Tag: matter in our surroundings class 9 notes ncert
Class 9 Science Chapter 1 Matter in Our Surroundings Notes
If you are class 9th student then this article “Class 9 Science Chapter 1 Matter in Our Surroundings Notes” is very important for you.
Matter encompasses anything possessing mass and occupying space. This includes substances like hydrogen and oxygen, sugar and sand, as well as air and water.
Matter is composed of tiny, diminutive particles. These particles are drawn together due to the attractive forces between them, despite the gaps that exist between them.

Class 9 Science Chapter 1 Matter in Our Surroundings Notes
NCERT Class 9 Science Chapter 1 Matter in Our Surroundings Notes
States of Matter
As per Class 9 Science Chapter 1 Matter in Our Surroundings Notes matter finds its classification as solid, liquid, or gas dependent on the interactions between particles and the manner in which they are arranged.
The transformation between these three states of matter can be achieved by adjusting pressure and temperature. For instance, by elevating the temperature, solid ice can transition into a liquid state.
| Property | Solid | Liquid | Gas |
|---|---|---|---|
| Shape and volume | Fixed shape and volume | No fixed shape but has volume | Neither definite shape nor volume |
| Energy | Lowest | Medium | Highest |
| Compressibility | Difficult | Nearly difficult | Easy |
| Arrangement of molecules | Regular and closely arranged | Random and little sparsely arranged | Random and more sparsely arranged |
| Fluidity | Cannot flow | Flows from higher to lower level | Flows in all directions |
| Movement | Negligible | Depends on interparticle attraction | Free, constant and random |
| Interparticle space | Very less | More | Large |
| Interparticle attraction | Maximum | Medium | Minimum |
| Density | Maximum | Medium | Minimum |
| Rate of diffusion | Negligible | It depends on interparticle attraction. | Maximum |
Atomic View of the Three States of Matter
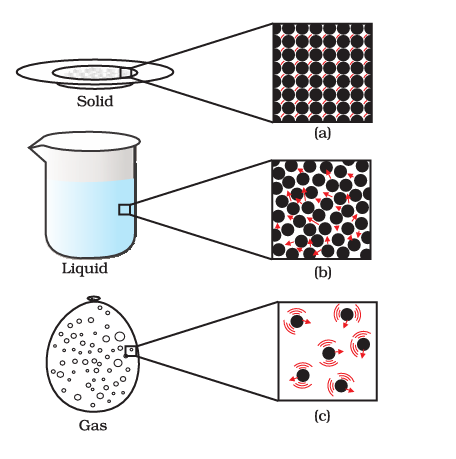
Physical Nature of Matter
A physical property denotes a facet of matter that is observable or quantifiable without altering its inherent character or composition.
Such properties remain unaffected by the quantity of matter in question. Examples of physical properties encompass visual attributes, coloration, scent, density, texture, melting and boiling points, solubility, and more.
Characteristics of Particles of Matter
Matter
Matter constitutes entities possessing mass and taking up space.
All that we can physically sense – touch, sight, hearing, taste, and even smell – falls under the category of matter.
Matter is comprised of incredibly minute particles that remain invisible to the naked eye.
The constitution of matter’s particles shapes its state and attributes, encompassing both physical and chemical aspects.
1. Particles within matter exhibit gaps between them.
This property underlies the concept of substances dissolving in others. For instance, when sugar dissolves in water, the water level remains unchanged because the sugar particles fit into the spaces between the water particles.
2. Matter’s particles are in constant motion.
Continuous and random movements result from the particles’ kinetic energy. Elevating the temperature increases their kinetic energy, intensifying their motion.
3. Particles of matter mutually attract each other.
Interparticle forces of attraction operate within every substance. These forces must be overcome to break a substance apart. The strength of this force varies among different substances.
Diffusion
The occurrence where matter’s particles spontaneously blend with one another is termed diffusion. An instance of this is the dispersion of ink in water.
Throughout diffusion, particles take up the interparticle gaps.
The pace of diffusion escalates as temperature rises, a result of heightened kinetic energy in the particles.
Can Matter Change Its State?
Effect of Change of Temperature on the State of Matter
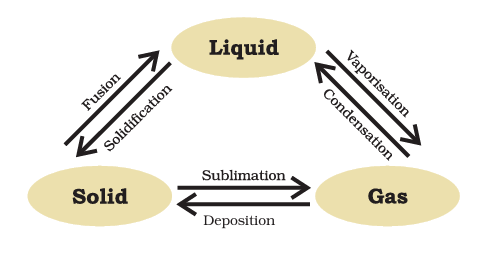
As temperature rises, the kinetic energy within matter’s particles escalates, leading to heightened vibrational energy. Consequently, the interparticle attractive force diminishes, causing particles to disengage from their positions and move with increased freedom.
