Category: Class 9
Law Of Conservation Of Energy
Law Of Conservation Of Energy: Energy is a fundamental concept that governs the behavior of the universe. It powers our homes, drives our cars, and fuels the processes of life itself.
The Law of Conservation of Energy is one of the most fundamental principles in physics, revealing the remarkable fact that energy cannot be created or destroyed but can only change from one form to another.
In this article, we will explore the essence of this law, its historical development, and its profound implications across various scientific disciplines.

Law Of Conservation Of Energy
Understanding the Law of Conservation of Energy
The Law of Conservation of Energy, also known as the First Law of Thermodynamics, states that the total energy of an isolated system remains constant over time. In simpler terms, energy cannot be created or destroyed within a closed system; it can only change its form or be transferred from one part of the system to another. This law underlies numerous physical phenomena and is the cornerstone of many scientific principles.
Historical Development
The concept of conservation of energy has a rich history of development:
- Early Ideas: The idea that something akin to energy conservation existed dates back to ancient philosophers, such as Aristotle, who postulated that the total amount of motion (kinetic energy) in the universe remains constant.
- Work by Julius Mayer and James Joule: In the mid-19th century, scientists like Julius Mayer and James Joule independently formulated the idea that energy is conserved. Joule’s famous experiments with mechanical work and heat led to the formulation of the mechanical equivalent of heat.
- Hermann Helmholtz: Hermann Helmholtz, a German physicist, further clarified the law by introducing the concept of conservation of energy in a broader sense, encompassing various forms of energy, including potential, kinetic, thermal, and chemical energy.
- The First Law of Thermodynamics: This principle was codified as the First Law of Thermodynamics, which articulates that the alteration in the internal energy of a closed system equals the heat added to the system minus the work performed by the system.
Implications and Applications
The Law of Conservation of Energy has far-reaching implications across various scientific fields:
- Physics: In classical physics, this law is crucial for understanding the behavior of mechanical systems, the motion of objects, and the transfer of energy in various forms.
- Thermodynamics: The law forms the foundation of thermodynamics, a branch of physics dealing with heat, work, and energy transfer. It underlies the principles governing engines, refrigerators, and the behavior of gases.
- Chemistry: In chemical reactions, energy conservation plays a vital role. Comprehending the conservation and transfer of energy is essential for predicting the outcomes of reactions and investigating the behavior of molecules.
- Engineering: Engineers rely on the conservation of energy when designing efficient systems and machinery. The law is essential in fields like electrical engineering, where energy transfer and conversion are fundamental.
- Environmental Science: In environmental science, the law is pivotal for assessing energy flows in ecosystems and understanding the impact of human activities on energy conservation and sustainability.
Challenges and Expanding Horizons
While the Law of Conservation of Energy has been a cornerstone of classical physics, it faced challenges and expansions with the advent of modern physics. The development of quantum mechanics and the theory of relativity introduced new perspectives on energy conservation, particularly at microscopic scales and in situations involving extremely high speeds or strong gravitational fields.
Applications Across Diverse Fields
The Law of Conservation of Energy has found applications in various scientific, technological, and everyday contexts:
- Renewable Energy: The development of renewable energy sources, such as solar panels and wind turbines, relies on energy conservation principles. These technologies harness energy from natural processes and convert it into electricity while adhering to the law.
- Electric Circuits: In electrical engineering, energy conservation is fundamental for analyzing and designing circuits. It ensures that electrical energy input equals the output, accounting for losses due to resistance and inefficiencies.
- Climate Science: Understanding the Earth’s energy balance is essential in climate science. The law helps scientists assess the energy absorbed from the Sun, radiated back into space, and retained in the atmosphere, influencing climate patterns.
- Medicine: Medical devices like pacemakers and defibrillators operate based on the principles of energy conservation, ensuring precise delivery of electrical energy to the heart to maintain proper function.
- Space Exploration: Space missions and satellite operations adhere to the law when planning energy budgets, ensuring that spacecraft have enough energy to perform their tasks efficiently.
Challenges and Expanding Horizons
While the Law of Conservation of Energy remains a fundamental pillar of physics, it faced challenges and expansions in the 20th century:
- Quantum Mechanics: At the quantum level, the conservation of energy takes on unique characteristics. Energy levels in quantum systems are quantized, leading to phenomena like electron energy states in atoms and energy conservation within subatomic particles.
- Theory of Relativity: Albert Einstein’s theory of relativity introduced the famous equation E=mc², demonstrating that energy (E) and mass (m) are interchangeable. This theory expanded our understanding of energy conservation in the context of space-time.
- Dark Energy and Dark Matter: In cosmology, the nature of dark energy and dark matter challenges our understanding of energy conservation on cosmic scales. These mysterious components of the universe may not adhere to conventional energy conservation laws.
Conclusion
The Law of Conservation of Energy, a cornerstone of physics, guides our understanding of energy’s role in the universe. It has applications ranging from everyday engineering and environmental science to cutting-edge research in quantum mechanics and cosmology. Despite challenges and expansions, this law endures as a fundamental principle, offering valuable insights into the workings of the physical world and opening doors to new discoveries and technological advancements. As science continues to evolve, the conservation of energy remains a timeless and indispensable concept.
Read More
- Difference Between Equinox And Solstice
- Molecular Weight Of K2Cr2O7
- Molecular Weight Of Phosphorus
- Molecular Weight Of SO2
- Molecular Weight of Na2CO3
Frequently Asked Questions (FAQs) On Law Of Conservation Of Energy
1. What is the Law of Conservation of Energy?
The Law of Energy Conservation in physics asserts the unchanging total energy of an isolated system throughout time. Energy cannot be created or destroyed but can only change from one form to another.
2. Who formulated the Law of Conservation of Energy?
Although the concept of conserving energy has ancient origins, the modern formulation of this principle is credited to 19th-century scientists such as Julius Mayer, James Joule, and Hermann Helmholtz.
3. What are the different forms of energy?
Energy can exist in various forms, including kinetic energy (associated with motion), potential energy (associated with position or configuration), thermal energy (associated with heat), chemical energy (associated with chemical reactions), and electromagnetic energy (associated with light and other electromagnetic waves), among others.
