Tag: introductory chemical engineering thermodynamics
Thermodynamics class 11 physics
Thermodynamics: Certainly, here is a more detailed explanation of the topics typically covered in a Class 11 Physics Thermodynamics course, with additional information and examples. This comprehensive guide should provide you with a deeper understanding of thermodynamics:

Thermodynamics
Introduction to Thermodynamics
Thermodynamics is a branch of physics that deals with the study of heat, work, and energy transfer. It provides essential insights into the behavior of matter and energy in various physical systems. Thermodynamics plays a crucial role in understanding natural processes, from engines and refrigerators to chemical reactions and the behavior of gases.
System and Surroundings
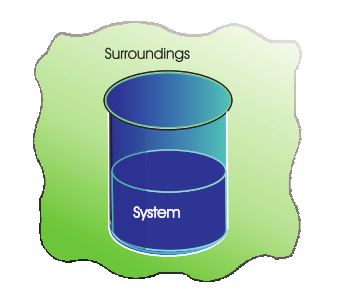
System
In thermodynamics, we define a system as a specific region of space or a quantity of matter under consideration. It is the part of the universe we are interested in studying. The surroundings are everything outside the system. We often use the terms “system” and “surroundings” to describe the boundaries and interactions in thermodynamic processes.
Types of Systems
- Open System: A system that can exchange both matter and energy with its surroundings. For example, an open cup of coffee where heat is lost to the surroundings, and you can add or remove coffee.
- Closed System: A system that can exchange energy but not matter with its surroundings. A sealed thermos containing hot coffee is an example.
- Isolated System: A system that cannot exchange either matter or energy with its surroundings. The universe itself is often considered an isolated system for some thermodynamic analyses.
Thermodynamic Processes
Thermodynamic processes describe how a system changes from one state to another. Several common processes include:
Isothermal Process
An isothermal process is one in which the temperature of the system remains constant throughout the process. For example, when a gas in a piston-cylinder system expands while being in contact with a hot reservoir, keeping its temperature constant.
Adiabatic Process
An adiabatic process is one in which there is no heat exchange between the system and its surroundings. In such processes, any change in the internal energy of the system is solely due to work done on or by the system. A rapid compression of a gas is an example of an adiabatic process.
Isobaric Process
An isobaric process is one in which the pressure of the system remains constant while other parameters like volume and temperature may change. Boiling of water at a constant atmospheric pressure is an isobaric process.
Isochoric Process
An isochoric process is one in which the volume of the system remains constant. It typically results in changes in pressure and temperature. Heating a sealed container with a fixed volume is an example of an isochoric process.
Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics states that if two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. In simpler terms, if two objects are at the same temperature as a third object, they are also at the same temperature as each other. This law allows us to define temperature scales.
First Law of Thermodynamics
The First Law of Thermodynamics, often called the Law of Conservation of Energy, states that energy cannot be created or destroyed in an isolated system. Instead, it can only change forms. Mathematically, it can be expressed as:
ΔU = Q – W
Where:
- ΔU represents the change in the internal energy of the system.
- Q represents the heat added to the system.
- W represents the work done by the system.
This law is fundamental and is applied to various processes and systems.
Internal Energy
Internal energy (U) is the sum of the kinetic and potential energies of the particles within a system. It depends on the state of the system and is a state function.
Heat Capacity and Specific Heat
Heat capacity (C) is the amount of heat required to raise the temperature of a substance by one degree Celsius (or one Kelvin). The specific heat (c) is the heat capacity per unit mass. Mathematically:
C = mc
Where:
- C is the heat capacity.
- m is the mass.
- c is the specific heat.
Work and Heat Transfer
Work Done
In thermodynamics, work is defined as the energy transferred to or from a system as a result of a force acting on a boundary and causing a displacement in the direction of the force. Work done on a system is considered positive, while work done by a system is considered negative. The formula for work in a piston-cylinder system is:
W = PΔV
Where:
- W is the work done.
- P is the pressure.
