Tag: alcohol phenol and ether previous year questions jee mains
Alcohols Phenols And Ethers Class 12
Alcohols Phenols And Ethers Class 12: Alcohols, phenols, and ethers are fundamental classes of organic compounds that play crucial roles in various chemical, industrial, and biological processes.
These compounds are characterized by the presence of oxygen atoms bonded to carbon atoms, creating a wide range of chemical properties and applications. In this article, we will delve into the structures, nomenclature, properties, and applications of alcohols, phenols, and ethers.

Alcohols Phenols And Ethers
I. Alcohols
Structure and Nomenclature: Alcohols are organic compounds that contain a hydroxyl (-OH) group attached to a carbon atom. The general formula for alcohols is R-OH, where R represents an alkyl or aryl group. Alcohols are named by replacing the -e at the end of the alkane name with -ol. For example, methane becomes methanol when a hydroxyl group is attached.
Properties:
- Alcohols exhibit a wide range of physical properties depending on the size and structure of the alkyl group. They can be liquids or solids at room temperature.
- Hydrogen bonding between the hydroxyl groups makes alcohols highly soluble in water.
- Alcohols can undergo various chemical reactions, including oxidation, esterification, and dehydration.
Applications:
- Methanol and ethanol are widely used as solvents in industries and laboratories.
- Ethanol is a common alcohol used in alcoholic beverages and as a biofuel.
- Alcohols are crucial intermediates in the synthesis of pharmaceuticals, plastics, and perfumes.
II. Phenols
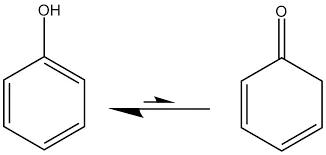
Structure and Nomenclature: Phenols are organic compounds with a hydroxyl group (-OH) attached directly to an aromatic benzene ring. The general formula for phenols is Ar-OH, where Ar represents an aromatic group. Phenols are typically named by adding the word “phenol” in front of the substituents on the benzene ring.
Properties:
- Phenols are slightly acidic due to the presence of the hydroxyl group, which can release a proton (H+) into solution.
- They have a distinct odor and are often used in perfumes and antiseptic products.
- Many phenolic compounds exhibit antioxidant properties.
Applications:
- Phenol itself is used in the production of resins, plastics, and pharmaceuticals.
- Compounds like cresol and xylenol are used as disinfectants.
- Natural phenols, such as those found in green tea and fruits, are known for their health benefits.
III. Ethers
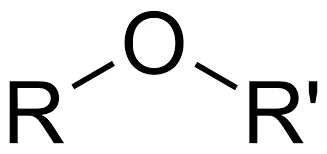
Structure and Nomenclature: Ethers consist of two organic groups (alkyl or aryl) bonded to an oxygen atom, which serves as the bridge between them. The general formula for ethers is R-O-R’, where R and R’ represent alkyl or aryl groups. Ethers are named by listing the two organic groups on either side of the oxygen atom, followed by the word “ether.”
Properties:
- Ethers have relatively low boiling points and are often volatile compounds.
- They are less polar than alcohols and less soluble in water but highly flammable.
- Ethers are relatively stable compounds.
Applications:
- Diethyl ether, historically known as “ether,” was once widely used as a general anesthetic.
- Ethers are used as solvents in laboratories and industries.
- Dimethyl ether (DME) is gaining attention as a clean-burning alternative fuel.
Conclusion
Alcohols, phenols, and ethers are essential organic compounds with diverse structures and properties, making them invaluable in various fields, including chemistry, industry, medicine, and everyday life. Their versatility in chemical reactions and their role as intermediates in the synthesis of various products underscore their importance in the world of organic chemistry. Understanding these compounds is essential for scientists and chemists working in a wide range of applications.
Read More
- Alcohols Phenols And Ethers
- Effect Of Magnet On Current Carying Wire
- Simple Harmonic Motion Formulas
- Dipole Uniform Magnetic Field
- Light Filtering Polaroid Films Polarization
Frequently Asked Questions (FAQs) Alcohols Phenols And Ethers
1. What are alcohols, phenols, and ethers?
Alcohols are organic compounds characterized by the presence of a hydroxyl (-OH) group attached to a carbon atom. Phenols are similar but have the -OH group directly attached to an aromatic benzene ring. Ethers consist of two organic groups bonded to an oxygen atom.
