Blog
History of Class 10th: Chapter Wise Complete Notes of NCERT
The NCERT history of class 10th Notes serve as indispensable study material, forming a pivotal component of the broader subject of Social Science. Given the abstract nature of History, students are required to craft meticulous and concise responses.
Securing commendable grades in History is equally imperative as excelling in other disciplines. NCERT Class 10 History delves into significant topics like Nationalism, the French Revolution, Globalisation, and Imperialism, all of which have left a profound impact on our society. Consequently, our NCERT Class 10 History Notes encompass a comprehensive understanding of these concepts.
These meticulously crafted notes are the handiwork of subject matter experts, tailored to facilitate effortless comprehension and retention for students.
Moreover, they prove invaluable for purposes of review and examination readiness. By diligently perusing these CBSE Class 10 Social Science Notes, students stand to achieve higher marks in their History assessments. Visit History 10th class to get chapter wise solution of all history chaptes.

NCERT Notes on History of Class 10th
The CBSE Class 10 History textbook comprises a grand total of 5 chapters. Within the provided table, we have meticulously assembled the complete set of CBSE Class 10 History notes spanning all 5 chapters. To access these notes, simply click on the corresponding chapter links. Each of these CBSE Notes has been meticulously curated, drawing from the most up-to-date NCERT Class 10 History syllabus as a point of reference.
- Chapter 1: The Rise of Nationalism in Europe
- Chapter 2: Nationalism in India
- Chapter 3: The Making of a Global World
- Chapter 4: The Age of Industrialisation
- Chapter 5: Print Culture and The Modern World
Benefits of Studying from NCERT History of Class 10th Notes
- Facilitating an effortless grasp of the concepts.
- These notes prove convenient for swift and comprehensive chapter reviews.
- Encompassing pivotal subjects, ideas, and chronological details.
- Perusing these notes guides students in identifying areas warranting heightened attention.
Frequently Asked Questions on NCERT History of Class 10th Notes
1. What are NCERT History Class 10 Notes?
NCERT History Class 10 Notes are concise and comprehensive study materials created to aid students in understanding and revising the concepts covered in the Class 10 History textbook published by the National Council of Educational Research and Training (NCERT).
2. Who prepares these NCERT History Class 10 Notes?
These notes are meticulously prepared by subject matter experts who have a deep understanding of the Class 10 History syllabus and curriculum.
3. How can I access these notes?
You can access these notes by downloading them from the provided links corresponding to each chapter. These links are often available on educational websites, forums, or study platforms.
4. How do these notes help in my studies?
NCERT History Class 10 Notes serve as valuable tools for better understanding and retention of the historical concepts. They provide a concise overview of important topics, making it easier for you to grasp the subject matter.
5. Are these notes a substitute for the NCERT textbook?
While these notes are designed to complement your study of the NCERT textbook, they are not a substitute for it. It’s important to refer to the official textbook for in-depth understanding and context.
Metals and Non Metals Class 10 NCERT Science Chapter 3 Notes
Metals and Non Metals Class 10: In the arrangement known as the periodic table, the elements present on our planet are systematically organized according to their ascending atomic numbers.
Our current understanding recognizes a grand total of 118 elements, among which 92 occur naturally, while the remaining 26 are synthesized artificially within laboratory settings.
These elements can be categorized into three distinct groups—Metalloids, Metals, and Non-Metals—based on their unique physical and chemical characteristics.
Metals and Non Metals Class 10 Notes: NCERT Science Chapter 3

Metals and Non Metals Class 10
Physical Properties: Metals and Non Metals Class 10
A quantifiable attribute that signifies a state of a physical system is known as a physical property. The physical properties of a system serve to define its shifts between momentary conditions. The term “observables” is employed to denote these discernible physical attributes.
Physical Properties of Metals
- Possessing significant hardness and high tensile strength – Carbon stands out as the sole non-metal with remarkably high tensile strength.
- Maintaining solidity at room temperature – While one non-metal, bromine, defies this by being a liquid at room temperature, others such as carbon and sulfur remain solid under these conditions.
- Exhibiting sonority – When struck, metals generate a distinctive ringing sound.
Proficient conductors of heat and electricity – Graphite notably excels as a conductor of both heat and electricity. - Demonstrating malleability, enabling the shaping into thin sheets.
Displaying ductility, allowing the drawing into fine wires. - Exhibiting notably high melting and boiling points (with exceptions like Caesium (Cs) and Gallium (Ga)) – An illustrative instance is graphite, a non-metal form of carbon, which boasts a high boiling point and remains solid at room temperature.
- Possessing density (excluding alkali metals), with Osmium exhibiting the highest density and lithium displaying the lowest.
- Exhibiting luster – The capacity to reflect light and be polished is characteristic of metals, exemplified by gold, silver, and copper. Iodine and carbon, although non-metals, also possess luster, albeit specific to certain forms like diamond and graphite for carbon.
- Generally showcasing a silver-grey hue (aside from gold and copper) – The typical coloration of metals tends to be silver or grey in nature.
Non-Metals
Nonmetals are elements that lack the characteristic properties of metals.
Physical Properties of Non-metals
- Exist in states of matter including solids, liquids, and gases at standard room conditions.
- Fragile
- Not capable of being shaped into thin sheets
- Not capable of being drawn into thin wires
- Lack a resonating sound when struck
- Poor conductors of heat and electricity
Exceptions in Physical Properties
- Alkali metals (Na, K, Li) can be cut using a knife.
- Mercury is a liquid metal.
- Lead and mercury are poor conductors of heat.
- Mercury expands significantly for the slightest change in temperature.
- Gallium and caesium have very low melting points.
- Iodine is non-metal, but it has lustre.
- Graphite conducts electricity.
- Diamond conducts heat and has a very high melting point.
Examples of Non-metals
- Hydrogen – Gas
- Nitrogen – Gas
- Oxygen – Gas
- Fluorine – Gas
- Chlorine – Gas
- Bromine – Liquid
- Iodine – Solid
- Carbon – Solid
- Sulphur – Solid
- Phosphorous – Solid
- Silicon – Solid
Chemical Properties: Metals and Non Metals Class 10
Chemical Properties of Metals
Alkali metals (such as Li, Na, K, etc.) display vigorous reactions with water and oxygen or air.
- Mg reacts with heated water.
- Al, Fe, and Zn undergo reactions with steam.
- Cu, Ag, Pt, and Au show no reactivity with water or weak acids.
Reaction of Metals with Oxygen (Burnt in Air)
When metals undergo combustion in the presence of atmospheric oxygen, they give rise to metal oxides. These oxides are a foundational type of substance discovered naturally, capable of altering the hue of red litmus paper to blue. To prevent interactions with oxygen, moisture, and carbon dioxide in the atmosphere, sodium and potassium metals are stored in kerosene oil.
