Tag: acids bases and salts class 10
Acids Bases and Salts Class 10 Notes of NCERT Science Ch. 2
Acids Bases and Salts Class 10 Notes: A salt is created through the replacement of hydrogen ions by a metal or an ammonium ion in the precens of acid. A substance referred to as a base undergoes a reaction with an acid, resulting in the production of water and salt exclusively. Consequently, the combination of an acid and a base leads to the formation of a salt.
Household cleaning products are used to remove oil from windows and floors contain bases. Bases found in items like soaps, toothpaste, egg whites, dishwashing liquids, and household ammonia.
This article will give you a overview and quick short notes on class 10 chapter 2: Acid bases and salts.
CBSE Class 10 Science Notes Chapter 2 Acids Bases And Salts
Acids Bases and Salts Class 10 Notes
Introduction of Acid Base and Salts: Short Notes
An acid is defined as a substance that exhibits a sour taste when dissolved in water, turns blue litmus paper to red, and has the ability to neutralize bases. Conversely, a base is a substance whose aqueous solution tastes bitter, turns red litmus paper to blue, and can neutralize acids. On the other hand, salt is a neutral substance that does not have any impact on litmus paper when dissolved in an aqueous solution.
Acids Bases and Salts can be classified based on:
a) Composition: This includes elements, compounds, and mixtures.
b) State: Matter can exist in the form of solids, liquids, or gases.
c) Solubility: Matter can be categorized as suspensions, colloids, or solutions based on its ability to dissolve.
Mixtures can be further classified as homogeneous or heterogeneous, depending on the uniformity of their composition. Compounds, on the other hand, can be categorised as covalent or ionic compounds based on the types of chemical bonds present within them.
What Is an Acid, Base and Salts
An acid is characterised as any substance that contains hydrogen and has the ability to donate a proton, which is a hydrogen ion, to another substance. On the other hand, a base refers to a molecule or ion that can accept a hydrogen ion from an acid. Acidic substances are commonly recognised by their sour taste, which is a distinctive characteristic.
Ionisable and Non-Ionisable Compounds
As per chapter 2 of class 10 Ionizable compounds are substances that have the ability to undergo ionization, which is the process of forming ions by gaining or losing charged particles (usually electrons or protons) when dissolved in a solvent like water. In other words, ionizable compounds can dissociate into ions in solution.
On the other hand, non-ionizable compounds are substances that do not readily undergo ionization when dissolved in a solvent. These compounds remain predominantly in their molecular form and do not dissociate into ions.
Arrhenius’ Theory of Acids and Bases
According to the Arrhenius definition, an acid is a substance that, when dissolved in water, dissociates to produce H+ (aq) or H3O+ ions. Conversely, an Arrhenius base is a substance that, when dissolved in water, dissociates to yield OH− ions.
Examples:
Acids:
- Hydrochloric acid (HCl)
- Sulphuric acid (H2SO4)
- Nitric acid (HNO3)
Bases:
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Calcium hydroxide (Ca(OH)2)
Bronsted Lowry Theory
A Bronsted acid is defined as a substance that donates an H+ (aq) ion in a chemical reaction. Conversely, a Bronsted base is a substance that accepts an H+ (aq) ion.
Example:
In the reaction: HCl (aq) + NH3 (aq) → NH+4(aq) + Cl− (aq)
- HCl acts as a Bronsted acid because it donates an H+ (aq) ion.
- Cl− is the conjugate base of HCl because it remains after the acid donates its H+ (aq) ion.
- NH3 acts as a Bronsted base because it accepts the H+ (aq) ion from HCl.
- NH+4 is the conjugate acid of NH3 because it is formed after accepting the H+ (aq) ion.
Physical Test for Acid and Base

Two physical tests can be employed to identify an acid or a base.
a. Taste: An acid typically has a sour taste, while a base has a bitter taste. However, this method of taste is not recommended as it can be unsafe due to potential contamination or corrosiveness of acids and bases.