2. How are alcohols named?
Alcohols are typically named by replacing the -e at the end of the alkane name with -ol. For example, methane becomes methanol when a hydroxyl group is attached.
3. Are alcohols soluble in water?
Yes, alcohols are often highly soluble in water due to hydrogen bonding between the hydroxyl groups and water molecules.
4. What is the acidity of phenols?
Phenols are slightly acidic because they can release a proton (H+) into solution due to the presence of the hydroxyl group.
5. What is the main characteristic of ethers?
Ethers are characterized by the presence of an oxygen atom that serves as a bridge between two organic groups.
Alcohols Phenols And Ethers
Alcohols Phenols And Ethers: Organic chemistry is a branch of chemistry that deals with the study of carbon-containing compounds, and within this realm, alcohols, phenols, and ethers are fundamental functional groups.
These compounds play pivotal roles in both the natural world and the realm of synthetic chemistry, impacting fields ranging from pharmaceuticals to agriculture, and from materials science to biochemistry.
In this extensive 2000-word article, we will delve deep into the fascinating world of alcohols, phenols, and ethers, exploring their structures, nomenclature, properties, reactions, and significant applications.

Alcohols Phenols And Ethers
Table of Contents
1. Alcohols
- 1.1 Introduction to Alcohols
- 1.2 Nomenclature of Alcohols
- 1.3 Classification of Alcohols
- 1.4 Physical Properties of Alcohols
- 1.5 Chemical Properties of Alcohols
- 1.6 Applications of Alcohols
2. Phenols
- 2.1 Introduction to Phenols
- 2.2 Nomenclature of Phenols
- 2.3 Physical Properties of Phenols
- 2.4 Chemical Properties of Phenols
- 2.5 Acidity of Phenols
- 2.6 Applications of Phenols
3. Ethers
- 3.1 Introduction to Ethers
- 3.2 Nomenclature of Ethers
- 3.3 Physical Properties of Ethers
- 3.4 Chemical Properties of Ethers
- 3.5 Applications of Ethers
1. Alcohols
1.1 Introduction to Alcohols
Alcohols are organic compounds characterized by the presence of one or more hydroxyl (-OH) functional groups attached to carbon atoms. These compounds are ubiquitous in nature and have a significant impact on human life, ranging from the ethanol in alcoholic beverages to the glycerol in skincare products.
1.2 Nomenclature of Alcohols
The International Union of Pure and Applied Chemistry (IUPAC) provides a systematic method for naming alcohols. The general rule is to replace the -e ending of the corresponding alkane with -ol. Here are some examples:
- Methane becomes methanol
- Ethane becomes ethanol
- Propane becomes propanol
For more complex molecules, a number is used to indicate the position of the hydroxyl group, and prefixes such as “iso-” or “tert-” are added to denote branching or multiple hydroxyl groups.
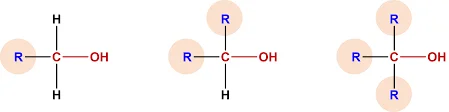
1.3 Classification of Alcohols
Alcohols can be classified into three main categories based on the number of alkyl or aryl groups bonded to the carbon atom bearing the hydroxyl group:
- Primary (1°) alcohols: The carbon atom bonded to the hydroxyl group is only attached to one other carbon atom.
- Secondary (2°) alcohols: The carbon atom bonded to the hydroxyl group is attached to two other carbon atoms.
- Tertiary (3°) alcohols: The carbon atom bonded to the hydroxyl group is attached to three other carbon atoms.
- This classification is essential because it influences the reactivity of the alcohol in various chemical reactions.
1.4 Physical Properties of Alcohols
Alcohols exhibit several notable physical properties:
Boiling and Melting Points: Alcohols generally have higher boiling and melting points compared to hydrocarbons of similar molecular weight. This is due to the presence of hydrogen bonding between alcohol molecules, which requires more energy to break.
- Solubility: Lower-molecular-weight alcohols (methanol, ethanol) are highly soluble in water due to their ability to form hydrogen bonds with water molecules. As the alkyl chain length increases, solubility in water decreases.