Metal + Oxygen → Metal oxide (basic)
● Sodium (Na) and potassium (K) are preserved within kerosene oil due to their vigorous reactivity with air, leading to combustion.
4K(s) + O2(g) → 2K2O(s) (highly energetic reaction)
● Magnesium (Mg), aluminum (Al), zinc (Zn), and lead (Pb) exhibit a gradual reaction with air, developing a protective layer that thwarts corrosion.
2Mg(s) + O2(g) → 2MgO(s) (Mg combustion produces intense white light)
4Al(s) + 3O2(g) → 2Al2O3(s)
● Silver, platinum, and gold remain inert, devoid of combustion or reactivity with air.
Basic Oxides of Metals
Metallic oxides are structured crystalline solids comprising a metal cation and an oxide anion. Commonly, they engage in reactions with water to generate bases or combine with acids to produce salts. The equation MO + H2O → M(OH)2 (where M represents a group 2 metal) exemplifies this pattern. Consequently, these compounds are frequently referred to as basic oxides.
Certain metallic oxides are soluble in water, forming alkalis. Their aqueous solutions exhibit the ability to convert red litmus paper to blue.
Examples:
Na2O(s) + H2O(l) → 2NaOH(aq)
K2O(s) + H2O(l) → 2KOH(aq)
Amphoteric Oxides of Metals
Amphoteric oxides display the unique ability to react with both acids and bases, resulting in the formation of salts and water.
For instance, compounds like Al2O3, ZnO, PbO, and SnO fall under this category.
Examples of reactions:
Al2O3(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2O(l)
Al2O3(s) + 2NaOH(aq) → 2NaAlO2(aq) + H2O(l)
ZnO(s) + 2HCl(aq) → ZnCl2(aq) + H2O(l)
ZnO(s) + 2NaOH(aq) → Na2ZnO2(aq) + H2O(l)
Reactivity Series
The activity series of metals, often referred to as the reactivity series, entails the organization of metals in a sequence based on their decreasing levels of reactivity.
The subsequent table presents the reactivity of metals, ranked from greatest to least reactivity.
| Symbol | Element |
|---|---|
| K | Potassium ( Highly Active Metal) |
| Ba | Barium |
| Ca | Calcium |
| Na | Sodium |
| Mg | Magnesium |
| Al | Aluminium |
| Zn | Zinc |
| Fe | Iron |
| Ni | Nickel |
| Sn | Tin |
| Pb | Lead |
| H | Hydrogen |
| Cu | Copper |
| Hg | Mercury |
| Ag | Silver |
| Au | Gold |
| Pt | Platinum |
Roasting
Undergoing vigorous heating in the presence of an abundant supply of air, it transforms sulphide ores into oxides. This process serves to eliminate volatile impurities as well.
Equation:
2ZnS(s) + 3O2(g) + Heat → 2ZnO(s) + 2SO2(g)
Calcination
Through intense heating within a restricted air supply, it transforms carbonate and hydrated ores into oxides while simultaneously purging volatile impurities.
Equations:
ZnCO3(s) + heat → ZnO(s) + CO2(g)
CaCO3(s) + heat → CaO(s) + CO2(g)
Al2O3.2H2O(s) + heat → 2Al2O3(s) + 2H2O(l)
2Fe2O3.3H2O(s) + heat → 2Fe2O3(s) + 3H2O(l)
Reaction of Metals with Water or Steam
Aluminium, iron, and zinc are metals that exhibit no reaction with water, be it cold or hot. However, when they encounter steam, they generate metal oxide along with hydrogen gas. In contrast, lead, copper, silver, and gold are metals that remain unreactive when in contact with water.
Metal + Water → Metal hydroxide or Metal oxide + Hydrogen
Examples:
2Na + 2H2O (cold) → 2NaOH + H2 + heat
Ca + 2H2O (cold) → Ca(OH)2 + H2
Mg + 2H2O (hot) → Mg(OH)2 + H2
2Al + 3H2O (steam) → Al2O3 + 3H2
Zn + H2O (steam) → ZnO + H2
3Fe + 4H2O (steam) → Fe3O4 + 4H2
Reaction of Metals with Acid
Upon immersion of a metal into an acid, it undergoes a reduction in size due to the consumption within a chemical process. Concurrently, the appearance of gas bubbles is evident, signifying the generation of hydrogen gas as a byproduct. This reactive hydrogen gas can be further demonstrated through its combustible nature, as evidenced by igniting it with a burning splint.
Metal + Dilute Acid → Salt + Hydrogen gas
Examples:
2Na(s) + 2HCl(dilute) → 2NaCl(aq) + H2(g)
2K(s) + H2SO4(dilute) → K2SO4(aq) + H2(g)
Only magnesium (Mg) and manganese (Mn) exhibit reactivity with highly diluted nitric acid, resulting in the liberation of hydrogen gas.
Examples:
Mg(s) + 2HNO3(dilute) → Mg(NO3)2(aq) + H2(g)
Mn(s) + 2HNO3(dilute) → Mn(NO3)2(aq) + H2(g)
Displacement Reaction
A higher-reactivity element replaces a lower-reactivity element within its compound or solution.
How Do Metals React with the Solution of Other Metal Salts
A metal that possesses greater reactivity has the ability to replace a metal with lesser reactivity within a solution of its salt, leading to what is termed a displacement reaction. This kind of reaction is commonly referred to as a metal displacement reaction. The reactivity of frequently employed metals has been organized in a descending order, constituting the reactivity or activity series.
The reaction takes the form:
Metal A + Salt of metal B → Salt of metal A + Metal B
For instance:
Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
This process finds application in thermite welding, where it contributes to the displacement of iron from its oxide using aluminum.
Moreover, it is a pivotal aspect of steel production, where carbon displaces iron from its oxide.
Furthermore, this principle finds predominant use in various metal extraction processes.
Reaction of Metals with Bases
Bases are characterized by a bitter flavor and a smooth, slick sensation. When a base is dissolved in water, it is termed an alkali. Upon interacting with acids, these substances yield salts through chemical reactions. Bases are recognized for their ability to shift the color of red litmus paper to blue.
The reaction follows the pattern:
Base + Metal → Salt + Hydrogen
Examples:
2NaOH(aq) + Zn(s) → Na2ZnO2(aq) + H2(g)
2NaOH(aq) + 2Al(s) + 2H2O(l) → 2NaAlO2(aq) + 2H2(g)
Extraction of Metals and Non-Metals
Applications of Displacement Reaction
Uses of displacement reaction
Metal Extraction
Production of Steel
Thermite Reaction: Al(s) + Fe2O3(s) → Al2O3 + Fe(molten)
The thermite reaction finds application in the welding of railway tracks, repairing cracked machine parts, and more.
Occurrence of Metals
The majority of elements, particularly metals, are naturally found in a bonded state alongside other elements. These combinations of metal compounds are categorized as minerals. However, only a select few among these minerals serve as practical reservoirs of the respective metal. These specific utilitarian reserves are referred to as ores.