4. Can energy be lost or created in everyday processes?
In everyday occurrences, energy remains conserved, undergoing conversions from one manifestation to another. In a car engine, fuel’s chemical energy converts into kinetic (motion) and thermal (heat) energy.
5. How is the Law of Conservation of Energy applied in technology and engineering?
The law is crucial in designing and analyzing systems, such as engines, power plants, and electrical circuits. It ensures that energy input equals the energy output, accounting for losses due to inefficiencies.
Difference Between Equinox And Solstice
Difference Between Equinox And Solstice: Equinox and solstice are astronomical events that mark significant points in the Earth’s orbit around the Sun, bringing about changes in seasons and daylight hours. Here’s a comparison of the key differences between equinox and solstice:
Difference Between Equinox And Solstice
1. Definition:
- Equinox: An equinox occurs when the Sun is directly above the Earth’s equator, resulting in nearly equal lengths of day and night. There are two equinoxes each year: the vernal equinox (spring equinox) and the autumnal equinox (fall equinox).
- Solstice: A solstice is an astronomical event when the Sun reaches its highest or lowest point in the sky at noon, marking the longest day (summer solstice) or shortest day (winter solstice) of the year.
2. Occurrence:
- Equinox: Equinoxes occur twice a year, around March 20-21 (vernal equinox) and September 22-23 (autumnal equinox) in the Northern Hemisphere. In the Southern Hemisphere, the dates are reversed.
- Solstice: Solstices also occur twice a year, around June 20-21 (summer solstice) and December 21-22 (winter solstice) in the Northern Hemisphere. In the Southern Hemisphere, the dates are reversed.
3. Daylight Hours:
- Equinox: During equinoxes, day and night are approximately of equal length in most parts of the world. This balance of daylight and darkness is often referred to as “equilux.”
- Solstice: Solstices mark the extremes of daylight hours. The summer solstice has the longest day and shortest night, while the winter solstice has the shortest day and longest night.
4. Sun’s Position:
- Equinox: In an equinox, the Sun is situated precisely above the Earth’s equator, resulting in the Sun rising directly in the east and setting directly in the west, regardless of the observer’s geographical location on Earth.
- Solstice: During a solstice, the Sun is at its highest (summer solstice) or lowest (winter solstice) point in the sky at noon, resulting in the longest or shortest noontime shadows, respectively.
5. Seasonal Significance:
- Equinox: Equinoxes signal the beginning of spring (vernal equinox) or fall (autumnal equinox) and are associated with the transition between the seasons.
- Solstice: Solstices mark the official start of summer (summer solstice) or winter (winter solstice) and represent the peak of the respective seasons.
6. Cultural and Celebratory Traditions:
- Equinox: Equinoxes have been celebrated in various cultures and religions as times of balance and renewal. For example, the vernal equinox is associated with Easter in Christianity.
- Solstice: Solstices have often been associated with major cultural and religious celebrations. For instance, the summer solstice is celebrated in festivals like Midsummer’s Eve in some cultures.
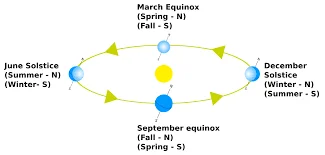
In summary, equinoxes and solstices are astronomical events that influence the length of day and night, the Sun’s position in the sky, and the changing of seasons. Equinoxes represent balance, while solstices represent extremes in daylight and are culturally significant in various parts of the world.
Read More
- Molecular Weight Of K2Cr2O7
- Molecular Weight Of Phosphorus
- Molecular Weight Of SO2
- Molecular Weight of Na2CO3
- Molecular Mass Of Sodium
Frequently Asked Questions (FAQs) On Difference Between Equinox And Solstice
1. What is the main difference between an equinox and a solstice?
The key difference lies in their astronomical significance. Equinoxes mark the times when day and night are nearly of equal length, signaling the transition between seasons, while solstices mark the moments when the Sun reaches its highest or lowest point in the sky, resulting in the longest or shortest day of the year.
2. How many equinoxes and solstices occur each year?
There are two equinoxes each year: the vernal (spring) equinox and the autumnal (fall) equinox. Likewise, there are two solstices: the summer solstice and the winter solstice.
3. When do equinoxes occur?
Equinoxes typically occur around March 20-21 (vernal equinox) and September 22-23 (autumnal equinox) in the Northern Hemisphere. The dates are reversed in the Southern Hemisphere.
4. When do solstices occur?
Solstices generally occur around June 20-21 (summer solstice) and December 21-22 (winter solstice) in the Northern Hemisphere, with reversed dates in the Southern Hemisphere.
5. How do equinoxes affect daylight hours?
Equinoxes bring nearly equal lengths of day and night, resulting in a balance of daylight and darkness.
Molecular Weight Of K2Cr2O7
Molecular Weight Of K2Cr2O7: Potassium dichromate, with the chemical formula K2Cr2O7, is a chemical compound widely used in various industries and laboratories for its oxidizing properties.
Understanding the molecular weight of potassium dichromate is essential in chemistry, particularly in analytical and stoichiometric calculations. In this article, we will delve into the mole weight of K2Cr2O7, its significance, and its applications in different fields.

Molecular Weight Of K2Cr2O7
Exploring the Atomic Structure of Potassium Dichromate (K2Cr2O7)
Before we calculate the mole weight of potassium dichromate, it’s crucial to examine its atomic composition:
- Potassium (K): Potassium has an atomic mass of approximately 39.10 atomic mass units (amu).
- Chromium (Cr): Chromium has an atomic mass of approximately 51.99 amu.
- Oxygen (O): Oxygen has an atomic mass of approximately 16.00 amu.
Potassium dichromate (K2Cr2O7) consists of two potassium atoms (K), two chromium atoms (Cr), and seven oxygen atoms (O).