- Odor and Taste: Some alcohols, such as ethanol, have distinctive odors and flavors, which contribute to their use in beverages and perfumes.
1.5 Chemical Properties of Alcohols
Alcohols exhibit a wide range of chemical properties:
- Oxidation: Alcohols can be oxidized to form aldehydes or ketones, depending on their classification. Primary alcohols are oxidized to aldehydes, which can further be oxidized to carboxylic acids. Secondary alcohols are oxidized to ketones. Tertiary alcohols do not undergo oxidation under normal conditions.
- Esterification: Alcohols can react with carboxylic acids to form esters, a reaction commonly used in the fragrance and flavor industry.
- Dehydration: Alcohols can undergo dehydration reactions to form alkenes. This is often achieved by heating the alcohol in the presence of a strong acid catalyst.
- Substitution Reactions: Alcohols can undergo substitution reactions, where the -OH group is replaced with another functional group. Common substitutions include the conversion of alcohols to alkyl halides.
1.6 Applications of Alcohols
Alcohols find applications in various industries and everyday life:
- Ethanol (Ethyl Alcohol): Perhaps the most well-known alcohol, ethanol, is used as a recreational beverage, industrial solvent, and biofuel. It’s also employed as an antiseptic and disinfectant.
- Methanol (Methyl Alcohol): Methanol is used as an industrial solvent, antifreeze, and fuel. It’s also a precursor in the production of formaldehyde and other chemicals.
- Glycerol: Glycerol is used extensively in the cosmetics and pharmaceutical industries due to its moisturizing properties. It’s also utilized in the production of nitroglycerin, a potent explosive.
- Butanol: Butanol is used as a solvent, in the manufacture of plastics, and as a fuel additive. It’s also a potential biofuel.
2. Phenols
2.1 Introduction to Phenols
- Phenols are organic compounds characterized by the presence of a hydroxyl (-OH) group attached to a benzene ring. These compounds have unique properties due to the combination of the aromatic benzene ring and the hydroxyl group.
2.2 Nomenclature of Phenols
- Nomenclature of phenols is relatively straightforward. The hydroxyl group is considered the functional group, and the compound is named by adding the word “phenol” to the name of the substituent(s) on the benzene ring. For example, if a hydroxyl group is attached to a benzene ring with a methyl group, it is called “methylphenol.”
2.3 Physical Properties of Phenols
Phenols exhibit several distinctive physical properties:
- Higher Melting Points: Phenols generally have higher melting points compared to their hydrocarbon counterparts due to hydrogen bonding between phenol molecules. This property makes them solid at room temperature.
- Acidity: Phenols are more acidic than alcohols because the hydroxyl group in phenols is directly attached to the benzene ring, making it more stable. The acidity of phenols allows them to react with strong bases to form phenoxide ions.
- Aroma and Taste: Some phenols contribute to the characteristic aroma and taste of various natural products. For example, phenol derivatives are found in essential oils and spices.
2.4 Chemical Properties of Phenols
Phenols exhibit a variety of chemical reactions:
- Electrophilic Aromatic Substitution: Phenols are highly susceptible to electrophilic aromatic substitution reactions due to the electron-rich nature of the benzene ring. This makes them valuable intermediates in the synthesis of numerous organic compounds.
- Esterification: Phenols can react with carboxylic acids to form phenolic esters, which have applications in the fragrance and flavor industry.
- Oxidation: Phenols can be oxidized to quinones, which are important in biological processes and as precursors in the synthesis of dyes and pigments.
2.5 Acidity of Phenols
- The increased acidity of phenols, compared to alcohols, is due to the resonance stabilization of the phenoxide ion formed when a proton is lost from the hydroxyl group. This makes phenols capable of reacting with strong bases, a property not shared by alcohols.
2.6 Applications of Phenols
Phenols have diverse applications in various industries:
- Antiseptics and Disinfectants: Phenol and its derivatives have been historically used as antiseptics and disinfectants. However, their use has diminished due to toxicity concerns.
- Phenolic Resins: Phenolic resins, produced by the condensation of phenol with formaldehyde, are widely used in the production of molded plastic objects, laminates, and coatings.