Notably, gold (Au) and platinum (Pt) are examples of elements that exist in their native or free form.
Extraction of Metals
The activity of retrieving metal ores from deep subterranean deposits is referred to as Mining. Within the Earth’s crust, metal ores are present in varying quantities. The extraction of metals from these ores is the pivotal step that enables us to harness the minerals concealed beneath the surface. It’s important to note that ores contrast significantly from the refined metals visible in structures like buildings and bridges. Ores comprise the sought-after metal compounds along with impurities and earthly materials collectively termed Gangue.
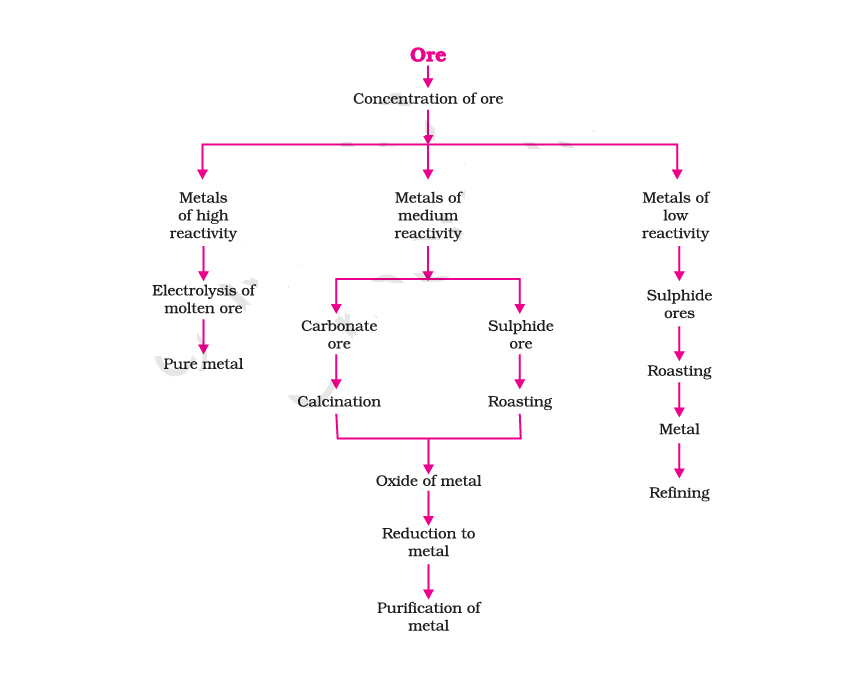
Enrichment of Ores
Purification involves the elimination of impurities or gangue from ore by employing diverse physical and chemical methods. The selection of a specific technique for a given ore hinges on the distinctions between the ore and the gangue in their properties.
In the realm of chemistry, gangue signifies an unwanted element or impurity that encases minerals within an ore deposit, which can be anything from sand and rock to other materials. This mineral presence is a frequent occurrence in mining activities.
Extracting Metals Low in Reactivity Series
By self-reduction- when the sulphide ores of less electropositive metals like Hg, Pb, Cu etc., are heated in air, a part of the ore gets converted to oxide, which then reacts with the remaining sulphide ore to give the crude metal and sulphur dioxide. In this process, no external reducing agent is used.
1. 2HgS(Cinnabar)+3O2(g)+heat→2HgO(crude metal)+2SO2(g)
2HgO(s)+heat→2Hg(l)+O2(g)
2. Cu2S(Copper pyrite)+3O2(g)+heat→2Cu2O(s)+2SO2(g)
2Cu2O(s)+Cu2S(s)+heat→6Cu(crude metal)+SO2(g)
3. 2PbS(Galena)+3O2(g)+heat→2PbO(s)+2SO2(g)
PbS(s)+2PbO(s)→2Pb(crudemetal)+SO2(g)
Extracting Metals in the Middle of Reactivity Series
Calcination signifies a process in which ore is subjected to heat in the absence of air or with limited air supply. Roasting, on the other hand, involves heating ore in the presence of air or oxygen, but below its melting point. This process is utilized to enhance or modify the properties of ores.
Smelting is a procedure that entails heating the previously roasted or calcined ore (metal oxide) to an elevated temperature alongside a suitable reducing agent. This results in the production of the raw metal in its molten form.
Example:
Fe2O3 + 3C (coke) → 2Fe + 3CO2
An aluminothermic reaction, known as the Goldschmidt reaction, is a profoundly exothermic reaction involving the heating of metal oxides, typically those of Fe and Cr, in the presence of aluminum at high temperatures.
Examples:
Fe2O3 + 2Al → Al2O3 + 2Fe + heat
Cr2O3 + 2Al → Al2O3 + 2Cr + heat
Extraction of Metals Towards the Top of the Reactivity Series
Electrolytic Reduction:
1. Down’s Process: Molten NaCl undergoes electrolysis within a specialized apparatus.
At the cathode (reduction):
Na+(molten) + e− → Na(s)
Metal is precipitated.
At the anode (oxidation):
2Cl−(molten) → Cl2(g) + 2e–
Chlorine gas is liberated.
2. Hall’s Process: An amalgamation of molten alumina and a fluoride solvent, typically cryolite (Na3AlF6), is subjected to electrolysis.
At the cathode (reduction):
2Al3+ + 6e– → 2Al(s)
Metal is deposited.
At the anode (oxidation):
6O2– → 3O2(g) + 12e–
Oxygen gas is released.
Metals positioned atop the reactivity series exhibit pronounced reactivity. These metals cannot be extracted from their compounds through heating with carbon, as their affinity for oxygen surpasses that of carbon. Thus, the electrolytic reduction method is employed for the extraction of such metals.
Refining of Metals: Metals and Non Metals Class 10
Metal refining involves the elimination of impurities or gangue from raw metal. This final phase in metallurgy relies on distinguishing the properties of the metal from those of the gangue.
Electrolytic Refining
Copper, zinc, nickel, silver, tin, gold, and similar metals undergo electrolytic refinement.
Anode: Contains impure or crude metal
Cathode: Comprises a slender strip of pure metal
Electrolyte: Consists of an aqueous solution of a metal salt
During anode oxidation: Metal ions are released into the solution
At cathode reduction: An equivalent quantity of metal from the solution gets deposited
Impurities gather at the base of the anode.
Electronic Configuration
Group 1 elements – Alkali metals
| Element | Electronic Configuration |
|---|---|
| Lithium(Li) | 2,1 |
| Sodium(Na) | 2,8,1 |
| Potassium(K) | 2,8,8,1 |
| Rubidium(Rb) | 2,8,18,8,1 |
Group 2 elements – Alkaline earth metals
| Element | Electronic Configuration |
|---|---|
| Beryllium(Be) | 2,2 |
| Magnesium(Mg) | 2,8,2 |
| Calcium(Ca) | 2,8,8,2 |
| Stronium(Sr) | 2,8,18,8,2 |
How Do Metals and Non-Metals React?
