Tag: Chemical Reaction and Equation Notes Class 10
Chemical Reaction and Equation Notes Class 10 NCERT Sci. Ch.1
Chemical Reaction and Equation Notes Class 10: Explore the fascinating world of Science, where we discover how things work and learn about chemical reactions that bring changes around us. From metal rusting to things breaking down, chemical reactions show us how tiny particles interact and create transformations. In Class 10 Science, Chapter 1 explains how substances change their form.
In the article “Chemical Reaction and Equation Notes Class 10” you can learn more about CBSE science class 10 chapter 1 Chemical Reaction and Equation. These notes are carefully made to give you the right amount of information without overwhelming you. They will help you prepare for exams smoothly. Understand how substances change and be ready to explore the amazing world of science. Start this educational journey now and discover the wonders of chemistry.
CBSE Science Chapter 1: Chemical Reaction and Equation Notes Class 10
Chemical Reactions
In a chemical reaction, one or more reactants change into one or more products. During this process, the atoms of the reactants get rearranged, leading to the creation of different substances as the end products.
Chemical reactions are transformations in which reactants change into products by forming or breaking bonds between different atoms.
During a chemical reaction, one set of chemical components undergoes a change to become another. These changes involve the movement of electrons in the formation and breaking of chemical bonds between atoms, with no alteration in the nuclei. Chemical equations are used to describe these reactions. The rate at which chemical reactions occur can be predicted at a given temperature and chemical concentration. Generally, reaction rates increase with higher temperatures since more thermal energy is available to reach the activation energy required to break bonds between atoms.
Types of Chemical Reactions Described in Chemical Reaction and Equation Notes Class 10
Considering various factors, chemical reactions can be categorized into multiple groups. Here are a few examples:
- Combination
- Decomposition
- Single Displacement
- Double displacement
- Redox
- Endothermic
- Exothermic
- Precipitation
- Neutralisation
Combination Reaction
A combination reaction is a type of chemical reaction where two or more substances react to form a single new compound. In this reaction, elements or compounds come together to create a more complex compound as the product.
Let’s take the example of the reaction between iron (Fe) and sulfur (S) to form iron(II) sulfide (FeS):
Fe + S → FeS
In this combination reaction, solid iron and solid sulfur combine to produce iron(II) sulfide, a new compound. This type of reaction can occur under appropriate conditions, such as when iron and sulfur are heated together. The atoms of iron and sulfur rearrange to form the compound FeS.
Combination reactions are essential in various chemical processes, ranging from the formation of simple compounds like water to more complex ones found in industrial applications and biological systems.
Decomposition Reaction
Decomposition reactions involve the breakdown of one compound into two or more compounds or elements. They are the opposite of combination reactions.
A typical decomposition reaction is represented as follows:
AB → A + B
Examples:
1. When hydrogen peroxide (H2O2) decomposes, it forms water (H2O) and oxygen gas (O2).
2H2O2(l) → 2H2O(l) + O2(g)
Hydrogen peroxide → Water + Oxygen gas
2. Silver chloride (AgCl) decomposes upon exposure to light, forming silver (Ag) and chlorine gas (Cl2).
2AgCl(s) + light → 2Ag(s) + Cl2(g)
Silver chloride + Light → Silver + Chlorine gas
Decomposition reactions are significant in various fields, such as chemistry, biology, and environmental science. They are involved in processes ranging from the breakdown of compounds in nature to chemical reactions in industrial applications.
a. Decomposition reactions that require heat are known as thermolytic decomposition or thermolysis.
Example:
Thermal decomposition of HgO:
2HgO(s) → 2Hg(l) + O2(g)
b. Decomposition reactions that require light are called photolytic decomposition or photolysis.
Example:
Photolytic decomposition of H2O2:
2H2O2(l) + light → 2H2O(l) + O2(g)
c. Decomposition reactions that require electricity are referred to as electrolytic decomposition or electrolysis.
Example:
Electrolytic decomposition of water:
2H2O(l) → 2H2(g) + O2(g)
In these specific types of decomposition reactions, the input of heat, light, or electricity triggers the breakdown of compounds into their constituent elements or simpler compounds.
Displacement Reaction
Displacement reactions, also known as Substitution Reactions or Single Displacement/Replacement Reactions, occur when a more reactive element displaces a less reactive element from a compound.
A typical displacement reaction can be expressed using a chemical equation as follows:
A + BC → AC + B
For a displacement reaction to occur, element ‘A’ must be more reactive than element ‘B’. If ‘B’ is more reactive than ‘A’, then ‘A’ will not displace ‘C’ from ‘BC’, and the reaction will not take place.