Example: Compounds like curd, lemon juice, orange juice, and vinegar possess a sour taste because they contain acids. Baking soda, on the other hand, has a bitter taste and serves as an example of a base.
b. Effect on Indicators by Acids and Bases: Indicators are chemical substances that undergo a change in their physical properties, such as color or odor, when in contact with an acid or a base. The following are commonly used indicators and the colors they exhibit:
a) Litmus:
- In a neutral solution: Purple
- In an acidic solution: Red
- In a basic solution: Blue
Litmus is available in the form of paper strips, with red litmus and blue litmus variants. An acid turns a moist blue litmus paper to red, while a base turns a moist red litmus paper to blue.
b) Methyl orange:
- In a neutral solution: Orange
- In an acidic solution: Red
- In a basic solution: Yellow
c) Phenolphthalein:
- In a neutral solution: Colorless
- In an acidic solution: Remains colorless
- In a basic solution: Pink
These indicators can be used to determine whether a substance is acidic or basic based on the observed color change.
Acid-Base Reactions for Class 10 Students
An acid-base neutralization reaction takes place when an acid reacts with a base. The outcome of this reaction is the formation of salt and water as the end products. In the standard approach, an acid-base neutralization reaction is represented as a double-replacement reaction.
Class 10 Reactions of Acids and Bases
a) Reaction of acids and bases with metals
Acids, in general, react with metals to produce salt and hydrogen gas. Bases, in general, do not react with metals and do not produce hydrogen gas.
Acid + active metal → salt + hydrogen + heat
2HCl + Mg → MgCl2 + H2 (↑)
Hydrochloric acid + Magnesium → Magnesium chloride + Hydrogen
Base + metal → salt + hydrogen + heat
2NaOH + Zn → Na2ZnO2 + H2 (↑)
Sodium hydroxide + Zinc → Sodium zincate + Hydrogen
A more reactive metal displaces the less reactive metal from its base.
2Na + Mg (OH) 2 → 2NaOH + Mg
Sodium + Magnesium hydroxide → Sodium hydroxide + Magnesium
b) Reaction of acids with metal carbonates and bicarbonates
Acids produce carbon dioxide, as well as metal salts and water, when they react with metal carbonates or metal bicarbonates. Sodium chloride, carbon dioxide, and water are formed when sodium carbonate interacts with hydrochloric acid. Allowing carbon dioxide gas to travel through lime water turns it milky.
Acid + metal carbonate or bicarbonate → salt + water + carbon dioxide.
2HCl + CaCO3 → CaCl2 + H2O + CO2
H2SO4 + Mg (HCO3)2 → MgSO4 + 2H2O + 2CO2
Effervescence indicates the liberation of CO2 gas.
c) Reaction of Acid with Base given in class 10
1. Reaction of metal oxides and hydroxides with acids
Metal oxides or metal hydroxides are basic in nature.
Acid + base → salt + water + heat
H2SO4 + MgO → MgSO4 + H2O
2HCl + Mg (OH) 2 → MgCl2 + 2H2O
2. Reaction of non-metal oxides with bases
Non-metal oxides are acidic in nature
Base + Nonmetal oxide → salt + water + heat
2NaOH + CO2→ Na2CO3 + H2O
3. Reaction of acids and base
A very common acid is hydrochloric acid. The reaction between strong acid, says hydrochloric acid and strong base say sodium hydroxide, forms salt and water. The complete chemical equation is shown below.
HCl (strong acid) + NaOH (strong base) → NaCl (salt) + H2O (water)
Water:
Water itself is a neutral substance and does not exhibit acidic or basic properties. However, it plays a crucial role in the dissociation of acids and bases when they are added to it. Acids and bases dissociate into their respective ions when dissolved in water, allowing them to conduct electricity.
Difference between a Base and an Alkali:
Base:
- Bases can undergo a neutralization reaction with acids.
- They are composed of metal oxides, metal hydroxides, metal carbonates, and metal bicarbonates.
- Most bases are insoluble or only slightly soluble in water.
Alkali:
- An alkali refers specifically to an aqueous solution of a base, primarily consisting of metallic hydroxides.
- Alkalis readily dissolve in water and dissociate to produce OH− ions.
- It’s important to note that while all alkalis are bases, not all bases are alkalis. The term “alkali” specifically applies to bases that are soluble in water and form alkaline solutions.