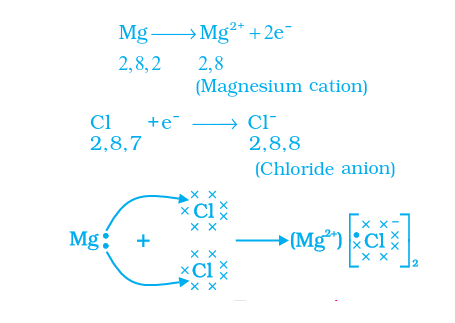
Metals shed valence electrons, leading to the creation of cations.
Non-metals acquire these electrons in their valence shell, resulting in the formation of anions.
The cation and anion are drawn together by a potent electrostatic force, culminating in the establishment of an ionic bond.
For instance, in calcium chloride, the ionic bond emerges from the attraction between oppositely charged calcium and chloride ions.
The calcium atom loses two electrons, achieving the electronic arrangement akin to the nearest noble gas (argon), resulting in a net charge of +2.
Ionic Compounds
Ionic compounds are balanced compounds comprising positively charged cations and negatively charged anions. Binary ionic compounds, those containing just two distinct elements, are named by listing the cation’s name followed by the anion’s name.
The compound’s cohesion arises from the electrostatic attractions between ions of opposing charges.
Illustrations include: MgCl2, CaO, MgO, NaCl, and more.
Properties of Ionic Compound
Ionic compounds:
Typically exist as crystalline solids composed of ions.
Exhibit elevated melting and boiling points.
Display electrical conductivity in aqueous solutions and when liquefied.
Generally dissolve readily in water and polar solvents.
Electric Conduction of Ionic Compounds
Ionic compounds exhibit electrical conductivity when they are in a molten or aqueous state, during which the ions are liberated and function as charge carriers. In their solid form, ions are firmly bound by electrostatic attraction and lack mobility, resulting in the inability to conduct electricity.
CBSE Class 10 Science notes Chapter 3 – 5
CBSE Class 10 Science notes Chapter 3 – 4
As an illustration, consider ionic compounds like NaCl, which do not conduct electricity while in a solid state. However, when these compounds are dissolved in water or in a molten condition, they become capable of conducting electricity.
Melting and Boiling Points of Ionic Compounds
Ionic compounds possess robust electrostatic forces that demand a substantial energy input to disrupt. Consequently, the melting and boiling points of an ionic compound are typically elevated.
Solubility of Ionic Compounds
The majority of ionic compounds tend to dissolve in water, a phenomenon attributed to the dispersion of ions within the water medium. This outcome is a result of water’s polar characteristic.
For instance, consider NaCl, a three-dimensional salt crystal comprised of Na+ and Cl− ions held together by electrostatic forces of attraction. When a NaCl crystal makes contact with water, the partially positively charged ends of water molecules interact with the Cl− ions, while the negatively charged ends of water molecules interact with the Na+ ions. This ion-dipole interaction between ions and water molecules contributes to the disruption of the robust electrostatic forces binding the crystal. As a result, the crystal becomes soluble in water.
Corrosion: Metals and Non Metals Class 10
Alloys
Alloys represent uniform blends of a metal combined with either other metals or nonmetals. The creation of alloys serves to amplify advantageous material properties like hardness, tensile strength, and resistance to corrosion.
Here are a few instances of alloys:
– Brass: amalgamation of copper and zinc
– Bronze: fusion of copper and tin
– Solder: combination of lead and tin
– Amalgam: mixture involving mercury and other metals
Corrosion
Progressive degradation of a substance, often a metal, due to the influence of moisture, air, or chemicals in its ambient surroundings.
Rusting:
4Fe(s) + 3O2(from air) + xH2O(moisture) → 2Fe2O3. xH2O(rust)
Copper Corrosion:
Cu(s) + H2O(moisture) + CO2(from air) → CuCO3.Cu(OH)2(green)
Silver Corrosion:
Ag(s) + H2S(from air) → Ag2S(black) + H2(gas)
Prevention of Corrosion
Preventions
1. Application of Coatings: Metal surfaces can be safeguarded from corrosion by applying protective coatings like paints, oil, or grease. These coatings create a barrier that excludes air and moisture.
2. Alloy Formation: Incorporating metals into alloys enhances their corrosion resistance. For instance, stainless steel is a notable example of an alloyed metal.
3. Galvanization: This involves the deposition of molten zinc onto iron articles. Zinc forms a protective layer that acts as a barrier against corrosion.
4. Electroplating: Through the application of an electric current, one metal can be coated onto another, providing not only protection but also an improved metallic appearance. Illustrations include silver plating and nickel plating.
5. Sacrificial Protection: Magnesium, being more reactive than iron, can be used as a sacrificial layer on iron or steel articles. Acting as a cathode, magnesium undergoes a reaction, sacrificing itself instead of the underlying iron or steel, thus shielding the articles from corrosion.
Read Also
- Chemical Reactions and Equations
- Acids, Bases and Salts
- Life Processes
- Control and Coordination
- How Do Organisms Reproduce?
- Heredity and Evolution
- Electricity
- Magnetic Effects of Electric Current
- Our Environment
- Human Eye and the Colourful World Notes Chapter 10 Science
Frequently Asked Questions on Metals and Non Metals Class 10
1. What are metals and non-metals?
Metals are elements that typically exhibit properties like malleability, ductility, and good conductivity of heat and electricity. Non-metals, on the other hand, often lack these properties and may be brittle and poor conductors.
2. How are metals and non-metals classified on the periodic table?
Metals are generally found on the left side and in the middle of the periodic table, while non-metals are primarily located on the right side.
3. What are metalloids?
Metalloids are elements that have properties intermediate between metals and non-metals. They show a combination of characteristics from both categories.
4. What is the reactivity series of metals?
The reactivity series is a ranking of metals based on their reactivity with water, acids, and other substances. It helps us understand their behavior in various chemical reactions.
5. How do metals react with water and acids?
Many metals react with water to form metal hydroxides and hydrogen gas. They can also react with acids to produce salts and hydrogen gas.
6. What are ionic compounds?
Ionic compounds are compounds generated through the transfer of electrons from a metal to a non-metal, culminating in the creation of positively charged cations and negatively charged anions.
7. What is the difference between metals and non-metals in terms of electron gain and loss?
Metals tend to lose electrons and form positively charged ions (cations), while non-metals tend to gain electrons and form negatively charged ions (anions).
8. How are alloys different from pure metals?
Alloys are mixtures of two or more metals, or a metal and a non-metal, that are combined to enhance specific properties. They often possess improved strength, durability, and resistance to corrosion compared to pure metals.
9. What is the process of corrosion?
Corrosion is the gradual degradation of metals due to the influence of factors like moisture, air, and chemicals in the environment. It often results in the formation of oxides, hydroxides, or other compounds on the metal’s surface.