- ΔV is the change in volume.
Heat Transfer
Heat transfer is the process of energy transfer due to temperature differences. There are three primary modes of heat transfer:
- Conduction: Heat transfer through direct contact between particles in a solid.
- Convection: Heat transfer through the movement of fluids (liquids or gases).
- Radiation: Heat transfer through electromagnetic waves (e.g., sunlight).
Applications of the First Law
Calorimetry
Calorimetry is the science of measuring heat. A calorimeter is a device used to measure the heat transfer in various processes. Calorimetry is often employed to determine specific heat capacities and heat of reaction in chemical reactions.
Enthalpy
Enthalpy (H) is a thermodynamic quantity used to describe the heat content of a system at constant pressure. It is defined as:
H = U + PV
Where:
- H is enthalpy.
- U is internal energy.
- P is pressure.
- V is volume.
The change in enthalpy (ΔH) is often used to calculate the heat exchanged in processes at constant pressure.
Second Law of Thermodynamics
The Second Law of Thermodynamics states that heat naturally flows from a region of higher temperature to a region of lower temperature. This law is often associated with the concept of entropy.
Heat Engines
A heat engine is a device that converts thermal energy into mechanical work. The efficiency of a heat engine is given by:
Efficiency = (Useful Work Output) / (Heat Input)
The most efficient heat engine theoretically possible is the Carnot engine, which operates between two heat reservoirs.
Entropy
Entropy (S) is a measure of the amount of disorder or randomness in a system. The Second Law of Thermodynamics can be expressed as the increase in entropy in an isolated system over time. It is given by:
ΔS ≥ Q/T
Where:
- ΔS is the change in entropy.
- Q is the heat added to the system.
- T is the absolute temperature in Kelvin.
Entropy is a state function and is used to analyze the direction of spontaneous processes.
Reversible and Irreversible Processes
In thermodynamics, a reversible process is one that can be reversed by an infinitesimal change in a property, such as temperature or pressure. In contrast, an irreversible process is one that cannot be reversed without some external influence. Real-world processes are often irreversible, and reversible processes are idealized for analysis.
Third Law of Thermodynamics
The Third Law of Thermodynamics states that as the temperature of a system approaches absolute zero (0 Kelvin), the entropy of the system approaches a minimum value. This law helps explain the behavior of matter at extremely low temperatures and is often used in the study of materials at cryogenic temperatures.
Entropy and Probability
Entropy can be interpreted in terms of probability. It is related to the number of microscopic configurations (ways particles can arrange themselves) consistent with the macroscopic properties of the system. In simple terms, systems tend to evolve toward states with higher entropy, which correspond to greater disorder and randomness.
Heat Transfer
Fourier’s Law of Heat Conduction
Fourier’s law describes the heat conduction in a material. It states that the rate of heat transfer through a material is directly proportional to the temperature gradient (change in temperature over distance) and is inversely proportional to the thermal conductivity of the material.
Newton’s Law of Cooling
Newton’s law of cooling describes the rate at which an object cools when placed in a cooler environment. It states that the rate of change of temperature of an object is directly proportional to the temperature difference between the object and its surroundings.
Carnot Cycle
The Carnot cycle is a theoretical thermodynamic cycle that represents the most efficient engine possible operating between two heat reservoirs. It consists of four reversible processes: two isothermal processes and two adiabatic processes. The Carnot engine is often used as an idealized model for the maximum efficiency of real heat engines.
Carnot’s Theorem
Carnot’s theorem states that no engine operating between two heat reservoirs can be more efficient than a Carnot engine operating between the same reservoirs. This theorem establishes an upper limit on the efficiency of real heat engines, and it highlights the importance of reversible processes in achieving maximum efficiency.
Heat Pumps and Refrigerators
In addition to heat engines, thermodynamics also applies to heat pumps and refrigerators. These devices transfer heat from a lower-temperature reservoir to a higher-temperature reservoir. The key difference between a heat pump and a refrigerator lies in their primary objectives:
- A heat pump is designed to heat a space or provide hot water by extracting heat from a colder environment and transferring it to a warmer one. Heat pumps are commonly used for home heating and cooling.