Hydronium Ion
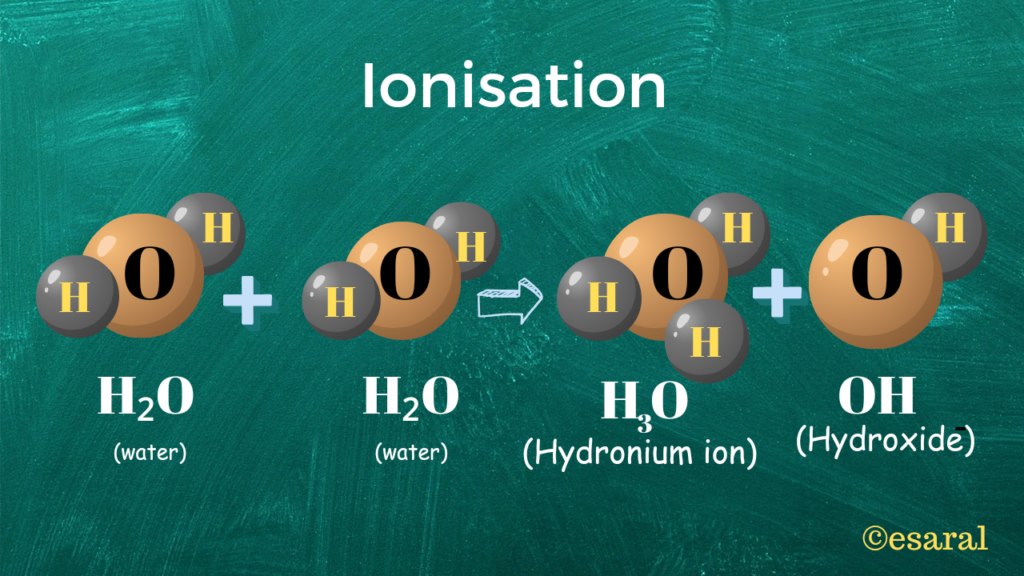
Acids Bases and Salts Class 10 Notes
A hydronium ion is created when a hydrogen ion (H+) accepts a lone pair of electrons from the oxygen atom of a water molecule. This interaction leads to the formation of a coordinate covalent bond between the hydrogen ion and the water molecule.
Dilution: Dilution refers to the process of decreasing the concentration of a solution by adding more solvent, usually water. This addition of solvent is typically an exothermic process, releasing heat. When diluting an acid, it is important to add the acid to water gradually, and not the other way around, to prevent potential splattering or hazardous reactions.
Strength of Acids and Bases: Strong acid or base: A strong acid or base is characterized by complete dissociation in water, where all the molecules of a given amount fully separate into their respective ions, resulting in the presence of H+(aq) ions for acids and OH−(aq) ions for bases.
Weak acid or base: In contrast, a weak acid or base only partially dissociates in water, with only a few of the molecules from a given amount breaking apart to form their respective ions. This results in the presence of H+(aq) ions for acids and OH−(aq) ions for bases, but in smaller quantities compared to strong acids or bases.
pH
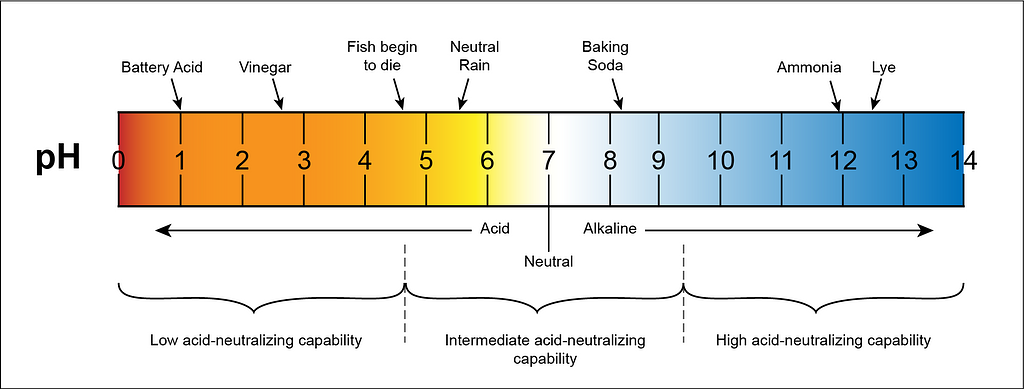
A universal indicator is a type of pH indicator that provides a pH range from 0 to 14, allowing it to indicate the acidity or alkalinity of a solution. It changes color depending on the pH value of the solution, providing a visual representation of its acid-base nature.