10. How can we prevent corrosion?
Corrosion prevention can be achieved through techniques such as applying protective coatings like paints or oils, creating alloys, galvanizing, electroplating, and employing sacrificial protection, which involves utilizing a more reactive metal to safeguard a less reactive metal.
Human Eye and the Colourful World Notes Chapter 10 Science
In the class 10 Science chapter, “The Human Eye and the Colourful World Notes,” students delve into the application of light knowledge and its properties, previously studied in other chapters. The focus is on understanding how these concepts relate to the human eye.
Additionally, students explore various optical phenomena, including the nature and formation of rainbows, the splitting of white light, and the intriguing phenomenon of the blue color of the sky.
Human Eye and the Colourful World Notes

Structure of a Human Eye
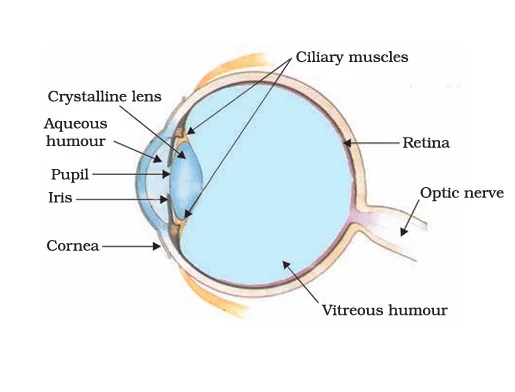
Among all the sense organs, the human eye holds paramount importance, as it grants us the ability to perceive the enchanting and colorful world that surrounds us.
This remarkable organ takes on a spherical shape, with an average diameter of 2.3 cm. Internally, the eye comprises several essential components, such as the cornea, iris, pupil, lens, ciliary muscles, retina, nerve cells, optic nerve, yellow spot, aqueous and vitreous humor, and suspensory ligament.
These intricately interwoven elements work in harmony to facilitate our vision and visual experiences.
The human eye’s lens system plays a crucial role in forming an image on the retina, the light-sensitive screen within the eye. Light enters through the thin membrane known as the cornea, where most of the light refraction occurs at its outer surface. Positioned behind the cornea is the iris, a dark muscular diaphragm responsible for controlling the size of the pupil. The pupil itself acts as a structure that regulates the amount of light entering the eye.
When light illuminates the retina, the light-sensitive cells are activated, triggering electrical signals. These signals are then transmitted to the brain via the optic nerves. The brain undertakes a complex analysis of these signals and processes the information, allowing us to perceive objects as they truly are. In this intricate process, our eyes enable us to perceive the world around us with clarity and precision.
Defects of a Human Eye
Various common eye disorders affect individuals due to a combination of factors, but fortunately, many of these conditions can be improved through corrective measures. Some of the prevalent eye defects include:
1. Myopia (Short-sightedness): Individuals with myopia have clear vision for nearby objects but struggle to see distant objects. A concave lens can effectively correct this condition.
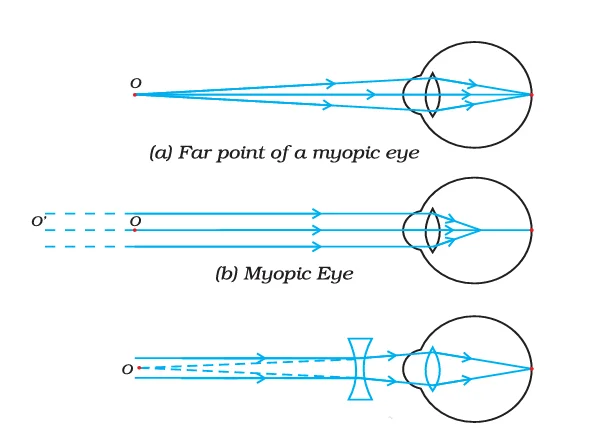
2. Hypermetropia (Farsightedness): People with hypermetropia experience clear vision for distant objects but face difficulty in seeing nearby objects. This defect can be corrected using a convex lens.
3. Presbyopia: This age-related condition arises from weakened ciliary muscles, hardened lens, and reduced lens flexibility. It leads to difficulties in focusing on nearby objects and makes reading and writing challenging.
4. Cataract: Another age-related condition, cataract, occurs when lens proteins erode, causing loss of lens transparency. This results in blurry vision and cloudy lenses, but it can be treated by replacing the affected lens with an artificial one.
In understanding and addressing these eye disorders, modern advancements in corrective procedures have significantly improved the quality of life for those affected.
Dispersion of White Light by a Glass Prism
When a prism is introduced to incident white light, it effectively separates it into a beautiful band of seven colors. This band of colored components is known as the spectrum of the light beam. The credit for the first successful observation of sunlight spectrum through a glass prism goes to Isaac Newton.
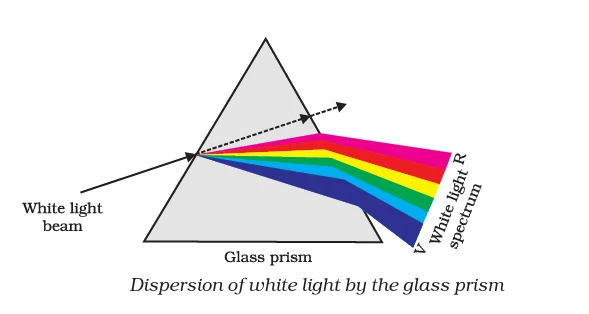
As light passes through a prism, different colors bend at varying angles with respect to the incident ray. Among them, red light bends the least, while violet light bends the most. Consequently, each color follows a distinct path, leading to their individual emergence and distinct appearance. This captivating phenomenon allows us to witness the mesmerizing array of colors constituting the visible spectrum.
Atmospheric Refraction
Atmospheric refraction refers to the bending of light by Earth’s atmosphere, caused by the varying optical densities of its different layers.
The twinkling effect of stars is a result of atmospheric refraction of starlight. When starlight passes through the atmosphere on its way to Earth, it undergoes continuous refraction due to the changing densities of the atmosphere. As a consequence, the path of light rays from the star slightly fluctuates, causing the apparent position of the star to waver. This continuous variation in the perceived position of the star leads to the flickering of starlight entering our eyes, giving rise to the captivating twinkling phenomenon we observe in the night sky.
Scattering of Light
As a light beam travels through a medium, it interacts with the particles present in the medium. During this interaction, some of the light rays are absorbed, while others are scattered in different directions. The extent of scattering and the intensity of the scattered light depend on both the size of the particles and the wavelength of the light.

Read Also
- Chemical Reactions and Equations
- Acids, Bases and Salts
- Life Processes
- Control and Coordination
- How Do Organisms Reproduce?