Examples:
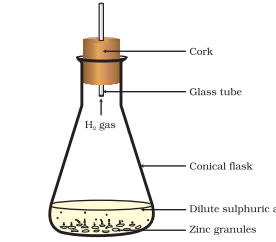
1. When zinc reacts with hydrochloric acid, it produces hydrogen gas and zinc chloride.
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
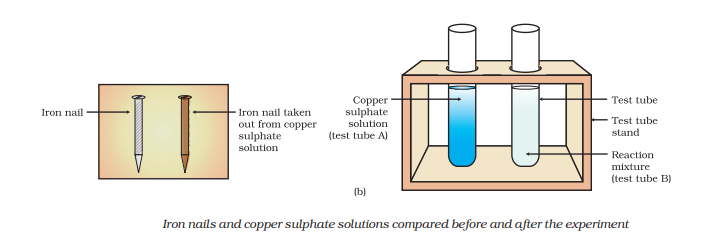
2. When zinc reacts with copper sulfate, it forms zinc sulfate and copper metal.
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
In these examples, zinc, being more reactive than hydrogen in hydrochloric acid and copper in copper sulfate, displaces them from the compounds, resulting in the formation of new products. Displacement reactions play a significant role in various chemical processes and have practical applications in different industries.
Double Displacement Reaction or Precipitation Reaction
Double displacement reactions, also known as Double Replacement Reactions, involve the exchange of ions between two reactants, resulting in the formation of new compounds.
The general representation of a double displacement reaction is:
AB + CD → AC + BD
Examples:
1. When a solution of barium chloride reacts with a solution of sodium sulphate, a white precipitate of barium sulphate is formed, along with sodium chloride.
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) (Precipitate) + 2NaCl(aq)
2. When sodium hydroxide (a base) reacts with hydrochloric acid, sodium chloride and water are formed.
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Note: A double displacement reaction that forms a precipitate is also referred to as a precipitation reaction. Neutralization reactions are also examples of double displacement reactions, where an acid and a base react to form a salt and water. Double displacement reactions play a crucial role in various chemical processes and are encountered in different applications, such as in the synthesis of salts and precipitation reactions.
Redox Reaction
A redox reaction occurs when the oxidation states of the substances involved change. Oxidation refers to the loss of electrons or an increase in the oxidation state of a chemical or its atoms, while reduction refers to the gain of electrons or a decrease in the oxidation state of a chemical or its atoms.
During a redox reaction, both oxidation and reduction processes happen simultaneously. In oxidation, a substance loses electrons, gains oxygen, or loses hydrogen. On the other hand, in reduction, a substance gains electrons, loses oxygen, or gains hydrogen.
An oxidizing agent is a substance that causes another substance to undergo oxidation while it itself gets reduced. Conversely, a reducing agent is a substance that causes another substance to undergo reduction while it itself gets oxidized.
Redox reactions play a crucial role in various chemical processes, including combustion, corrosion, and energy production. They are essential for the transfer of electrons between substances, leading to significant transformations in the properties and behavior of chemicals.

Exothermic reaction
During a reaction, heat is released. Many combination reactions are exothermic, meaning they release heat.
Examples:
1. Al + Fe2O3 → Al2O3 + Fe + heat
In this reaction, when aluminum reacts with iron(III) oxide, it forms aluminum oxide and iron, releasing heat in the process.
2. CH4 + 2O2 → CO2 + 2H2O + heat
When methane reacts with oxygen, it produces carbon dioxide and water, along with the release of heat.
In both these combination reactions, the formation of new compounds leads to the release of heat energy, making them exothermic reactions. Exothermic reactions are common in nature and industry and play a significant role in various chemical processes, providing valuable energy and driving various natural phenomena.
Endothermic
Energy in the form of heat is necessary to drive the reaction.
6CO2 + 6H2O + Sunlight → C6H12O6 + 6O2
Glucose
In this reaction, glucose is produced from carbon dioxide and water in the presence of sunlight, and oxygen is released. The reaction requires the input of heat energy from sunlight to proceed.
Most of the decomposition reactions are endothermic, meaning they absorb heat from the surroundings to proceed. In these reactions, energy is required to break down a compound into its constituent elements or simpler compounds.
Chemical Reaction and Equation Notes Class 10
Corrosion
The gradual deterioration of a material, often a metal, due to the influence of moisture, air, or chemicals in the surrounding environment is known as corrosion.
Rusting:
4Fe(s) + 3O2(from air) + xH2O(moisture) → 2Fe2O3.xH2O(rust)
Corrosion of copper:
Cu(s) + H2O(moisture) + CO2(from air) → CuCO3.Cu(OH)2(green)
Corrosion of silver:
Ag(s) + H2S (from air) → Ag2S(black) + H2(g)
In these examples, rusting, copper corrosion, and silver corrosion all occur due to the interaction with elements in the environment, such as moisture, air, and chemicals. These reactions lead to the formation of new compounds on the surface of the materials, causing them to deteriorate over time. Corrosion is a common phenomenon that affects various materials, and it is a significant concern in various industries and everyday applications.