Calculating the Molecular Weight of K2Cr2O7
To calculate the mole weight of potassium dichromate (K2Cr2O7), we sum the atomic masses of its constituent atoms according to the chemical formula:
Molecular Weight of K2Cr2O7 = (2 × Atomic Mass of K) + (2 × Atomic Mass of Cr) + (7 × Atomic Mass of O)
Molecular Weight of K2Cr2O7 = (2 × 39.10 amu) + (2 × 51.99 amu) + (7 × 16.00 amu)
The Molecular Weight of K2Cr2O7 = 78.20 amu + 103.98 amu + 112.00 amu
Molecular Weight of K2Cr2O7 ≈ 294.18 amu
Therefore, the molecular weight of potassium dichromate (K2Cr2O7) is approximately 294.18 atomic mass units (amu) or 294.18 grams per mole (g/mol).
Significance of Molecular Weight in Potassium Dichromate
The molecular weight of potassium dichromate is significant for various reasons:
- Analytical Chemistry: Potassium dichromate is widely used as a primary standard in titrations, particularly for the determination of reducing agents. Its accurate molecular weight is vital for precise volumetric analysis.
- Environmental Analysis: It finds application in laboratories dedicated to environmental testing, where it is instrumental in analyzing diverse water and soil samples. Its primary role is in accurately assessing the concentrations of organic compounds and pollutants.
- Safety Considerations: Potassium dichromate is a hazardous chemical with various industrial applications, including as a corrosion inhibitor and in the manufacturing of pigments. Knowledge of its molecular weight aids in proper handling and safety protocols.
- Educational and Research Purposes: In educational settings and research laboratories, potassium dichromate is used for experiments and studies related to redox reactions and stoichiometry.
Conclusion
The molecular weight of potassium dichromate (K2Cr2O7), approximately 294.18 amu or 294.18 g/mol, is a fundamental property that underlies its applications in chemistry, analytical science, environmental testing, and industry. Whether it’s ensuring the accuracy of analytical results, adhering to safety guidelines, or conducting research, understanding the molecular weight of potassium dichromate is crucial for achieving reliable and meaningful outcomes in various scientific and practical endeavors.
Read More
- Molecular Weight Of Phosphorus
- Molecular Weight Of SO2
- Molecular Weight of Na2CO3
- Molecular Mass Of Sodium
- Molecular Mass of Sucrose
Frequently Asked Questions (FAQs) Molecular Weight Of K2Cr2O7
What is the molecular weight of potassium dichromate (K2Cr2O7)?
The mole weight of potassium dichromate (K2Cr2O7) is roughly 294.18 atomic mass units (amu) or 294.18 grams per mole (g/mol). This determination is made by summing the atomic masses of its constituent atoms, as specified by its chemical formula.
What is potassium dichromate used for?
Potassium dichromate serves diverse purposes, functioning as an oxidizing agent in chemical reactions, a primary standard in analytical chemistry, a corrosion inhibitor, and a constituent in pigment manufacturing. Additionally, it plays a pivotal role in environmental testing.
Why is it important to know the molecular weight of K2Cr2O7?
Understanding the molecular weight of potassium dichromate is crucial for precise analytical chemistry, accurate dosing in experiments, and adhering to safety guidelines. It ensures the correct proportions of reactants and products in chemical reactions.
Is potassium dichromate hazardous?
Indeed, potassium dichromate is classified as a hazardous chemical due to its toxic, carcinogenic properties. Its potential to induce severe skin and respiratory irritation. When working with it, it is imperative to adhere to strict safety protocols, which include the use of personal protective equipment.
What are the environmental applications of potassium dichromate?
Potassium dichromate finds application in environmental testing laboratories for the examination of water and soil samples, where its significance lies in accurately assessing the concentrations of organic compounds and pollutants.
Molecular Weight Of Phosphorus
Molecular Weight of Phosphorus: Phosphorus, symbolized by “P” in the periodic table, is a fundamental element present in all living organisms and holds a central position in numerous biochemical processes.
Understanding the mole weight of phosphorus is fundamental in chemistry, biology, and environmental science, as it provides insights into the behavior of this element in different contexts. In this article, we will explore the mole weight of phosphorus, its significance, and its diverse applications.

Molecular Weight Of Phosphorus
Exploring the Atomic Structure of Phosphorus
Before delving into the mole weight of phosphorus, it’s essential to examine its atomic structure:
- Atomic Number: Phosphorus is defined by its atomic number, which is 15. This indicates that a phosphorus atom harbors 15 protons within its nucleus.
- Electron Configuration: The electron configuration of phosphorus is 2-8-5, meaning it has two electrons in its first energy level, eight in its second energy level, and five in its third energy level.
- Isotopes: Phosphorus has several isotopes, with phosphorus-31 (P-31) being the most common and stable isotope. P-31 has 16 neutrons in addition to its 15 protons.
Calculating the Molecular Weight of Phosphorus (P)
To calculate the mole weight of phosphorus (P), we consider the atomic mass of its most abundant and stable isotope, phosphorus-31 (P-31):
- The atomic mass of phosphorus-31 is approximately 30.97 atomic mass units (amu) or 30.97 grams per mole (g/mol).
- Therefore, the mole weight of phosphorus (P) is approximately 30.97 g/mol or 30.97 amu.
Significance of Molecular Weight in Phosphorus
The mole weight of phosphorus holds significant importance in various fields and applications:
- Biochemistry: Phosphorus is a crucial component of DNA, RNA, and ATP (adenosine triphosphate), which are essential molecules in living organisms. Understanding its molecular weight aids in studying these biomolecules and their functions.
- Agriculture: Phosphorus stands as an indispensable nutrient crucial for fostering plant growth and is widely employed in fertilizers. Understanding its molecular weight holds significant importance in the formulation of fertilizers and agricultural strategies.
- Chemical Reactions: Phosphorus participates in various chemical reactions, such as the synthesis of phosphoric acid (used in the production of soft drinks and detergents). Its molecular weight is essential for stoichiometry in chemical equations.
- Environmental Science: Phosphorus is a concern in environmental science due to its role in water pollution (e.g., eutrophication). Understanding its molecular weight is crucial for monitoring and mitigating its impact on aquatic ecosystems.