- A refrigerator is designed to remove heat from the interior of the refrigerator and expel it to the room, keeping the contents of the refrigerator cool.
Both heat pumps and refrigerators operate using a cycle similar to the Carnot cycle but with certain modifications.
Phase Transitions
Thermodynamics also includes the study of phase transitions, which are changes in the state of matter. Common phase transitions include:
- Melting: The transition from a solid to a liquid state.
- Freezing: The transition from a liquid to a solid state.
- Vaporization: The transition from a liquid to a gas state, which includes both boiling (at a specific temperature and pressure) and evaporation (at any temperature).
- Condensation: The transition from a gas to a liquid state.
- Sublimation: The transition from a solid directly to a gas state without passing through the liquid state.
- Deposition: The transition from a gas directly to a solid state without passing through the liquid state.
The energy changes associated with these phase transitions are fundamental to understanding various physical processes, including the operation of refrigeration systems and the behavior of substances under different conditions.
State Functions
In thermodynamics, certain properties are defined as state functions because their values depend only on the current state of the system, not on how the system arrived at that state. Examples of state functions include internal energy (U), enthalpy (H), entropy (S), and pressure (P). Path functions, on the other hand, depend on the path taken to reach a particular state and include work (W) and heat (Q).
Maxwell’s Relations
Maxwell’s relations are a set of equations that relate the partial derivatives of the state functions, providing valuable relationships between thermodynamic properties. These relations are derived from the basic thermodynamic laws and are used to simplify calculations and analyze complex thermodynamic systems.
Thermodynamic Diagrams
In thermodynamics, graphical representations are often used to visualize and analyze processes. Two common diagrams include:
- P-V Diagram: A pressure-volume diagram represents the changes in pressure and volume of a system during a thermodynamic process. It helps in understanding processes like expansion, compression, and heat exchange.
- T-S Diagram (Temperature-Entropy Diagram): A temperature-entropy diagram illustrates changes in temperature and entropy during a thermodynamic process. It is particularly useful in analyzing the performance of heat engines and refrigeration cycles.
Applications of Thermodynamics
Thermodynamics finds applications in various fields, including:
- Chemical Engineering: Thermodynamics is crucial in chemical reactions, industrial processes, and the design of chemical reactors.
- Aerospace Engineering: It plays a pivotal role in the design and analysis of propulsion systems, such as jet engines and rockets.
- Environmental Science: Thermodynamics is used to study energy transfer in ecosystems and climate systems.
- Materials Science: It helps understand the behavior of materials under different conditions, aiding in material selection and design.
- Biological Systems: Thermodynamics is applied to analyze energy transfer and metabolic processes in biological systems.
Gibbs Free Energy
Gibbs free energy (G) is a thermodynamic potential that combines enthalpy and entropy to predict whether a chemical reaction will be spontaneous or non-spontaneous under constant temperature and pressure conditions. The Gibbs free energy change (ΔG) of a reaction is related to its spontaneity:
- If ΔG < 0, the reaction is spontaneous (exergonic).
- If ΔG > 0, the reaction is non-spontaneous (endergonic).
- If ΔG = 0, the system is at equilibrium.
The concept of Gibbs free energy is particularly important in chemistry and biochemistry to predict reaction outcomes.
Thermodynamic Equilibrium
Thermodynamic equilibrium occurs when a system’s properties (such as temperature, pressure, and chemical composition) no longer change with time because there is no net exchange of energy or matter with its surroundings. Different types of equilibrium include thermal, mechanical, and chemical equilibrium.
Thermodynamic Laws in Chemistry
In chemistry, thermodynamics plays a crucial role in understanding chemical reactions and equilibria. The following concepts are significant:
- Chemical Potential: The chemical potential (μ) represents the change in Gibbs free energy with respect to changes in the number of particles (e.g., moles of a substance). It is essential for understanding phase equilibria and chemical reactions.
