced closer together. This can lead to a change in the state of the gas. For example, at high pressures, gases that are normally in a gaseous state might become liquid or solid. This is known as “condensation” or “liquefaction.”
2. Decrease in Pressure: Lowering the pressure on a gas allows its molecules to spread out more. If the pressure drops significantly, gases can change from a liquid to a gas (vaporization) or from a solid to a gas (sublimation) without passing through the liquid phase.
3. Phase Diagrams: The relationship between pressure and temperature for different states of matter can be represented on a phase diagram. Phase diagrams show the conditions under which a substance exists as a solid, liquid, or gas. Critical points, where the distinction between gas and liquid phases becomes less clear, can also be shown on these diagrams.
It’s important to note that the effects of pressure changes are more pronounced at low temperatures and become less significant at higher temperatures. Additionally, not all substances behave the same way with changes in pressure; each substance has its own unique phase behavior.
Evaporation
According to Class 9 Science Chapter 1 Matter in Our Surroundings Notes Evaporation is a process through which a liquid substance transforms into a gaseous state at temperatures below its boiling point. Unlike boiling, which occurs throughout the liquid, evaporation takes place only at the liquid’s surface.
During evaporation, molecules with higher kinetic energy near the liquid’s surface gain enough energy to break the intermolecular forces holding them within the liquid. These molecules then escape into the air as vapor. This process leads to cooling of the remaining liquid due to the energy loss caused by the escaping molecules.
Evaporation occurs naturally and continuously, even at temperatures below the boiling point. Factors like temperature, surface area, humidity, and air movement influence the rate of evaporation.
For example, on a hot day, clothes dry faster because the increased temperature speeds up the evaporation of water from the fabric. Evaporation plays a significant role in the water cycle, as water bodies continuously lose water to the atmosphere through this process.
Factors Affecting Evaporation
In Class 9 Science Chapter 1 Matter in Our Surroundings Notes, Several factors that influence the rate of evaporation are discussed:
- Temperature: Higher temperatures provide more energy to the molecules in the liquid, increasing their kinetic energy and the likelihood of escaping the liquid’s surface. As a result, higher temperatures generally lead to faster evaporation.
- Surface Area: A larger exposed surface area allows more liquid molecules to come into contact with the air, promoting faster evaporation. This is why liquids in wider containers tend to evaporate more quickly.
- Air Movement: Air movement, often referred to as wind, helps carry away the vapor molecules that have escaped the liquid’s surface. This reduces the concentration of vapor near the liquid and enhances evaporation.
- Humidity: Humidity is the amount of moisture already present in the air. When the air is humid, it’s already saturated with moisture, which makes it more difficult for the vapor from the liquid to be absorbed by the air. In drier air, evaporation happens more efficiently.
- Pressure: Lower pressure environments can facilitate faster evaporation. This is why liquids tend to evaporate more quickly at higher altitudes where the atmospheric pressure is lower.
Cooling Due to Evaporation
In the process of evaporation, the particles within a liquid gather energy from their environment to surmount the intermolecular forces that bind them together, leading to a change in phase. This act of absorbing heat from the surroundings causes a cooling effect on those surroundings.
A familiar example is the cooling sensation we experience through sweating, where the body’s moisture evaporates, taking away heat energy and cooling the body.
What Applications of Evaporative Cooling Described in Class 9 Science Chapter 1 Matter in Our Surroundings Notes
Water is often stored in earthenware containers to maintain its cool temperature. Much like the pores found in cotton fabric, the pores on the surface of earthen pots facilitate enhanced evaporation. This process aids in keeping the water’s temperature down.
For our own comfort, we sweat profusely to cool down our bodies. In essence, sweating is a form of evaporation. As the water on our skin evaporates, it consumes energy and thereby reduces our body temperature.
In the summer, we commonly opt for cotton clothing. Cotton possesses a remarkable ability to absorb water, allowing greater contact between the sweat on our skin and the air. This encourages more efficient evaporation. Consequently, wearing cotton clothing contributes to a cooling sensation due to the enhanced evaporative effect it creates.