Conclusion
The molecular weight of phosphorus, approximately 30.97 g/mol or 30.97 amu, is a fundamental property that underpins its significance in the fields of chemistry, biology, and environmental science. Whether it’s its role in DNA, its use as a fertilizer, its participation in chemical reactions, or its impact on aquatic environments, understanding the molecular weight of phosphorus is essential for unraveling its multifaceted behavior and applications. This versatile element continues to be a cornerstone of scientific and practical advancements in various disciplines.
Read More
- Molecular Weight Of SO2
- Molecular Weight of Na2CO3
- Molecular Mass Of Sodium
- Molecular Mass of Sucrose
- Molecular Mass of Chlorine
Frequently Asked Questions (FAQs) Molecular Weight Of Phosphorus
What is the molecular weight of phosphorus?
The molecular weight of phosphorus (P) is roughly 30.97 grams per mole (g/mol) or 30.97 atomic mass units (amu). This determination is based on the atomic mass of its most prevalent and stable isotope, phosphorus-31 (P-31).
Why is it important to know the molecular weight of phosphorus?
Understanding the molecular weight of phosphorus is crucial in various scientific fields. It aids in stoichiometry in chemical reactions, is essential in the study of biochemical processes, and is significant in agriculture for fertilizer formulation.
What are the key roles of phosphorus in biological systems?
Phosphorus plays a pivotal role in biological systems, serving as an essential component within DNA, RNA, and ATP (adenosine triphosphate). These molecules are central to genetic information storage and energy transfer processes. Additionally, phosphorus is of critical importance in maintaining the structure of cell membranes.
How is phosphorus utilized in agriculture?
Phosphorus is an essential nutrient for plant growth. It is commonly used in the form of phosphorus-containing fertilizers to enhance crop yield and quality. Phosphorus promotes root development, flowering, and fruiting in plants.
What is the significance of phosphorus in chemical reactions?
Phosphorus is actively involved in numerous chemical reactions, one of which is the production of phosphoric acid. This acid finds application in the manufacturing of soft drinks and detergents. The molecular weight of phosphorus is of paramount importance in stoichiometric calculations, ensuring the precise ratios of reactants and products in chemical processes.
Molecular Weight Of SO2
Molecular Weight Of SO2: Sulfur dioxide, with the chemical formula SO2, is a compound of great importance in various fields, including environmental science, chemistry, and industry.
It is a gas with a distinctive, pungent odor and is produced both naturally and through human activities. In this article, we will delve into the molecular weight of sulfur dioxide, its significance, and its various applications.

Molecular Weight Of SO2
Decoding the Composition of Sulfur Dioxide (SO2)
To understand the molecular weight of sulfur dioxide (SO2), let’s examine its atomic composition:
- Sulfur (S): Sulfur has an atomic mass of approximately 32.07 atomic mass units (amu).
- Oxygen (O): Oxygen has an atomic mass of approximately 16.00 amu.
- Sulfur dioxide (SO2) consists of one sulfur atom (S) and two oxygen atoms (O).
Calculating the Molecular Weight of SO2
To calculate the mole weight of sulfur dioxide (SO2), we sum the atomic masses of its constituent atoms according to the chemical formula:
Molecular Weight of SO2 = Atomic Mass of S + (2 × Atomic Mass of O)
Molecular Weight of SO2 = 32.07 amu + (2 × 16.00 amu)
The Molecular Weight of SO2 = 32.07 amu + 32.00 amu
Molecular Weight of SO2 ≈ 64.07 amu
Therefore, the molecular weight of sulfur dioxide (SO2) is approximately 64.07 atomic mass units (amu) or 64.07 grams per mole (g/mol).
Significance of Molecular Weight in Sulfur Dioxide
The molecular weight of sulfur dioxide holds significant importance in various scientific and practical applications:
- Environmental Monitoring: Sulfur dioxide is a common air pollutant produced by combustion processes, particularly in industrial settings. Monitoring its levels in the atmosphere is crucial for assessing air quality and environmental impact.
- Chemical Reactions: Sulfur dioxide participates in various chemical reactions, including those in the atmosphere (e.g., in acid rain formation) and industrial processes (e.g., in the production of sulfuric acid). Understanding its molecular weight aids in stoichiometry and reaction calculations.
- Preservation: Sulfur dioxide is used as a preservative in the food and beverage industry to prevent spoilage and oxidation. Knowledge of its molecular weight is essential for accurate dosing.
- Analytical Chemistry: Sulfur dioxide can be employed in analytical chemistry as a reducing agent and reagent in chemical tests. Precise molecular weight values are vital for analytical procedures.
- Environmental Remediation: In environmental science and engineering, sulfur dioxide can be employed for the removal of pollutants such as nitric oxide (NO) from gas streams. Its molecular weight is essential for designing and optimizing these processes.
Conclusion
The mole weight of sulfur dioxide (SO2), approximately 64.07 amu or 64.07 g/mol, plays a crucial role in numerous scientific and practical applications. Whether it’s assessing air quality, conducting chemical reactions, preserving food, or analyzing environmental processes, understanding the mole weight of SO2 is fundamental for achieving desired outcomes and addressing environmental and industrial challenges. This versatile compound continues to be a key player in various fields, underscoring the importance of accurate molecular weight knowledge.
Read More
- Molecular Weight of Na2CO3
- Molecular Mass Of Sodium
- Molecular Mass of Sucrose
- Molecular Mass of Chlorine
- Molar Mass Of C2H5OH
Frequently Asked Questions (FAQs) Molecular Weight Of SO2
What is the molecular weight of sulfur dioxide (SO2)?
The molecular weight of sulfur dioxide (SO2) is approximately 64.07 atomic mass units (amu) or 64.07 grams per mole (g/mol). This value is calculated based on the atomic masses of sulfur (S) and oxygen (O) in the chemical formula.
What is sulfur dioxide (SO2) used for?
Sulfur dioxide is used in various applications, including as an air pollutant monitor, a preservative in the food and beverage industry, a reducing agent in chemical reactions, and in environmental remediation processes.
Why is it important to know the molecular weight of SO2?