- Heredity and Evolution
- Electricity
- Metals and Non Metals Class 10 NCERT Science Chapter 3 Notes
- Magnetic Effects of Electric Current
- Our Environment
Frequently Asked Questions on Human Eye and the Colourful World Notes
Q 1: How does the eye work Class 10 notes?
The human eye functions like a complex optical system. Light enters the eye through the cornea and pupil. The iris adjusts the size of the pupil to regulate the amount of light entering. The light then passes through the lens, which focuses it onto the retina.
The retina contains light-sensitive cells called rods and cones, which convert light into electrical signals. These signals are transmitted through the optic nerve to the brain for processing. The brain interprets these signals to create visual perceptions. This intricate process enables us to see and perceive the world around us.
Q 2: What are the parts of the eye Class 10 notes?
On average, the eye is a spherical organ with a diameter of approximately 2.3 cm. Internally, it comprises several essential structures, including the cornea, iris, pupil, lens, ciliary muscles, retina, nerve cells, optic nerve, yellow spot, aqueous and vitreous humor, as well as the suspensory ligament. These intricately interconnected components work in harmony to enable vision and the processing of visual information, facilitating our perception of the surrounding world.
Q 3: What are the 5 main parts of the eye?
The key components responsible for your vision include:
1. Cornea: Positioned at the front of your eye, the cornea is a dome-shaped layer that bends the incoming light, directing it further into your eye.
2. Pupil: Acting as a gateway for light, the pupil is the black dot situated in the center of your eye. It expands in dim light and contracts in bright light, its size regulated by the iris.
3. Iris: Commonly known as your eye color, the iris is a muscular structure controlling the pupil’s size and regulating the amount of light entering your eye.
4. Lens: Located behind the iris and pupil, the lens collaborates with the cornea to focus incoming light, much like a camera lens. It sharpens the image before you, allowing clear and detailed vision.
5. Retina: Situated at the back of the eye, the retina is a layer of tissue that converts incoming light into electrical signals. These signals are then transmitted to the brain, where they are interpreted as images, enabling you to see the world around you.
Q 4: Which gland is found in human eye?
The meibomian glands are oil glands located along the edge of the eyelids, where the eyelashes are present. These glands secrete an oily layer that forms the outer part of the tear film, which helps prevent tears from evaporating too rapidly. Various eye problems can be associated with the meibomian glands and their proper functioning.
Q 5: What is eye number?
“Eye number” typically refers to a measurement of refractive error in the eyes, also known as the prescription for corrective lenses. It is denoted in units called diopters (D). When light enters the eye, it should focus precisely on the retina for clear vision. However, in some individuals, the light either falls short of or goes beyond the retina, causing blurry vision. This condition can be categorized into myopia (nearsightedness), hypermetropia (farsightedness), and astigmatism. Eye number determines the strength of lenses needed to correct these refractive errors, allowing the light to focus correctly on the retina and providing clear vision.
Class 10th Chapter 11 Science Notes for NCERT Students
Class 10th Chapter 11 Science: The Class 10 science chapter 11, titled ‘Electricity,’ explores the fundamental aspects of electricity. It seeks to unravel the nature of electricity, the factors influencing the flow of electric current, and the functioning of electric circuits.
Additionally, the chapter delves into the heating effects of electric current and explores its various practical applications. In our pursuit of comprehending electric current’s makeup, the chapter also delves into the discussion of atomic structure.
Class 10th Chapter 11 Science Notes for NCERT Students
Atomic Structure
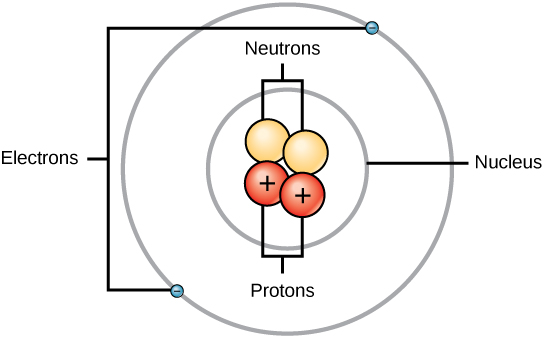
Within an atom, a positively charged nucleus is surrounded by negatively charged electrons that orbit around it. In metals, valence electrons are not tightly bound and are free to move within the conductor, forming an electric current.
Charge
Charge is an inherent characteristic of matter that enables it to exert electromagnetic force.
Conductors and Insulators: Class 10th Chapter 11 Science Notes
A conductor is a substance that allows current to flow through it with relatively little resistance, while an insulator is a substance that offers higher resistance to the flow of current. Copper, iron, and steel are examples of conductors, while glass, dry wood, and cotton are examples of insulators.
Electric Potential and Potential Difference
Electric potential at a point is characterized as the work required to bring a unit positive charge from infinity to that specific point. The potential difference between two points is the discrepancy in electric potentials at those locations. Electrons only move when there exists a difference in electric pressure, known as the potential difference. One Volt is defined as the energy consumption of one joule per electric charge of one coulomb.
Mathematically, the electric potential between two points can be expressed as:
V = W/Q
where V represents the potential difference, \(W\) stands for the work done, and \(Q\) denotes the electric charge.
Electric Current(I)
Electric current is the term used to describe the movement of electric charges. Mathematically, it is represented as I = Q/t, where I denotes the current, Q represents the electric charge, and t signifies the time taken for the charge to flow.
Models of Electric Current
Drift Velocity of Electron
Inside a metallic conductor, when an electric field is applied due to a potential difference, electrons attain an average velocity. This average velocity is known as the drift velocity.
Battery and Its Working
A cell acts as a source of potential difference, generated through internal chemical reactions within it.
At the anode: Cu(s) ⇌ Cu2+(aq) + 2e−
At the cathode: Ag(aq) + 2e− ⇌ 2Ag(s)
When multiple cells are combined, it forms a battery.
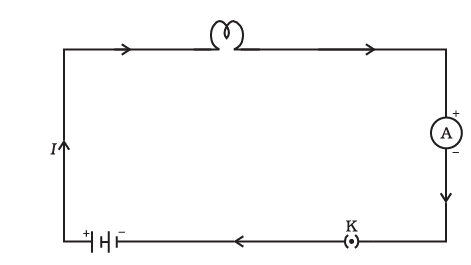
Electric Circuit
Electric Circuit and Circuit Diagram
An electric circuit refers to a closed-loop path through which a current flows. A circuit diagram is a visual representation of the electric circuit using symbols and lines.
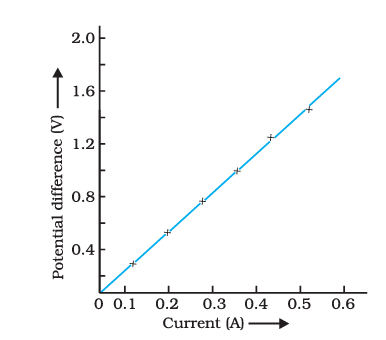
Resistance and Ohm’s Law
Ohm’s Law
Ohm’s Law establishes that the current passing through an ohmic conductor is directly proportional to the potential difference applied across its two ends. In other words, the relationship between the potential difference across the conductor and the current flowing through it is defined by Ohm’s Law.