Read Also:
- CBSE Previous Year Question Papers Class 9 Maths with Solutions
- Sample Question Paper for Class 9 CBSE Hindi Course B
- Matter In Our Surroundings Class 9 Sample Paper With Answers
- MCQ for Class 9 Science Chapter 1 Matter in Our Surroundings
Frequently Asked Questions – FAQs on Class 9 Science Chapter 1 Matter in Our Surroundings Notes
What is the main focus of Class 9 Science Chapter 1 Matter in Our Surroundings Notes?
Chapter 1, “Matter in Our Surroundings,” primarily explores the different states of matter, their characteristics, and the factors that influence their behavior.
What are the three states of matter discussed in Class 9 Science Chapter 1 Matter in Our Surroundings Notes?
The three states of matter covered in this chapter are solids, liquids, and gases. Their properties, behavior, and phase changes are explained.
How is the concept of diffusion explained in Class 9 Science Chapter 1 Matter in Our Surroundings Notes?
Diffusion, the process of particles spreading out in a substance, is elaborated upon. It discusses how gases and liquids diffuse and factors affecting the rate of diffusion.
What is evaporation, and how is it relevant to the chapter?
Evaporation, the conversion of a liquid into a gas at temperatures below its boiling point, is discussed. Its role in cooling, sweat’s cooling effect, and its relevance to the water cycle are explained.
How does the Class 9 Science Chapter 1 Matter in Our Surroundings Notes discuss the relationship between temperature and the state of matter?
The chapter explains how changing temperature affects the state of matter. It covers concepts such as melting, boiling, and the effect of temperature on the kinetic energy of particles.
CBSE Previous Year Question Papers Class 9 Maths with Solutions
CBSE Previous Year Question Papers Class 9 Maths with Solutions: When it comes to acing the Central Board of Secondary Education (CBSE) Class 9 Mathematics exam, practice and familiarity with the exam pattern play a crucial role.
One of the most effective tools for preparation is the utilization of CBSE previous year question papers with solutions.
These papers offer students a comprehensive insight into the exam format, marking scheme, and the types of questions that are likely to be asked. In this article, we will delve into the significance of using CBSE Class 9 Maths previous year question papers with solutions as a part of your study strategy.

CBSE Previous Year Question Papers Class 9 Maths with Solutions
- Maths Class 9 Previous year Question Paper 2020
- Maths Class 9 Previous year Question Paper 2019-20 – Set – 1
- Maths Class 9 Previous year Question Paper – SA 2 (2016)
- Maths Class 9 Previous year Question Paper – A 2 (2016), Marking scheme
- Maths Class 9 Previous year Question Paper – SA 1 2015
- Maths Class 9 Previous year Question Paper – SA 1 2011
Read Also
- Sample Question Paper for Class 9 CBSE Hindi Course B
- Class 9 Science Chapter 1 Matter in Our Surroundings Notes
- Matter In Our Surroundings Class 9 Sample Paper With Answers
- MCQ for Class 9 Science Chapter 1 Matter in Our Surroundings
Importance of CBSE Previous Year Question Papers:
- Familiarity with Exam Pattern: One of the significant advantages of studying previous year question papers is that students become familiar with the structure of the exam. They get a clear idea of the distribution of marks, the number of questions from each chapter, and the types of questions (short answer, long answer, multiple-choice, etc.). This familiarity helps students plan their time effectively during the exam.
- Understanding Question Trends: CBSE question papers often exhibit certain patterns over the years. By reviewing multiple years’ papers, students can identify which topics are frequently covered and which are relatively less emphasized. This insight enables focused preparation, allowing students to allocate more time to areas that are likely to carry more weight in the exam.
- Exposure to Diverse Questions: The question papers from previous years encompass a wide range of questions that test different aspects of each topic. This exposure helps students develop a comprehensive understanding of each concept and enables them to tackle various question formats confidently.
- Time Management: Solving previous year question papers under timed conditions helps students practice time management, a crucial skill during the actual exam. It aids in gauging how much time should be allocated to each question and prevents the situation of running out of time before completing the paper.
- Self-Assessment: Attempting these papers and then comparing the answers with the provided solutions allows students to assess their own understanding of the subject. It highlights areas where they need to improve and provides an opportunity for self-directed learning.
The Role of Solutions:
While solving previous year question papers is beneficial, having access to accurate and comprehensive solutions is equally important. Solutions provide step-by-step explanations of how to approach and solve each question.