Knowing the molecular weight of sulfur dioxide is important for stoichiometry in chemical reactions, environmental monitoring, dosing in food preservation, and analytical chemistry procedures. It provides essential information for accurate calculations and assessments in these fields.
Is sulfur dioxide a natural or human-made compound?
Sulfur dioxide can be both natural and human-made. It occurs naturally as a result of volcanic eruptions and is also generated as a byproduct of specific biological processes.. However, a significant amount of sulfur dioxide in the atmosphere results from human activities, such as the burning of fossil fuels.
How does sulfur dioxide contribute to environmental pollution?
Sulfur dioxide is a major contributor to air pollution, particularly in industrial areas. It can lead to the formation of acid rain, which has harmful effects on the environment, including damage to vegetation and aquatic ecosystems.
Molecular Weight of Na2CO3
Molecular Weight of Na2CO3: Sodium carbonate, with the chemical formula Na2CO3, is a compound commonly known as soda ash or washing soda.
It plays a significant role in various industrial processes and applications, including glass production, detergent manufacturing, and water treatment.
Understanding the molecular weight of Na2CO3 is essential for both theoretical and practical purposes. In this article, we will explore the molecular weight of sodium carbonate and its significance in chemistry and industry.

Molecular Weight of Na2CO3
The Composition of Sodium Carbonate (Na2CO3)
Before diving into the molecular weight of sodium carbonate, let’s break down its chemical composition:
- Sodium (Na): Sodium has an atomic mass of approximately 22.99 atomic mass units (amu).
- Carbon (C): Carbon has an atomic mass of approximately 12.01 amu.
- Oxygen (O): Oxygen has an atomic mass of approximately 16.00 amu.
- Sodium carbonate (Na2CO3) consists of two sodium atoms (Na), one carbon atom (C), and three oxygen atoms (O).
Calculating the Molecular Weight of Na2CO3
To find the molecular weight of sodium carbonate (Na2CO3), we sum the atomic masses of its constituent atoms according to the chemical formula:
Molecular Weight of Na2CO3 = (2 × Atomic Mass of Na) + Atomic Mass of C + (3 × Atomic Mass of O)
The Molecular Weight of Na2CO3 = (2 × 22.99 amu) + 12.01 amu + (3 × 16.00 amu)
Molecular Weight of Na2CO3 = 45.98 amu + 12.01 amu + 48.00 amu
Molecular Weight of Na2CO3 ≈ 105.99 amu
So, the mol weight of sodium carbonate (Na2CO3) is approximately 105.99 atomic mass units (amu) or 105.99 grams per mole (g/mol).
Significance of Molecular Weight in Sodium Carbonate
The mole weight of sodium carbonate is crucial for several reasons:
- Stoichiometry: In chemical reactions involving sodium carbonate, its molecular weight is used to calculate the amounts of reactants and products. This is essential for balancing chemical equations and determining reaction yields.
- Dosing in Water Treatment: Sodium carbonate is used in water treatment to adjust pH levels and soften water. Knowledge of its molecular weight helps in dosing calculations to achieve the desired treatment results.
- Manufacturing Processes: Industries such as glass manufacturing and detergent production rely on sodium carbonate as a key ingredient. Accurate molecular weight values are vital for quality control and formulation.
- Analytical Chemistry: In analytical chemistry, sodium carbonate can be used as a standard for titrations. Precise molecular weight values are essential for accurate analytical procedures.
Conclusion
The mole weight of sodium carbonate (Na2CO3), approximately 105.99 amu or 105.99 g/mol, is a fundamental property that underlies its various applications in chemistry and industry. Whether used as a reactant in chemical processes, a water treatment agent, or an essential component in manufacturing, understanding the molecular weight of sodium carbonate is essential for ensuring the efficiency and effectiveness of these processes. This versatile compound continues to be a vital player in numerous industrial and scientific endeavors.
Read More
- Molecular Mass Of Sodium
- Molecular Mass of Sucrose
- Molecular Mass of Chlorine
- Molar Mass Of C2H5OH
- Molecular Weight of Co2
Frequently Asked Questions (FAQs) Molecular Weight of Na2CO3
What is the mole weight of Na2CO3?
The mole weight of sodium carbonate (Na2CO3) is approximately 105.99 atomic mass units (amu) or 105.99 grams per mole (g/mol). It is calculated by adding the atomic masses of its constituent atoms according to the chemical formula.
Why is it important to know the molecular weight of Na2CO3?
Knowing the molecular weight of sodium carbonate is crucial for various reasons, including stoichiometry in chemical reactions, dosing in water treatment, formulation in manufacturing processes, and analytical chemistry procedures.
What are some common uses of sodium carbonate (Na2CO3)?
Sodium carbonate, also known as soda ash or washing soda, is used in a wide range of applications. It is used in glass production, detergent manufacturing, water treatment for pH adjustment, and as a cleaning agent in household and industrial settings.
How is the mole weight of Na2CO3 calculated?
The mole weight of Na2CO3 is calculated by summing the atomic masses of its constituent atoms. In the case of sodium carbonate, you add the atomic masses of two sodium (Na) atoms, one carbon (C) atom, and three oxygen (O) atoms.
Can sodium carbonate be used in food or as a food additive?
Yes, sodium carbonate is used in the food industry as a food additive with the code E500. It is used in some food products, including baked goods, as a leavening agent to help dough rise.
Molecular Mass Of Sodium
Molecular Mass Of Sodium: Sodium, with the chemical symbol “Na” derived from the Latin word “natrium,” is a chemical element that plays a crucial role in various aspects of our daily lives.
Understanding the molecular mass of sodium is fundamental in chemistry, as it helps scientists and researchers predict how sodium interacts with other elements and compounds. In this article, we will delve into the molecular mass of sodium, its significance, and its applications.

Molecular Mass Of Sodium
The Atomic Structure of Sodium
To comprehend the molecular mass of sodium, it is important to first explore its atomic structure:
- Atomic Number: Sodium is identified by its atomic number, which is 11. This signifies that a sodium atom contains 11 protons in its nucleus.
- Electron Configuration: Sodium has an electron configuration of 2-8-1, meaning it has two electrons in its first energy level, eight in its second energy level, and one in its third energy level.