PH Scale for Acids Bases and Salts
A neutral solution, which is neither acidic nor alkaline, has a pH value of 7.
The pH of a solution can be calculated using the equation pH = -log10[H+]. In pure water, the concentrations of both hydrogen ions ([H+]) and hydroxide ions ([OH-]) are equal to 10^-7 mol/L. Therefore, the pH of pure water is 7.
The pH scale spans from 0 to 14, where values below 7 indicate an acidic solution, values above 7 indicate a basic (alkaline) solution, and a pH of 7 represents a neutral solution.
Importance of pH in Everyday Life: Class 10 Notes
1. pH sensitivity of plants and animals:
Plants and animals exhibit sensitivity to pH levels. Vital biological processes such as food digestion, enzyme functions, and hormonal activities rely on specific pH values for proper functioning.
2. pH of soil:
For optimal plant growth and cultivation, the pH of soil is ideally maintained between 6.5 and 7.0. This range provides favorable conditions for nutrient availability and absorption by plants.
3. pH in the digestive system:
The digestive process within our stomach occurs at a specific pH range of 1.5 to 4. The acidic environment, influenced by hydrochloric acid (HCl), aids in the breakdown of food. Enzymatic activities during digestion are also dependent on the pH conditions within the stomach.
4. pH in tooth decay:
Tooth decay occurs when teeth are exposed to an acidic environment with a pH of 5.5 and below. Acidic conditions promote the erosion of tooth enamel, leading to decay and cavities.
5. pH in self-defence mechanisms of animals and plants:
Both animals and plants employ acidic substances as a means of self-defense. For instance, bees and plants like nettles secrete highly acidic substances to deter threats. These secreted acidic substances possess specific pH levels that contribute to their defensive properties.
Manufacture of Acids and Bases

Acids Bases and Salts Class 10 Notes
a) Nonmetal oxide + water → acid
SO2(g) + H2O(l) → H2SO3(aq)
SO3(g) + H2O(l) → H2SO4(aq)
4NO2(g) + 2H2O(l) + O2(g) → 4HNO3(aq)
Non-metal oxides are thus referred to as acid anhydrides.
b) Hydrogen + halogen → acid
H2(g) + Cl2(g) → 2HCl(g)
HCl(g) + H2O(l) → HCl(aq)
c) Metallic salt + conc. sulphuric acid → salt + more volatile acid
2NaCl(aq) + H2SO4(aq) → Na2SO4(aq) + 2HCl(aq)
2KNO3(aq) + H2SO4(aq) → K2SO4(aq) + 2HNO3(aq)
d) Metal + oxygen → metallic oxide (base)
4Na(s) + O2(g) → 2Na2O(s)
2Mg(s) + O2(g) → 2MgO(s)
e) Metal + water → base or alkali + hydrogen
Zn(s) + H2O(steam) → ZnO(s)+ H2(g)
f) Few metallic oxides + water → alkali
Na2O(s) + H2O(l) → 2NaOH(aq)
g) Ammonia + water → ammonium hydroxide
NH3(g) + H2O(l) → NH4OH(aq)
Salts
Salt is a general term used to refer to a class of compounds composed of ions held together by ionic bonds. In chemistry, salt specifically refers to a compound formed when the hydrogen ions of an acid are replaced by metal ions or ammonium ions. This process is known as neutralization. Salts are typically solid crystals with high melting points and can be either soluble or insoluble in water.
Common examples of salts include sodium chloride (table salt), calcium carbonate, potassium nitrate, and magnesium sulfate. Salts play essential roles in various biological, chemical, and industrial processes. They are widely used in cooking, food preservation, manufacturing, and as nutrients for plants and animals.
Types of Salts
Common Salt:
Sodium Chloride (NaCl) is commonly known as common salt because it is extensively used worldwide for culinary purposes.
Family of Salts:
Salts that share the same cation or anion belong to the same family. For instance, NaCl, KCl, and LiCl are members of the same salt family.
pH of Salts:
A salt resulting from the combination of a strong acid and a strong base will be neutral in nature, indicated by a pH value of around 7.
A salt formed from a weak acid and a strong base will be basic, with a pH value greater than 7.
Conversely, a salt produced from a strong acid and a weak base will be acidic, with a pH value below 7.