- Equilibrium Constants: Thermodynamics provides the foundation for understanding and calculating equilibrium constants (K) for chemical reactions. The equilibrium constant expresses the ratio of product concetrations to reactant concentrations at equilibrium.
- Le Chatelier’s Principle: This principle states that if a system at equilibrium is subjected to an external change (e.g., temperature, pressure, or concentration), the system will adjust to partially counteract that change and restore equilibrium. Thermodynamics helps explain how and why these adjustments occur.
- Thermodynamics and Climate Science
- Thermodynamics plays a vital role in climate science and the study of Earth’s climate system. Concepts such as heat transfer, greenhouse gas effects, and phase changes are essential for understanding climate patterns, climate change, and the impact of human activities on the environment.
- Thermodynamics in Engineering
- Thermodynamics is a cornerstone of engineering disciplines, including:
- Mechanical Engineering: It is essential for the design and analysis of engines, turbines, and HVAC (heating, ventilation, and air conditioning) systems.
- Chemical Engineering: Thermodynamics is used to design and optimize chemical processes, including reactions, separations, and heat exchangers.
- Electrical Engineering: It is applied to the design of power generation systems, such as thermoelectric generators and power plants.
- Materials Science: Understanding thermodynamic principles helps in material selection, phase diagrams, and heat treatment processes.
- Thermodynamics in Biology
- Thermodynamics is also relevant in biology, particularly in the study of living organisms and biochemical processes. The principles of thermodynamics help explain how cells obtain and utilize energy, how enzymes function, and how metabolic pathways operate.
- Modern Advances in Thermodynamics
- Modern physics and engineering continue to push the boundaries of thermodynamics. Concepts like non-equilibrium thermodynamics explore systems that are far from equilibrium, such as those involving turbulence, fluid dynamics, and biological systems. Additionally, quantum thermodynamics extends thermodynamic principles to the quantum scale, providing insights into the behavior of very small systems.
Conclusion
Thermodynamics is a broad and fundamental field with applications spanning various scientific and engineering disciplines. Its principles help us understand and predict the behavior of energy and matter in diverse systems, from the macroscopic to the microscopic, and from natural processes to industrial applications. A strong foundation in thermodynamics is essential for scientists and engineers working in fields where energy transfer, heat, and work are critical components of their research and designs.
Read More
- Photosynthesis In Higher Plants
- Motion In A Plane
- Basic Properties Electrical Charge
- Anatomy Of Flowering Plants
- Elastic Behaviour Of Materials
Frequently Asked Question (FAQs) Thermodynamics
1. What is thermodynamics?
Thermodynamics is a branch of physics that deals with the study of heat, work, and energy transfer in various physical systems. It provides fundamental principles for understanding the behavior of matter and energy.
2. What are the key laws of thermodynamics?
The key laws of thermodynamics are:
- Zeroth Law: If two systems are in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
- First Law: Energy cannot be created or destroyed; it can only change forms. This law is also known as the law of conservation of energy.
- Second Law: Heat naturally flows from a region of higher temperature to a region of lower temperature. This law is associated with the concept of entropy.
- Third Law: As temperature approaches absolute zero (0 Kelvin), the entropy of a perfect crystal approaches a minimum value.
3. What are the different types of thermodynamic systems?
Thermodynamic systems can be categorized into three types:
- Open System: Allows the exchange of both matter and energy with the surroundings.
- Closed System: Allows the exchange of energy but not matter with the surroundings.
- Isolated System: Does not allow the exchange of either matter or energy with the surroundings.
4. What are thermodynamic processes?
Thermodynamic processes describe how a system changes from one state to another. Common processes include isothermal, adiabatic, isobaric, and isochoric processes, each with specific characteristics.
5. How is heat related to thermodynamics?
Heat is a form of energy transfer that plays a central role in thermodynamics. It can enter or leave a system during various processes and is typically represented by the symbol Q.