- Isotopes: Sodium has several isotopes, but the most abundant and stable isotope is sodium-23 (Na-23), which has 12 neutrons in addition to its 11 protons.
Molar Mass vs. Molecular Mass
It is essential to differentiate between molar mass and molecular mass. Molar mass refers to the mass of one mole of a substance, often expressed in grams per mole (g/mol). Molecular mass (or molecular weight) typically refers to the mass of a molecule, which consists of two or more atoms chemically bonded together. Sodium, as an element, does not form diatomic molecules like hydrogen (H2) or oxygen (O2), so its molecular mass is the same as its atomic mass.
Calculating the Molecular Mass of Sodium (Na)
To calculate the molecular mass of sodium, we can use the atomic mass of sodium-23 (Na-23):
- The atomic mass of sodium-23 is approximately 22.99 atomic mass units (amu) or 22.99 g/mol.
- Therefore, the molecular mass of sodium (Na) is approximately 22.99 g/mol or 22.99 amu.
Significance of Molecular Mass in Sodium
The molecular mass of sodium is a crucial parameter with significant implications in various fields:
- Chemical Reactions: Sodium readily forms compounds with other elements, such as chlorine (NaCl) or oxygen (Na2O). The molecular mass of sodium is essential for stoichiometry, helping determine the amounts of reactants and products in chemical reactions.
- Biological Processes: Sodium ions (Na+) are vital in biology, playing a key role in nerve impulse transmission, muscle contraction, and maintaining electrolyte balance in the human body.
- Metallurgy: Sodium is used in the extraction of certain metals, such as titanium and zirconium, from their ores. Its molecular mass is relevant in metallurgical processes.
- Alloys: Sodium can be incorporated into alloys, such as sodium-potassium alloy, which has applications in heat transfer and cooling systems.
- Nuclear Reactors: In certain nuclear reactors employing liquid metal cooling systems, sodium serves as a coolant. The mole mass of sodium is important in the design and operation of such reactors.
Conclusion
Sodium, with a mole mass of approximately 22.99 g/mol or 22.99 amu, is a fundamental element in chemistry and biology. Its molecular mass is a crucial parameter in various chemical reactions, biological processes, and industrial applications. Sodium’s role in our daily lives extends from table salt (sodium chloride) to essential bodily functions, illustrating the significance of understanding its molecular mass and its behavior in various contexts.
Read More
- Molecular Mass of Sucrose
- Molecular Mass of Chlorine
- Molar Mass Of C2H5OH
- Molecular Weight of Co2
- Molecular Mass of Water
Frequently Asked Questions (FAQs) mole Mass Of Sodium
What is the mole mass of sodium?
The mole mass of sodium (Na) is approximately 22.99 grams per mole (g/mol) or 22.99 atomic mass units (amu). It represents the mass of one mole of sodium atoms.
How is the mole mass of sodium calculated?
The mole mass of sodium is calculated using the atomic mass of its most abundant and stable isotope, sodium-23 (Na-23), which has an atomic mass of approximately 22.99 amu or 22.99 g/mol.
What is the significance of knowing the mole mass of sodium?
Knowing the mole mass of sodium is important in various fields, including chemistry, biology, and industry. It is crucial for stoichiometry in chemical reactions, understanding the behavior of sodium compounds, and its role in biological processes such as nerve signaling and muscle contraction.
What are some common compounds of sodium?
Sodium forms numerous compounds, including sodium chloride (table salt, NaCl), sodium hydroxide (caustic soda, NaOH), sodium carbonate (soda ash, Na2CO3), and sodium bicarbonate (baking soda, NaHCO3), among others. These compounds have various industrial, culinary, and chemical applications.
Why is sodium important in biological processes?
Sodium ions (Na+) are essential in biological processes such as nerve impulse transmission, muscle contraction, and the maintenance of electrolyte balance in the human body. Sodium channels in cell membranes allow for the passage of sodium ions, facilitating electrical signaling.
Molecular Mass of Sucrose
Molecular Mass of Sucrose: Sucrose, commonly known as table sugar, is one of the most familiar and widely used carbohydrates in the world. Its sweet taste makes it a favorite ingredient in many culinary creations, from desserts to beverages.
But beyond its taste, sucrose has a fascinating chemical structure with a specific molecular mass that plays a crucial role in various applications and industries. In this article, we will explore the molecular mass of sucrose and its significance in both the culinary world and scientific research.

Molecular Mass of Sucrose
The Chemical Structure of Sucrose
Sucrose is a disaccharide, which means it consists of two simpler sugar molecules chemically bonded together. Specifically, it is composed of one molecule of glucose and one molecule of fructose linked by a glycosidic bond. This unique combination of glucose and fructose is what gives sucrose its distinctive sweet taste.
The molecular formula of sucrose is C12H22O11, representing 12 carbon (C) atoms, 22 hydrogen (H) atoms, and 11 oxygen (O) atoms. The molecular mass of sucrose is calculated by summing the atomic masses of these elements in the given ratio.
Calculating the Molecular Mass of Sucrose (C12H22O11)
To determine the molecular mass of sucrose, we need to consider the atomic masses of carbon (C), hydrogen (H), and oxygen (O):
- The atomic mass of carbon (C) is approximately 12.01 atomic mass units (amu).
- The atomic mass of hydrogen (H) is approximately 1.01 amu.
- The atomic mass of oxygen (O) is approximately 16.00 amu.
Now, let’s calculate the mol mass of sucrose:
The mol Mass of Sucrose = (12 × Atomic Mass of C) + (22 × Atomic Mass of H) + (11 × Atomic Mass of O)
mol Mass of Sucrose = (12 × 12.01 amu) + (22 × 1.01 amu) + (11 × 16.00 amu)
mol Mass of Sucrose ≈ 342.30 amu
Therefore, the mol mass of sucrose is approximately 342.30 atomic mass units (amu).
Significance of mol Mass in Sucrose
he mol mass of sucrose is a fundamental parameter with significant implications in various fields:
- Culinary Science: In cooking and baking, knowing the molecular mass of sucrose is crucial for precise recipe measurements. It helps determine the sweetness level, texture, and overall quality of dishes and desserts.