The pH of a salt derived from a weak acid and a weak base can be determined by conducting a pH test.
Chemicals from Common Salt
Sodium chloride is a widely recognized common salt, represented by its molecular formula NaCl. It is an essential component of our meals, serving as both a flavor enhancer and a preservative in our food. From common salt, we can derive the following four compounds:
1. Sodium hydroxide, also known as lye or caustic soda.
2. Baking soda, also referred to as sodium hydrogen carbonate or sodium bicarbonate.
3. Washing soda, scientifically known as sodium carbonate decahydrate.
4. Bleaching powder, which is calcium hypochlorite.
Preparation of Sodium Hydroxide:
Sodium hydroxide (NaOH) is a strong base with wide applicability. Caution must be exercised when preparing a sodium hydroxide solution by dissolving it in water, as the reaction is highly exothermic and can generate significant heat. It is crucial to ensure safety during this process to prevent splattering or boiling. The following steps outline a safe procedure for manufacturing a sodium hydroxide solution, along with recipes for different NaOH strengths:
Chemical formula: NaOH
Also known as: Caustic soda
Bleaching Powder:
Bleaching powder, which is soluble in water, is widely employed as a bleaching agent in textile industries. It serves as an oxidising agent and a disinfectant in various industrial applications. Bleaching powder is synthesised by reacting chlorine gas with dry slaked lime, represented as Ca(OH)2.
Chemical formula: Ca(OCl)Cl or CaOCl2
Preparation: Ca(OH)2(aq) + Cl2(g) → CaOCl2(aq) + H2O(l)
Upon interaction with water, bleaching powder releases chlorine, which is responsible for its bleaching action.
Uses of Bleaching Powder:
1. It is used for bleaching soiled clothes in laundry and as a bleaching agent for cotton and linen in the textile industry.
2. As a strong oxidizing agent, it finds applications in various industries.
3. It is used as a disinfectant for purifying water, making it potable.
Baking Soda:
Sodium bicarbonate, commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It consists of a sodium cation (Na+) and a bicarbonate anion (HCO3). Sodium bicarbonate exists as a white, crystalline substance, often found in the form of a fine powder. It has a slightly salty and alkaline taste, similar to washing soda (sodium carbonate).
Chemical name: Sodium hydrogen carbonate
Chemical formula: NaHCO3
Preparation (Solvay process):
a. Limestone is heated: CaCO3 → CaO + CO2
b. CO2 is passed through a concentrated solution of sodium chloride and ammonia:
NaCl(aq) + NH3(g) + CO2(g) + H2O(l) → NaHCO3(aq) + NH4Cl(aq)
Uses:
1. It reduces acidity in the stomach and acts as an antacid for treating stomach upset and indigestion.
2. It is utilized as a water softener in the process of washing.
3. Sodium bicarbonate finds applications in various household cleaning products.
Washing Soda:
Washing soda is another name for sodium carbonate decahydrate, which is a chemical compound with the formula Na2CO3·10H2O. It is commonly used in the glass, soap, and paper industries. Washing soda also functions as a water softener and is utilized as a domestic cleaner.
Chemical name: Sodium carbonate decahydrate
Chemical formula: Na2CO3·10H2O
Preparation (Solvay process):
a. Limestone is heated: CaCO3 → CaO + CO2
b. CO2 is passed through a concentrated solution of sodium chloride and ammonia:
NaCl(aq) + NH3(g) + CO2(g) + H2O(l) → NaHCO3(aq) + NH4Cl(aq)
Uses:
1. It finds applications in the glass, soap, and paper industries.
2. Washing soda is utilized as a water softener to counteract hard water.
3. It serves as a domestic cleaner for various cleaning purposes.
Crystals of Salts:
Certain salts have the ability to form crystals by combining with a specific proportion of water. The water molecules that join with the salt in a crystal are referred to as water of crystallization. Crystallization is the process by which a solid substance arranges its atoms or molecules into a highly organized structure called a crystal. This process can occur through precipitation from a solution, freezing, or, in rare cases, direct deposition from a gas.
Examples:
Table salt (sodium chloride or halite crystals), sugar (sucrose), and snowflakes are common examples of materials that can form crystals. Many gemstones, such as quartz and diamond, also exhibit a crystal structure.