- Consequently, the state of matter undergoes transformation.
- Solids transition to liquids through a phase change.
- Likewise, liquids experience phase changes to become gases.
Melting Point
The temperature at which a solid transforms into a liquid under atmospheric pressure is known as its melting point.
At the melting point, there exists an equilibrium between these two phases, namely solid and liquid. This means that both the solid and liquid states coexist simultaneously at this specific temperature.
Fusion
Nuclear fusion is a process where two atomic nuclei come together to form a heavier nucleus, releasing a significant amount of energy in the process.
This is the process that powers stars like our Sun, where hydrogen atoms combine to form helium through fusion, releasing a tremendous amount of energy in the form of light and heat.
In a broader context, fusion can also refer to the merging or joining of different elements or entities to create something new.
Class 9 Science Chapter 1 Matter in Our Surroundings Notes
Boiling Point
The boiling point of a substance is the temperature at which it transitions from its liquid phase to its gaseous phase under a specific pressure, typically atmospheric pressure.
At this temperature, the vapor pressure of the liquid equals the atmospheric pressure, allowing bubbles of vapor to form within the liquid and escape into the air, causing the liquid to boil.
The boiling point is a characteristic property of a substance and can vary depending on the atmospheric pressure.
Latent Heat of Fusion
The latent heat of fusion refers to the amount of heat energy required to change a substance from its solid state to its liquid state without a change in temperature.
This energy is used to overcome the forces of attraction between the particles within the solid, allowing them to transition into a more disordered arrangement in the liquid phase.
During the process of fusion, or melting, the substance absorbs energy without a temperature increase. Once the particles are in the liquid state, they have higher energy due to the additional heat energy provided.
The reverse process, where a liquid turns into a solid, also releases the same amount of latent heat of fusion. This phenomenon is crucial in understanding the energy exchange involved in phase transitions and is specific to each substance.
Latent Heat of Vaporisation
The latent heat of vaporization refers to the amount of heat energy needed to transform a substance from its liquid state to its gaseous state at a constant temperature and pressure.
Unlike the process of raising the temperature of a substance, during which its temperature increases, the latent heat of vaporization occurs at a consistent temperature while the substance changes its phase from liquid to gas.
When a substance vaporizes, such as water turning into steam, it absorbs energy to break the intermolecular forces holding the liquid together and allow its molecules to transition into the gas phase.
This energy is later released when the gas condenses back into a liquid. The latent heat of vaporization is a critical factor in processes like evaporation, boiling, and condensation and varies for different substances.
Sublimation
Sublimation is a phase transition process in which a substance directly transforms from its solid state to its gaseous state without passing through the intermediate liquid phase.
This occurs when the pressure and temperature conditions are such that the substance’s vapor pressure at its solid phase is greater than the atmospheric pressure.
During sublimation, the solid substance absorbs heat energy, causing its particles to gain enough energy to break the intermolecular forces holding them together.
These particles then transition into the gaseous state without first becoming a liquid. Dry ice (solid carbon dioxide) sublimating into carbon dioxide gas is a common example of this phenomenon.
Sublimation is distinct from other phase transitions like melting and boiling, which involve changes between solid and liquid or liquid and gas phases, respectively.
Effect of Change in Pressure on the State of Matter
A change in pressure can have a significant impact on the state of matter, particularly for gases. The behavior of solids and liquids is less affected by pressure changes compared to gases due to their relatively higher intermolecular forces and closely packed structures.
For gases:
1. Increase in Pressure: When the pressure on a gas is increased, the gas molecules are forced closer together. This can lead to a change in the state of the gas. For example, at high pressures, gases that are normally in a gaseous state might become liquid or solid. This is known as “condensation” or “liquefaction.”
2. Decrease in Pressure: Lowering the pressure on a gas allows its molecules to spread out more. If the pressure drops significantly, gases can change from a liquid to a gas (vaporization) or from a solid to a gas (sublimation) without passing through the liquid phase.
3. Phase Diagrams: The relationship between pressure and temperature for different states of matter can be represented on a phase diagram. Phase diagrams show the conditions under which a substance exists as a solid, liquid, or gas. Critical points, where the distinction between gas and liquid phases becomes less clear, can also be shown on these diagrams.
It’s important to note that the effects of pressure changes are more pronounced at low temperatures and become less significant at higher temperatures. Additionally, not all substances behave the same way with changes in pressure; each substance has its own unique phase behavior.