- Chemical Analysis: Sucrose can be quantified in food and beverage products through various analytical techniques. Understanding its molecular mass aids in accurate concentration calculations.
- Biological Processes: In biology and nutrition, the molecular mass of sucrose is essential for understanding how it is metabolized in the human body and its impact on health.
- Chemical Reactions: Sucrose can undergo various chemical reactions, such as hydrolysis, which breaks it down into its constituent sugars, glucose, and fructose. The molecular mass is essential for stoichiometry in such reactions.
- Food Industry: The sugar content of food and beverages is often determined by measuring sucrose concentration, and its molecular mass is used to calculate nutritional information.
Conclusion
Sucrose, the sweetener found in everyday table sugar, has a mol mass of approximately 342.30 atomic mass units (amu). This molecular mass is a vital factor in the world of culinary arts, scientific research, and various industries where sucrose plays a significant role. Understanding the molecular mass of sucrose enables precise measurements, ensures the quality of food products, and contributes to our comprehension of the chemistry behind the sweet taste we all enjoy.
Read More
- Molecular Mass Of O2
- Molecular Mass of H2
- Molecular Mass Of NH3
- Molecular Mass Of Ch4
- Nitric Acid Molar Mass
Frequently Asked Questions (FAQs) mol Mass of Sucrose
What is the mol mass of sucrose?
The mol mass of sucrose (C12H22O11), commonly known as table sugar, is approximately 342.30 atomic mass units (amu).
How is the molecular mass of sucrose calculated?
The mol mass of sucrose is calculated by summing the atomic masses of the carbon (C), hydrogen (H), and oxygen (O) atoms in its chemical formula in the given ratio. The atomic masses are obtained from the periodic table.
What is the significance of knowing the mol mass of sucrose?
Understanding the mol mass of sucrose is essential in various fields, including culinary science, chemical analysis, nutrition, and the food industry. It helps in precise recipe measurements, determining sweetness levels, and calculating nutritional information, among other applications.
Why is sucrose referred to as a disaccharide?
Sucrose is classified as a disaccharide because it is composed of two simpler sugar molecules, specifically glucose and fructose. They chemically bonded together through a glycosidic bond.
How is sucrose metabolized in the human body?
Sucrose is broken down into its constituent sugars, glucose and fructose, during digestion. These sugars are then absorbed into the bloodstream and utilized for energy or stored in the body.
Molecular Mass of Chlorine
Molecular Mass of Chlorine: Chlorine is a chemical element with the symbol “Cl” and the atomic number 17. It is a vital element in chemistry and plays crucial roles in various aspects of our lives, from disinfecting swimming pools to serving as a feedstock in the production of plastics and chemicals. To understand the properties and applications of chlorine, it’s essential to explore its molecular mass or atomic weight.

Molecular Mass of Chlorine
Chlorine’s Atomic Structure
Before diving into the molecular mass of chlorine, let’s take a brief look at its atomic structure. Chlorine has 17 electrons, arranged in three energy levels. Its atomic mass is approximately 35.45 atomic mass units (amu). It has two stable isotopes: chlorine-35 (about 75% abundance) and chlorine-37 (about 25% abundance), which contribute to its average atomic mass.
Molecular Mass vs. Atomic Mass
It’s important to distinguish between atomic mass and molecular mass. Atomic mass refers to the mass of a single atom of an element, while molecular mass (also known as molecular weight or molar mass) is the mass of a molecule, which is composed of two or more atoms. In the case of chlorine, it naturally exists as a diatomic molecule, Cl2, in its elemental form. To calculate the molecular mass of chlorine (Cl2), we add the atomic masses of the two chlorine atoms.
Calculating the Molecular Mass of Chlorine (Cl2)
To determine the molecular mass of Cl2, we need to consider the atomic masses of the chlorine atoms.
- The atomic mass of chlorine-35 (the more abundant isotope) is approximately 35.45 amu.
- The atomic mass of chlorine-37 is approximately 36.45 amu.
Now, we can calculate the molecular mass of Cl2:
Molecular Mass of Cl2 = (2 × Atomic Mass of Cl)
M – Cl2 = (2 × 35.45 amu)
Molecular Mass of Cl2 ≈ 70.90 amu
Therefore, the molecular mass of chlorine gas (Cl2) is approximately 70.90 atomic mass units (amu).
Significance of Mole Mass of Chlorine
Understanding the molecular mass of chlorine is important for various reasons:
- Chemical Reactions: It helps chemists predict the stoichiometry of reactions involving chlorine. For example, when chlorine gas reacts with sodium metal, it forms sodium chloride (table salt) with a known ratio based on molecular mass.
- Dosage in Water Treatment: Chlorine is commonly used to disinfect drinking water and swimming pools. Knowledge of its molecular mass helps determine the appropriate dosage required for effective disinfection.
- Industrial Processes: In industrial applications, chlorine is used in the production of chemicals like PVC (polyvinyl chloride) and as a bleach. Accurate calculations of molecular mass are critical for process control.
- Environmental Impact: Understanding the molecular mass of chlorine and its compounds is essential for assessing its environmental impact and behavior in natural systems.
Conclusion
Chlorine, a fundamental element in chemistry, has a molecular mass of approximately 70.90 atomic mass units (amu) when it exists as the diatomic molecule Cl2. This molecular mass is vital for various chemical applications, including water treatment, industrial processes, and environmental assessments. By comprehending the molecular mass of chlorine and its chemical properties, scientists and engineers can harness its potential for a wide range of beneficial applications while ensuring its responsible and safe use.
Read More
- Molar Mass Of C2H5OH
- Molecular Weight of Co2
- Molecular Mass of Water
- Difference Between Cyclone And Hurricane
- Molecular Weight Of Na
Frequently Asked Questions (FAQs) Mole Mass of Chlorine
What is the mole mass of chlorine?
The mole mass of chlorine (Cl2) is approximately 70.90 atomic mass units (amu).
How is the mole mass of chlorine calculated?