Plaster of Paris
Plaster of Paris is a widely used chemical compound that finds extensive application in sculpting materials and gauze bandages. It is a white powdery substance composed of hydrated calcium sulfate, which is typically obtained by calcining gypsum. In other words, Plaster of Paris is manufactured by heating gypsum at a high temperature.
Plaster of Paris is chemically expressed as CaSO4·1/2H2O, indicating that it contains one molecule of water of crystallization.
Uses:
Plaster of Paris, also known as gypsum plaster, is employed for creating casts to aid in the healing of fractures.
Summery: Acids Bases and Salts Class 10 Notes
The “Acids Bases and Salts Class 10 Notes” provide comprehensive information on the properties and reactions of acids, bases, and salts.
The notes of Class 10’s chapter 2 “Acid bases and salts” cover various aspects, including the definition of acids and bases, their characteristics, pH scale, neutralization reactions, and the preparation of chemicals from common salt.
Additionally, the summary highlights the importance of pH in everyday life, the differences between bases and alkalis, and the types of salts.
The notes also elaborate on the formation of crystals in certain salts and the uses of Plaster of Paris. This detailed summary serves as a valuable resource for students studying these topics in their class 10 curriculum.
Read Also:
- Class 10 Notes for Science NCERT
- Life Process Notes Class 10 NCERT Science Chapter 5
- Control and Coordination Notes Class 10: NCERT Science Ch. 6
- Notes on Magnetic Effect of Electric Current Class 10 NCERT
- Notes of Our Environment Class 10: NCERT Science Chapter 13
- Class 10th How Do Organisms Reproduce Notes: Science Ch. 7
- Class 10th Heredity and Evolution Notes: NCERT Science Ch. 8
- Chemical Reaction and Equation Notes Class 10 NCERT Sci. Ch.1
- Acids Bases and Salts Class 10 Notes of NCERT Science Ch. 2
- Class 10th Chapter 11 Science Notes for NCERT Students
- Human Eye and the Colourful World Notes Chapter 10 Science
Frequently Asked Question – FAQ on Acids Bases and Salts Class 10 Notes
Q 1. In the absence of water, acids do not exhibit their characteristic acidic behaviour.
Ans: Acids require water to display their typical acidic behavior due to a process known as ionization or dissociation. Acids release positively charged hydrogen ions (H+) into the water. These hydrogen ions are responsible for the acidic properties of the solution, as they can donate protons to other substances, leading to various acid-base reactions.
In the absence of water, there is no medium for the acids to dissociate into ions. Therefore, without water, acids cannot release hydrogen ions, and as a result, they do not exhibit their usual acidic characteristics such as the ability to lower pH, react with bases, or corrode certain materials.
Q 2. What are the three properties of Acids bases and salts?
Ans: The three main properties of acids are:
1. Sour taste: Many acids have a sour taste. Tasting acids is not recommended due to their potential for high corrosiveness and harm to health.
2. Acidic pH: Acids typically have a pH value lower than 7. The pH scale ranges from 0 to 14, considering 7 as neutral, and classifying values below 7 as acidic and values above 7 as alkaline (basic). The lower the pH value, the stronger the acid.
3. Acid-base reactions: Acids have the ability to react with bases to form salts and water in a process known as neutralization. In these reactions, acids donate hydrogen ions (H+) to bases, which then accept the ions to form water. This neutralization reaction results in the reduction of acidic or basic properties, respectively.
It’s important to handle acids with care, as some can be highly corrosive and pose significant risks to health and safety. When handling acids, it’s essential to prioritize safety and follow proper laboratory protocols.
Q 3. Write the pH range for acids and alkalis?
Ans: The pH scale ranges from 0 to 14, where:
- pH values below 7 indicate acidity
- pH 7 is neutral
- pH values above 7 indicate alkalinity (also known as basic).
Q 4. What is the chemical name of gypsum?
Ans: The chemical name of gypsum is “calcium sulfate dihydrate.” The gypsum (CaSO4·2H2O) made up of calcium ions (Ca2+), sulfate ions (SO42-), and two water molecules (H2O) in each unit.
Q 5. What is the common name of the compound CaOCl2?
Ans: The common name of the compound CaOCl2 is “bleaching powder” or “chlorinated lime.” It is a white or pale grayish powder with a strong smell of chlorine.