Rancidity
Food undergoes a process called rancidity when fats and oils in it undergo oxidation over an extended period. This results in the food developing a foul smell and unpleasant taste. Consuming rancid food can lead to stomach infections and discomfort.
Prevention methods include:
(i) Using air-tight containers to minimize exposure to air and oxygen.
(ii) Packaging food with nitrogen to create a protective atmosphere.
(iii) Refrigeration to slow down the oxidation process.
(iv) Adding antioxidants or preservatives to inhibit the oxidation of fats and oils.
By employing these prevention measures, we can prolong the freshness and quality of food, ensuring safer and more enjoyable consumption.
Word Equation
A word equation represents a chemical reaction using words instead of chemical formulas, making it easier to identify the reactants and products involved.
In this shorthand representation, the names of the reactants appear on the left side of the word equation. If there is more than one reactant, their names are separated by a plus sign (+). Similarly, the products are listed on the right side of the word equation, with their names separated by a plus sign (+) if there is more than one product.
For instance,
Sodium + Chlorine → Sodium chloride
The equation above can be read as: “Sodium reacts with chlorine to form sodium chloride.
Symbols of Elements and Their Valencies used in Chemical Reaction and Equation Notes Class 10
A symbol serves as a chemical code for an element. Every element is represented by a one or two-letter atomic symbol, typically an abbreviation of its name.
Valency refers to the combining capacity of an element. It represents the number of electrons that an atom gains, loses, or shares when it combines with another atom to form a molecule.
Writing Chemical Equations
A chemical equation is the representation of a chemical reaction using symbols and chemical formulas for the reactants and products.
In chemical equations:
- “(s)” is used for solids.
- “(l)” is used for liquids.
- “(g)” is used for gases.
- “(aq)” is used for aqueous solutions.
- “(↑)” is used to indicate a gas produced in the reaction.
- “(↓)” is used to indicate a precipitate formed in the reaction.
Ex: Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) ↑
Physical and Chemical Changes
Chemical change happens when one or more new substances are created, and these substances have different physical and chemical properties compared to the original ones.
In a physical change, there is a modification in color or state, but no new substance is produced. For instance, when water is boiled and changes into steam, no new substance is formed (even though water and steam appear different, they react in the same way when exposed to sodium, producing identical products). This change solely involves a shift in the state of matter, from liquid to vapor.
Observations that Help Determine a Chemical Reaction
To identify a chemical reaction, you can make use of the following observations:
a) The release of a gas
b) A change in temperature
c) The formation of a precipitate
d) A change in color
e) A change in state
Balancing of a Chemical Reaction
Law of Conservation of Mass
The Law of Conservation of Mass states that in a chemical reaction, atoms cannot be created or destroyed. Therefore, the total number of atoms of each element on the reactants side must be equal to the total number of atoms on the products side.
In simpler terms, the total mass of the products formed during a chemical reaction is always equal to the total mass of the reactants involved in the reaction. Mass is conserved throughout the entire process.
Balanced chemical equation
A chemical equation is considered balanced when the number of atoms for each element on the reactants side is equal to the number of atoms on the products side. Such an equation is referred to as a balanced chemical equation.
Steps for Balancing Chemical Equations
A chemical equation represents the changes that occur during a chemical reaction.
Reactants → Products
For a chemical equation to be balanced, the equilibrium must be maintained, meaning the number of each type of atom must be the same on both sides of the arrow.
Scientists use coefficients to balance chemical equations. Coefficients are numerical values added in front of chemical symbols or formulas, indicating the number of atoms or molecules involved.
Place coefficients as needed to achieve a balanced chemical equation, ensuring the same number of each type of atom appears in both reactants and products.
For instance:
Zn + HCl → ZnCl2 + H2
The balanced equation is:
Zn + 2HCl → ZnCl2 + H2
To balance the equation, you may use the hit and trial method, adjusting the coefficients until the number of atoms of each element is the same on both sides of the chemical equation.
Read Also:
- Acids Bases and Salts Class 10 Notes of NCERT Science Ch. 2
- Life Process Notes Class 10 NCERT Science Chapter 5
- Control and Coordination Notes Class 10: NCERT Science Ch. 6
- Class 10th How Do Organisms Reproduce Notes: Science Ch. 7
- Class 10th Heredity and Evolution Notes: NCERT Science Ch. 8
- Class 10th Chapter 11 Science Notes for NCERT Students
- Notes of Our Environment Class 10: NCERT Science Chapter 13
Frequently Asked Questions on Chemical Reaction and Equation Notes Class 10
Q 1. How can you identify a chemical reaction?