Resistance
Resistance quantifies the opposition presented to the flow of current within an electric circuit. It is expressed in ohms. Every material exhibits some degree of resistance to current flow. These materials can be broadly categorized into two groups: conductors and insulators. Conductors allow the flow of current with relatively low opposition, while insulators impede current flow to a greater extent.
Factors Affecting Resistance & How They Affect
Resistance is influenced by several factors:
- It is directly proportional to the length of the conductor.
- It is directly proportional to the nature of the conductor.
- It is directly proportional to the temperature of the conductor.
- It is inversely proportional to the cross-sectional area of the conductor.
Mathematically, this relationship is represented as:
Where is the resistance, (rho) is the resistivity of the material (a property related to the nature of the conductor), is the length of the conductor, and is the cross-sectional area of the conductor.
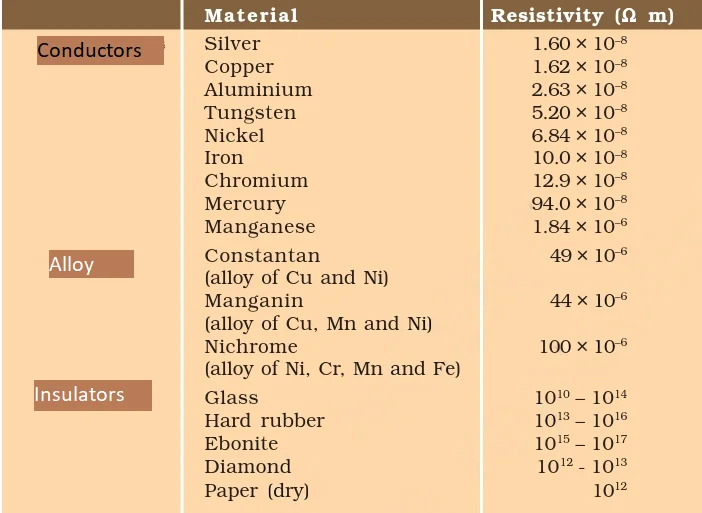
Resistivity
Resistivity refers to the electrical resistance exhibited by a substance with a length of one unit and a cross-sectional area of one unit.
Ohmic and Non-Ohmic Resistors
Resistors that adhere to Ohm’s Law are termed Ohmic resistors, while those that deviate from it are known as Non-Ohmic resistors.
Superconductors
Superconductors are conductors that exhibit zero resistance to the flow of current. Prominent examples of superconductors include aluminum, niobium, magnesium diboride, as well as certain compounds known as cuprates, such as yttrium barium copper oxide and iron pnictides.
Combination of Resistors
Combination of Resistors
When two resistors are arranged in series, they carry the same current through them.
On the other hand, when two resistors are arranged in parallel, they experience the same potential difference applied across them.
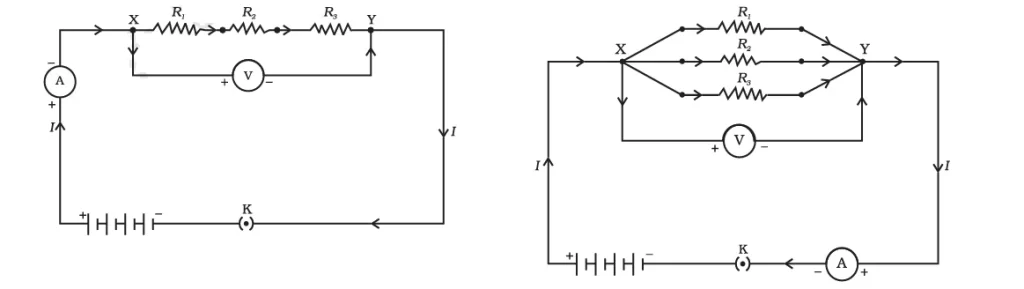
Equivalent Resistance of a System of Resistors
The equivalent resistance of two resistors can be calculated as follows:
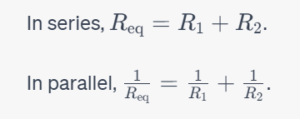
EMF and Terminal Voltage
EMF (Electromotive Force) refers to the potential difference between the two terminals of a cell when there is no current flowing through the circuit.
Terminal voltage, on the other hand, represents the potential difference between the two terminals of a cell when current is actively flowing through the circuit.
Electric Power and AC
Heating Effect of Current
Joule’s Law states the following relationships:
1. Heat (H) is directly proportional to the square of the current (I).
2. Heat (H) is directly proportional to the resistance of the given circuit.
3. Heat (H) is directly proportional to the time (t) for which the current flows through the conductor.
Moreover, when a potential difference is established, it induces the movement of electrons, resulting in the flow of current.
Uses of Heating Effect of Electric Current
The heating effect of current finds practical application in electrical heating appliances like electric kettles, electric irons, room heaters, water heaters (geysers), and similar devices.
Electric Power
Electric power is the measure of the rate at which work is done or electrical energy is consumed. It is denoted by the symbol P and can be calculated as P = W/t, where W is the work done in time t.
The standard unit for electric power is the Watt (W). One watt of power is consumed when a current of 1 Ampere (A) flows at a potential difference of 1 Volt (V).
The commercial unit of electrical energy is the kilowatt-hour (kWh), which is equivalent to 3,600,000 Joules (J) or 3.6×106 J.
Electric power can also be represented in terms of current and resistance as P = I^2R or in terms of potential difference and resistance as P = V^2/R.
One kilowatt-hour is defined as the amount of energy consumed when 1 kilowatt (kW) of power is used for 1 hour.
Read Also:
- Chemical Reactions and Equations
- Acids, Bases and Salts
- Life Processes
- Control and Coordination
- How Do Organisms Reproduce?
- Heredity and Evolution
- Magnetic Effects of Electric Current
- Our Environment
- Human Eye and the Colourful World
- Metals and Non Metals Class 10 NCERT Science Chapter 3 Notes
Frequently Asked Questions on Class 10th Chapter 11 Science Notes for NCERT Students
Q 1: What is the significance of Chapter 11 in Class 10 Science?
Chapter 11 in Class 10 Science covers topics related to electricity, electrical circuits, and power. It provides fundamental knowledge about electrical concepts and their practical applications.
Q 2: What are the key topics covered in Chapter 11 Science Notes?
The key topics covered in Chapter 11 Science Notes include electric current, electric circuits, Ohm’s Law, resistors, heating effect of current, electric power, and the commercial unit of electrical energy (kWh).
Q 3: How can I understand the concept of electric current better?
To understand electric current better, you can start by learning the definition of electric current and its unit (Ampere). You should also study the factors affecting the flow of current in a conductor, including resistance and potential difference.