They help students understand the reasoning behind each solution, aiding in the development of problem-solving skills. Additionally, solutions often include alternative methods of solving the same question, demonstrating the versatility of mathematical techniques.
How to Make the Most of CBSE Previous Year Question Papers:
- Regular Practice: Allocate specific time slots to solve these papers regularly. As consistency is key, make it a part of your study routine.
- Systematic Analysis: After solving a paper, analyze your performance. Identify areas where you struggled and revisit those concepts to strengthen your understanding.
- Use of Solutions: While reviewing the solutions, focus on the logic behind each step. If you encounter a different method while solving, compare it with the provided solution to broaden your approach.
- Time-Bound Practice: Attempting the papers under timed conditions mimics the exam environment. Work on improving your speed without compromising on accuracy.
- Revision: Revise the concepts that you found challenging during the solving process. Reattempt the related questions to solidify your grasp.
- Mock Exams: As the final exams approach, simulate the actual exam day by attempting full question papers in one sitting. This will help you refine your time management skills.
In conclusion, CBSE Previous Year Question Papers for Class 9 Maths, along with their solutions, are invaluable resources for students preparing for their exams.
These papers provide insights into the exam pattern, question trends, and a wide variety of question types.
By incorporating these papers into your study routine and utilizing the provided solutions effectively, you can enhance your understanding of mathematical concepts, improve problem-solving skills, and approach the final exam with confidence.
Frequently Asked Questions – FAQs
Why are CBSE Previous Year Question Papers important for Class 9 Maths preparation?
CBSE Previous Year Question Papers offer insights into the exam pattern, question trends, and types of questions that could appear in the actual exam. They help students practice time management, self-assess their preparation, and develop a comprehensive understanding of each topic.
Where can I find CBSE Class 9 Maths Previous Year Question Papers with Solutions?
You can find CBSE Class 9 Maths Previous Year Question Papers with Solutions on various educational websites, official CBSE portals, or by using study resources provided by coaching institutes.
How can solving previous year question papers improve my performance in the final exam?
Regular practice with previous year question papers helps you become familiar with the exam format, refine your problem-solving skills, and identify your strengths and weaknesses. It also boosts your confidence by giving you a realistic preview of the actual exam.
Can I solely rely on solving previous year papers for my Class 9 Maths exam preparation?
While solving previous year papers is an excellent practice, it’s essential to complement it with thorough studying of the textbook, classroom notes, and other reference materials. Use the question papers as a tool to enhance your understanding and test your knowledge.
How do I effectively use the solutions provided with the question papers?
Use the solutions to understand the step-by-step approach to solving each question. Focus on the reasoning behind each step, and compare your solution (if you have attempted it differently) with the provided one to learn alternative methods.
Sample Question Paper for Class 9 CBSE Hindi Course B
Sample Question Paper for Class 9 CBSE Hindi Course B: In order to establish a strong foundation for your upcoming academic phase it is essential to begin practicing a diverse range of questions sourced from CBSE Class 9 Hindi Sample Papers.
By embracing the guidance offered by NCERT Solutions designed for Class 9 Hindi, you will find yourself drawing closer to your aspirational destination.
Take a moment to explore: CBSE 9th Sample Papers 2023 for both Term 1 and Term 2, spanning all subject areas.

To gain insights into the question patterns frequently encountered in board examinations, avail yourself of the opportunity to download CBSE Sample Papers for Class 9 Hindi, along with the accompanying Marking Scheme PDF. This article is dedicated to providing you with the CBSE Hindi Sample Papers tailored for Class 9.
Sample Question Paper for Class 9 CBSE Hindi Course B Term 1
- Hindi course B term 1 Sample paper of year 2021-22
- Hindi course B term 2 Sample paper of year 2021-22
- Hindi course B Sample paper of year 2020-21
- Hindi course B Sample paper of year 2019-20
- Hindi course B Sample paper of year 2018-19
- Hindi course B Sample paper of year 2017-18
- Hindi course B Sample paper of year 2016-17
- Hindi course B Sample paper of year 2015-16
Benefits Of Solving Sample Question Paper for Class 9 CBSE Hindi Course B
We have already emphasized the importance of engaging with the Class 9 Hindi Sample Paper 2023 for both Term 1 & Term 2. Now, let’s underscore the advantages of this practice. Consider the following points:
1. Regularly solving papers equips you with the ability to effectively manage time during exams. This skill becomes crucial in the exam hall, where time management is as important as answering questions from the sample papers.