Several observable changes and indicators can identify a chemical reaction. During a chemical reaction, it forms new substances with different properties than the original reactants.
These changes can be evident through alterations in color, the evolution of gas, the formation of a precipitate, and variations in temperature. Some reactions emit light or produce distinct odors, providing further evidence of chemical change.
Additionally, the irreversibility of most chemical reactions sets them apart from physical changes. Observing effervescence or the rapid release of gas in the form of bubbles is also a common clue. Scientists rely on these recognizable signs to study and understand chemical processes across various disciplines.
Identifying chemical reactions is essential for advancing research, developing new materials, and comprehending the transformations that occur in the world around us.
Q 2. Why chemical equation balancing important?
In chemistry, the balancing of chemical equations is of paramount importance as it upholds the fundamental principle of the Law of Conservation of Mass. This law states that the total mass of the reactants in a chemical reaction must be equal to the total mass of the products. By balancing the equation, the same number of atoms of each element appears on both sides of the reaction, guaranteeing the preservation of mass.
Balanced chemical equations accurately represent the stoichiometry of a reaction, illustrating the correct proportions in which the reactants combine and the products are formed. This information is crucial for understanding the quantitative aspects of the reaction and for predicting the amounts of reactants and products needed or obtained.
Furthermore, chemical equation balancing is essential for performing stoichiometric calculations in laboratories and industries, allowing efficient synthesis and production of substances. Furthermore, accurate reproducibility and standardized study of the reaction require clear communication among scientists and researchers.
In summary, chemical equation balancing is the backbone of chemical understanding and enables precise calculations, ensuring the consistent application of chemical principles in research, industry, and education.
Q 3. How do chemical reactions impact our daily lives and the environment?
Chemical reactions have a profound impact on our daily lives and the environment. In our daily routines, chemical reactions are integral to numerous processes, such as cooking, digestion, and combustion in vehicles and appliances. They provide us with essential products like medicines, plastics, and cleaning agents. Chemical reactions in food preservation and fermentation play a vital role in food industry practices.
However, certain chemical reactions also pose environmental challenges. Combustion of fossil fuels releases carbon dioxide and other greenhouse gases, contributing to climate change. Industrial processes and emissions generate air and water pollutants, leading to environmental degradation and health concerns. Chemical reactions in fertilizers and pesticides can harm ecosystems and disrupt biodiversity.
Balancing these effects is crucial. To minimize the negative environmental impact of chemical reactions, we employ sustainable practices, green chemistry, and waste reduction. Understanding and managing chemical reactions contribute to finding solutions for environmental sustainability and improving our overall quality of life.
Q 4. How do chemical reactions play a role in food preparation and cooking?
Chemical reactions play a crucial role in food preparation and cooking, transforming raw ingredients into delicious and nutritious dishes. The Maillard reaction is responsible for the browning and development of savory flavors in grilled meats, roasted vegetables, and toasted bread. Caramelization of sugars produces a rich and sweet taste in dishes like caramelized onions and desserts. Fermentation, driven by yeast and bacteria, enhances the taste and texture of foods like bread, cheese, and pickles.
Chemical leavening agents like baking powder and baking soda release carbon dioxide, making dough and batters rise, resulting in light and fluffy cakes and bread. Denaturation of proteins through heat and acids changes their structure and texture in foods like eggs and meat.
Understanding these chemical processes empowers cooks and chefs to manipulate ingredients and cooking techniques, creating a wide array of flavors, textures, and appearances in culinary delights. Chemical reactions, when harnessed skillfully, are the key to transforming raw ingredients into delectable and visually appealing dishes that tantalize our taste buds and nourish our bodies.
Q 5. What are some safety precautions to consider when conducting chemical reactions in the laboratory?
Safety is of utmost importance when conducting chemical reactions in the laboratory to prevent accidents, injuries, and exposure to hazardous substances. Here are some essential safety precautions to consider:
1. Wear Appropriate Personal Protective Equipment (PPE): Always wear safety goggles, lab coats, gloves, and closed-toe shoes to protect eyes, skin, and clothing from chemical splashes and spills.
2. Work in a Well-Ventilated Area: Ensure proper ventilation to minimize exposure to fumes and gases. Use fume hoods when handling volatile or toxic substances.
3. Label and Store Chemicals Properly: Clearly label all chemicals and store them in designated, secure locations, following safety data sheet guidelines.
4. Know Emergency Procedures: Familiarize yourself with emergency protocols, including eyewash stations, safety showers, and fire extinguishers.
5. Do Not Taste or Smell Chemicals: Avoid tasting or smelling chemicals, as some substances can be toxic, corrosive, or harmful.
By observing these safety precautions and practicing responsible laboratory conduct, researchers can minimize risks and create a safe working environment for themselves and others.