- Pharmaceuticals: Phenols are important intermediates in the synthesis of pharmaceuticals, agrochemicals, and dyes.
3. Ethers
3.1 Introduction to Ethers
- Ethers are organic compounds characterized by an oxygen atom (-O-) bonded to two alkyl or aryl groups. The general structure of ethers is R-O-R’, where R and R’ represent alkyl or aryl groups. Ethers are known for their unique chemical properties, especially their low reactivity compared to other functional groups.
3.2 Nomenclature of Ethers
- The nomenclature of ethers is based on the names of the alkyl or aryl groups bonded to the oxygen atom, followed by the word “ether.” For example, diethyl ether has two ethyl groups bonded to the oxygen atom.
3.3 Physical Properties of Ethers
Ethers possess several notable physical properties:
- Boiling Points: Ethers generally have lower boiling points compared to alcohols of similar molecular weight. This is because ethers do not form hydrogen bonds with one another, unlike alcohols.
- Solubility: Ethers are typically more soluble in organic solvents than in water. However, their solubility in water is still higher than that of most hydrocarbons due to their oxygen atom.
- Flammability: Some ethers are highly flammable, which can be a safety concern.
3.4 Chemical Properties of Ethers
- Ethers are known for their relative chemical stability compared to other functional groups. They do not readily undergo reactions with common reagents under normal conditions. However, they can be cleaved by strong acids to form alcohol and alkyl halide products, a reaction known as “acid-catalyzed cleavage of ethers.”
3.5 Applications of Ethers
Despite their low reactivity, ethers have several practical applications:
- Solvents: Ethers are valuable solvents in laboratories and industries. Diethyl ether, for instance, is commonly used as a solvent for various chemical reactions.
- Anesthetics: Diethyl ether was historically used as a general anesthetic before safer alternatives became available.
- Fuel Additives: Methyl tertiary-butyl ether (MTBE) and ethyl tertiary-butyl ether (ETBE) are ethers used as fuel additives to improve octane ratings and reduce emissions in gasoline.
- Crown Ethers: Crown ethers, a subclass of ethers, are widely used in chemistry for their ability to complex with metal ions, aiding in separations and ion transport.
4. Conclusion
In conclusion, alcohols, phenols, and ethers are essential functional groups in organic chemistry, each with its unique properties, nomenclature, and reactivity. These compounds play crucial roles in a wide range of applications, from the production of pharmaceuticals and plastics to the formulation of perfumes and the development of new materials. Understanding the chemistry of alcohols, phenols, and ethers is fundamental to both students embarking on a journey in chemistry and professionals working in fields where these compounds are integral. As our understanding of these compounds continues to grow, their applications and significance in our world will undoubtedly expand as well.
Read More
- Effect Of Magnet On Current Carying Wire
- Simple Harmonic Motion Formulas
- Dipole Uniform Magnetic Field
- Light Filtering Polaroid Films Polarization
- Animals Biology Science Lion Zoology
Frequently Asked Questions (FAQs) Alcohols Phenols And Ethers
What are alcohols, phenols, and ethers?
Alcohols are organic compounds characterized by the presence of one or more hydroxyl (-OH) groups attached to carbon atoms. Phenols have a hydroxyl group attached to a benzene ring, while ethers have an oxygen atom bonded to two alkyl or aryl groups.
How are alcohols named?
Alcohols are named by replacing the -e ending of the corresponding alkane with -ol. For example, methane becomes methanol, and ethane becomes ethanol.
What is the difference between primary, secondary, and tertiary alcohols?
Alcohols are classified into three categories based on the carbon atom bearing the hydroxyl group. Primary alcohols have one alkyl group attached, secondary alcohols have two, and tertiary alcohols have three.
Why do alcohols have higher boiling points compared to hydrocarbons of similar molecular weight?
Alcohols have higher boiling points due to hydrogen bonding between alcohol molecules, which requires more energy to break than the weaker van der Waals forces in hydrocarbons.
What are the main chemical reactions of alcohols?
Alcohols can undergo oxidation to form aldehydes or ketones, esterification to form esters, and dehydration to produce alkenes, among other reactions.