2. The process of solving these papers grants you ample time to recognize and rectify your errors before you step into the final examination. This proactive approach aids in refining your performance.
3. Familiarity with the latest question trends enhances your confidence gradually. You become attuned to the types of questions that are likely to appear, bolstering your overall preparedness.
4. Immersing yourself in the sample test papers ensures that all significant topics receive your attention. This focused engagement contributes to a comprehensive understanding of the subject matter.
5. Through analyzing the sample test papers, you gain a comprehensive overview of the distribution of marks and the marking schemes. This knowledge provides clarity on the weightage assigned to different sections.
6. Regular practice with a variety of questions from the sample papers offers numerous opportunities to assess your performance. This ongoing self-assessment aids in tracking your progress and identifying areas that require improvement.
7. Pre-exposure to the types of questions you’ll encounter in the examination hall grants you a level of familiarity and readiness, contributing to a more composed and confident performance during the actual exam.
Read Also:
- CBSE Class 10 Sample Question Paper Social Science PDF
- CBSE Class 10 Science Sample Question Paper 2018 PDF
- NCERT Class 10 English Sample Paper PDF Download
- CBSE Hindi Sample Paper for Class 10th Download PDF
- CBSE Previous Year Question Papers Class 9 Maths with Solutions
- Class 9 Science Chapter 1 Matter in Our Surroundings Notes
- Matter In Our Surroundings Class 9 Sample Paper With Answers
- MCQ for Class 9 Science Chapter 1 Matter in Our Surroundings
CBSE Class 9 Hindi Exam Pattern 2023
In addition to engaging with sample test papers, it is essential to stay updated with the current CBSE exam pattern for Class 9 Hindi. Given that there are yearly adjustments or alterations to the pattern, paying close attention to the finer details is crucial. Understanding the distribution of marks holds significance.
This familiarity provides insights into the different sections involved. By observing attentively, you can gauge the extent of effort required for each segment, thereby acquiring a clear understanding of where to allocate your energies.
The CBSE Class 9 Hindi Exam Pattern for 2023 consists of the following components:
- Total Marks: The examination is typically conducted for a certain total mark value.
- Types of Questions: The question paper comprises different types of questions, such as Multiple Choice Questions (MCQs), Short Answer Questions, Long Answer Questions, etc.
- Section-wise Distribution: The marks are distributed across various sections or parts of the question paper.
- Internal Assessment: Some marks might be allocated to internal assessment components, which can include classwork, homework, projects, etc.
- Duration: The examination has a specified time duration within which students need to complete the paper.
- Topics and Chapters: The exam covers specific topics and chapters from the syllabus.
It’s important to refer to the official CBSE guidelines or your school’s curriculum to get the exact and updated exam pattern for CBSE Class 9 Hindi in 2023, as there might be specific changes or variations each year.
Frequently Asked Question – FAQs
1. What is a Sample Question Paper for Class 9?
A Sample Question Paper for Class 9 is a model or practice paper designed by educational boards or institutions to simulate the format and content of the actual examination. It contains a set of questions that students can use for practice and to get a sense of the question patterns, difficulty level, and marking scheme.
2. Why should I use Sample Question Papers for Class 9?
Using Sample Question Papers for Class 9 helps you familiarize yourself with the type of questions that might appear in the actual exam. It allows you to practice under exam-like conditions and assess your readiness, helping you identify your strengths and areas that need improvement.
3. Where can I find Sample Question Papers for Class 9?
You can find Sample Question Papers for Class 9 from various sources such as educational websites, official board websites (like CBSE, ICSE), study guides, and reference books. Your school or teachers might also provide sample papers for practice.
4. How can Sample Question Papers help me prepare for exams?
Sample Question Papers provide an opportunity to practice solving different types of questions, understand the exam pattern, and manage time effectively. By repeatedly solving these papers, you can enhance your problem-solving skills and gain confidence in your subject knowledge.
5. Are Sample Question Papers based on the actual syllabus?
Yes, typically Sample Question Papers are designed based on the official syllabus provided by educational boards. They cover the important topics and concepts outlined in the curriculum, ensuring that you practice questions relevant to your exams.