Evaporation
According to Class 9 Science Chapter 1 Matter in Our Surroundings Notes Evaporation is a process through which a liquid substance transforms into a gaseous state at temperatures below its boiling point. Unlike boiling, which occurs throughout the liquid, evaporation takes place only at the liquid’s surface.
During evaporation, molecules with higher kinetic energy near the liquid’s surface gain enough energy to break the intermolecular forces holding them within the liquid. These molecules then escape into the air as vapor. This process leads to cooling of the remaining liquid due to the energy loss caused by the escaping molecules.
Evaporation occurs naturally and continuously, even at temperatures below the boiling point. Factors like temperature, surface area, humidity, and air movement influence the rate of evaporation.
For example, on a hot day, clothes dry faster because the increased temperature speeds up the evaporation of water from the fabric. Evaporation plays a significant role in the water cycle, as water bodies continuously lose water to the atmosphere through this process.
Factors Affecting Evaporation
In Class 9 Science Chapter 1 Matter in Our Surroundings Notes, Several factors that influence the rate of evaporation are discussed:
- Temperature: Higher temperatures provide more energy to the molecules in the liquid, increasing their kinetic energy and the likelihood of escaping the liquid’s surface. As a result, higher temperatures generally lead to faster evaporation.
- Surface Area: A larger exposed surface area allows more liquid molecules to come into contact with the air, promoting faster evaporation. This is why liquids in wider containers tend to evaporate more quickly.
- Air Movement: Air movement, often referred to as wind, helps carry away the vapor molecules that have escaped the liquid’s surface. This reduces the concentration of vapor near the liquid and enhances evaporation.
- Humidity: Humidity is the amount of moisture already present in the air. When the air is humid, it’s already saturated with moisture, which makes it more difficult for the vapor from the liquid to be absorbed by the air. In drier air, evaporation happens more efficiently.
- Pressure: Lower pressure environments can facilitate faster evaporation. This is why liquids tend to evaporate more quickly at higher altitudes where the atmospheric pressure is lower.
Cooling Due to Evaporation
In the process of evaporation, the particles within a liquid gather energy from their environment to surmount the intermolecular forces that bind them together, leading to a change in phase. This act of absorbing heat from the surroundings causes a cooling effect on those surroundings.
A familiar example is the cooling sensation we experience through sweating, where the body’s moisture evaporates, taking away heat energy and cooling the body.
What Applications of Evaporative Cooling Described in Class 9 Science Chapter 1 Matter in Our Surroundings Notes
Water is often stored in earthenware containers to maintain its cool temperature. Much like the pores found in cotton fabric, the pores on the surface of earthen pots facilitate enhanced evaporation. This process aids in keeping the water’s temperature down.
For our own comfort, we sweat profusely to cool down our bodies. In essence, sweating is a form of evaporation. As the water on our skin evaporates, it consumes energy and thereby reduces our body temperature.
In the summer, we commonly opt for cotton clothing. Cotton possesses a remarkable ability to absorb water, allowing greater contact between the sweat on our skin and the air. This encourages more efficient evaporation. Consequently, wearing cotton clothing contributes to a cooling sensation due to the enhanced evaporative effect it creates.
Read Also:
- CBSE Previous Year Question Papers Class 9 Maths with Solutions
- Sample Question Paper for Class 9 CBSE Hindi Course B
- Matter In Our Surroundings Class 9 Sample Paper With Answers
- MCQ for Class 9 Science Chapter 1 Matter in Our Surroundings
Frequently Asked Questions – FAQs on Class 9 Science Chapter 1 Matter in Our Surroundings Notes
What is the main focus of Class 9 Science Chapter 1 Matter in Our Surroundings Notes?
Chapter 1, “Matter in Our Surroundings,” primarily explores the different states of matter, their characteristics, and the factors that influence their behavior.
What are the three states of matter discussed in Class 9 Science Chapter 1 Matter in Our Surroundings Notes?
The three states of matter covered in this chapter are solids, liquids, and gases. Their properties, behavior, and phase changes are explained.
How is the concept of diffusion explained in Class 9 Science Chapter 1 Matter in Our Surroundings Notes?
Diffusion, the process of particles spreading out in a substance, is elaborated upon. It discusses how gases and liquids diffuse and factors affecting the rate of diffusion.
What is evaporation, and how is it relevant to the chapter?
Evaporation, the conversion of a liquid into a gas at temperatures below its boiling point, is discussed. Its role in cooling, sweat’s cooling effect, and its relevance to the water cycle are explained.
How does the Class 9 Science Chapter 1 Matter in Our Surroundings Notes discuss the relationship between temperature and the state of matter?
The chapter explains how changing temperature affects the state of matter. It covers concepts such as melting, boiling, and the effect of temperature on the kinetic energy of particles.