The mole mass of chlorine is calculated by adding the atomic masses of the two chlorine atoms in a chlorine molecule (Cl2). The atomic mass of chlorine is approximately 35.45 amu.
What is the difference between atomic mass and mole mass?
Atomic mass refers to the mass of a single atom of an element, while molecular mass (or molecular weight) is the mass of a molecule, which consists of two or more atoms. In the case of chlorine, it naturally exists as Cl2, a diatomic molecule.
Why is knowing the mole mass of chlorine important?
Comprehending the molecular weight of chlorine is essential across a range of chemical contexts. This knowledge aids in predicting chemical reactions, establishing precise disinfection dosages for water treatment, and maintaining control over industrial processes that rely on chlorine.
What are the common applications of chlorine in chemistry?
Chlorine finds application in multiple areas, serving as a disinfectant for purifying drinking water and maintaining the cleanliness of swimming pools. Additionally, it acts as a fundamental component in the synthesis of chemicals such as PVC (polyvinyl chloride), plays a pivotal role in the production of bleach, and holds significance as a critical element in various industrial processes.
Molar Mass Of C2H5OH
Molar Mass Of C2H5OH: The molar mass of a chemical compound is a crucial concept in chemistry. It represents the mass of one mole of that compound and is expressed in grams per mole (g/mol).
Calculating the molar mass of a compound is fundamental for various applications in chemistry, such as determining the quantity of reactants and products in chemical reactions. In this article, we will explore the calculation of the molar mass of C2H5OH, which is the chemical formula for ethanol, a widely-used alcohol in beverages, industry, and research.

Molar Mass Of C2H5OH
Molar Mass and its Significance
The molar mass of a compound is the sum of the atomic masses of all the atoms present in its chemical formula. It is calculated using the atomic masses of each element as they appear in the formula, taking into account the number of atoms of each element.
In the case of ethanol (C2H5OH), the molar mass calculation involves adding up the atomic masses of carbon (C), hydrogen (H), and oxygen (O) in the given ratio.
Atomic Masses of Elements in C2H5OH
To calculate the molar mass of C2H5OH, we need to know the atomic masses of the elements it contains:
- Carbon (C) has an atomic mass of approximately 12.01 g/mol.
- Hydrogen (H) has an atomic mass of approximately 1.01 g/mol.
- Oxygen (O) has an atomic mass of approximately 16.00 g/mol.
Calculating the Molar Mass of C2H5OH (Ethanol)
The chemical formula C2H5OH indicates that there are two carbon atoms (C), six hydrogen atoms (H), and one oxygen atom (O) in one molecule of ethanol (C2H5OH).
To calculate the molar mass, we sum the atomic masses of these elements, taking into account their respective quantities in one molecule of ethanol:
Molar Mass of C2H5OH = (2 × Atomic Mass of C) + (6 × Atomic Mass of H) + (1 × Atomic Mass of O)
Molar Mass of C2H5OH = (2 × 12.01 g/mol) + (6 × 1.01 g/mol) + (1 × 16.00 g/mol)
Mol Mass of C2H5OH = 24.02 g/mol + 6.06 g/mol + 16.00 g/mol
Mol Mass of C2H5OH ≈ 46.08 g/mol
So, the molar mass of ethanol (C2H5OH) is approximately 46.08 grams per mole (g/mol).
Significance of Molar Mass in Chemistry
The molar mass of a compound is a critical parameter in various aspects of chemistry:
- Stoichiometry: It is essential for balancing chemical equations, determining reactant and product quantities, and predicting the outcomes of chemical reactions.
- Concentration: In solutions, mol mass is used to calculate the concentration of a solute, which is vital in analytical chemistry and laboratory experiments.
- Chemical Analysis: Molar mass helps identify and quantify unknown compounds through techniques such as mass spectrometry and gas chromatography.
- Determining Molecular Weight: It provides valuable information about the size and weight of molecules, which is crucial in polymer chemistry and material science.
- Mole Concept: It relates the mass of a substance to the number of moles, facilitating the conversion between mass and moles in chemical calculations.
Conclusion
The mol mass of a compound, such as C2H5OH (ethanol), is a fundamental concept in chemistry. It represents the mass of one mole of that compound and is calculated by summing the atomic masses of the elements in its chemical formula. In the case of ethanol, its molar mass is approximately 46.08 g/mol. Understanding molar mass is essential for various applications in chemistry, including stoichiometry, concentration calculations, chemical analysis, and the determination of molecular weights, making it a cornerstone of chemical science.
Read More
- Molecular Weight of Co2
- Molecular Mass of Water
- Difference Between Cyclone And Hurricane
- Molecular Weight Of Na
- Molecular Mass Of O2
Frequently Asked Questions (FAQs) Molar Mass Of C2H5OH
What is the molar mass of C2H5OH (ethanol)?
The mol mass of ethanol (C2H5OH) is approximately 46.08 grams per mole (g/mol). This value is calculated by summing the atomic masses of the carbon (C), hydrogen (H), and oxygen (O) atoms in one molecule of ethanol.
How is the molar mass of C2H5OH calculated?
The mol mass of C2H5OH is calculated by adding up the atomic masses of its constituent elements in the given ratio. For ethanol, it’s (2 × Atomic Mass of C) + (6 × Atomic Mass of H) + (1 × Atomic Mass of O), which results in approximately 46.08 g/mol.
Why is knowing the molar mass of C2H5OH important in chemistry?
The molar mass is crucial in chemistry for various reasons, including stoichiometry, balancing chemical equations, determining concentrations in solutions, identifying unknown compounds, and understanding molecular weights in chemical reactions and material science.
What is the significance of the molar mass in stoichiometry?
In stoichiometry, molar mass is essential for balancing chemical equations and determining the quantitative relationships between reactants and products. It helps calculate the amount of substance in moles based on mass or vice versa.
How can the molar mass of C2H5OH be used in practical applications?
The molar mass of ethanol is used in various applications, such as determining the alcohol content in beverages, calculating the amount of ethanol needed in chemical reactions, and ensuring the proper mixing of fuel blends, like ethanol and gasoline.