Q 4: What is Ohm’s Law, and how is it represented?
Ohm’s Law defines the relationship between current (I), potential difference (V), and resistance (R) in an electrical circuit. It is represented by the equation V = IR, where V is the potential difference, I is the current, and R is the resistance.
Q 5: How is electrical power calculated, and what are its units?
Electric power (P) is calculated as P = VI or P = I^2R or P = V^2/R, where V is potential difference, I is current, and R is resistance. The unit of electric power is the Watt (W).
Class 10 Board Exam Papers: CBSE Previous Year Questions
Class 10 Board Exam Papers serve as valuable study materials for students preparing for their board examinations. Regularly solving these past papers not only boosts students’ self-confidence but also alleviates examination stress. By practicing with these question papers, students gain insight into essential topics and the question formats commonly encountered in the Class 10 board exams.
Furthermore, the CBSE board question papers for Class 10, accompanied by answer PDFs, offer students an understanding of the exam pattern and marking scheme. This insight into the answers’ writing style helps them familiarize themselves with the expected approach for CBSE previous year question papers. As a result of their practice, students feel more assured and self-assured when facing the Class 10 examination.

Class 10 Board Exam Paper Subject Wise pdf Download
Easily accessible through the provided links are the CBSE Class 10 Previous Year Question Papers for Maths, Science, Social Science, and English. These question papers span from 2010 to 2022 and come with separate solution PDFs. The solutions offer step-by-step explanations for each question paper, aiding students in comprehending the answer writing process for CBSE Class 10.
Additionally, some of the previous years’ class 10 board exam papers are available without solutions, intended for students’ practice and self-assessment. This comprehensive resource empowers students to enhance their exam preparation and build confidence in tackling the Class 10 board examinations effectively.
Question Paper for Class X Examination 2023
| Subject name | Download |
| Apparel | Download |
| Arabic | Download |
| Artificial intelligence | Download |
| Assamese | Download |
| Automotive | Download |
| Bhasa melayu | Download |
| Banking insurance | Download |
| Beauty & wellness | Download |
| Bengali | Download |
| Bhutia | Download |
| Bodo | Download |
| Computer application | Download |
| Carnatic music (vocal)(theory) | Download |
| Carnatic music (mel ins)(theory) | Download |
| Carnatic music (per ins) | Download |
| Data science | Download |
| Elements of book-keeping & accountancy | Download |
| Elements of business | Download |
| English literature | Download |
| Food production | Download |
| French | Download |
| Front office operation | Download |
| German | Download |
| Gujarati | Download |
| Gurung | Download |
| Health care | Download |
| Hindi a | Download |
| Hindi b | Download |
| Hindustani music (mel ins) | Download |
| Hindustani music per ins | Download |
| Hindustani music vocal | Download |
| Home science | Download |
| Information technology | Download |
| Introduction tourism | Download |
| Introduction financial markets | Download |
| Japanese | Download |
| Kannada | Download |
| Kashmiri | Download |
| Lepcha | Download |
| Limboo | Download |
| Malayalam | Download |
| Manipuri | Download |
| Marathi | Download |
| Marketing & sales | Download |
| Mathematics basic | Download |
| Mathematics standard | Download |
| Mizo | Download |
| Multi skill foundation course | Download |
| Multimedia | Download |
| Ncc | Download |
| Nepali | Download |
| Odia | Download |
| Painting | Download |
| Persian | Download |
| Physical activity trainer | Download |
| Punjabi | Download |
| Rai | Download |
| Russian | Download |
| Sanskrit | Download |
| Science | Download |
| Security | Download |
| Sherpa | Download |
| Sindhi | Download |
| Spanish | Download |
| Social science | Download |
| Tamang | Download |
| Tamil | Download |
| Tangkhul | Download |
| Telugu | Download |
| Telugu telangana | Download |
| Thai | Download |
| Tibetain | Download |
| Urdu a | Download |
| Urdu b | Download |
Question Paper for Class X Examination 2022
| Subject name | Download |
| Agriculture | download |
| Apparel | download |
| Arabic | download |
| Artificial intelligence | download |
| Assamese | download |
| Automotive | download |
| Bhasa melayu | download |
| Banking insurance | download |
| Beauty & wellness | download |
| Bengali | download |
| Bhutia | download |
| Bodo | download |
| Computer application | download |
| Elements of book-keeping & accountancy | download |
| Elements of business | download |
| English literature | download |
| Food production | download |
| French | download |
| Front office operation | download |
| German | download |
| Gujarati | download |
| Gurung | download |
| Health care | download |
| Hindi a | download |
| Hindi b | download |
| Hindustani music (mel ins) | download |
| Hindustani music per ins | download |
| Hindustani music vocal | download |
| Home science | download |
| Information technology | download |
| Introduction tourism | download |
| Introduction financial markets | download |
| Japanese | download |
| Kannada | download |
| Kashmiri | download |
| Lepcha | download |
| Limboo | download |
| Malayalam | download |
| Manipuri | download |
| Marathi | download |
| Marketing & sales | download |
| Mathematics basic | download |
| Mathematics standard | download |
| Mizo | download |
| Multi skill foundation course | download |
| Multimedia | download |
| Ncc | download |
| Nepali | download |
| Odia | download |
| Painting | download |
| Persian | download |
| Physical activity trainer | download |
| Punjabi | download |
| Rai | download |
| Retail | download |
| Russian | download |
| Sanskrit | download |
| Science | download |
| Security | download |
| Sherpa | download |
| Sindhi | download |
| Spanish | download |
| Social science | download |
| Tamang | download |
| Tamil | download |
| Tangkhul | download |
| Telugu | download |
| Telugu telangana | download |
| Thai | download |
| Tibetain | download |
| Urdu a | download |
| Urdu b | download |
Question Paper for Class X (Examination 2020)
| Subject name | Download |
| Apparel | download |
| Arabic | download |
| Assamese | download |
| Automotive | download |
| Banking and insurance | download |
| Agriculture | download |
| Beauty and wellness | download |
| Bengali | download |
| Bhasamelayu | download |
| Bhutia | download |
| Bodo | download |
| Carnatic music | download |
| Retailing | download |
| Elements of book-keeping and accountancy (commerce) | download |
| Elements of business (commerce) | download |
| English language & literature | download |
| English comm | download |
| E-publishing and e-office(commerce) | download |
| Food production | download |
| Information technology | download |
| French | download |
| Front office operations | download |
| German | download |
| Gujarati | download |
| Gurung | download |
| Health care services | download |
| Hindi a | download |
| Hindi b | download |
| Hindustani music | download |
| Home science | download |
| Introduction to financial markets | download |
| Introduction to tourism | download |
| Japanese | download |
| Kannada | download |
| Kashmiri | download |
| Lepcha | download |
| Limboo | download |
| Malayalam | download |
| Manipuri | download |
| Marathi | download |
| Marketing and sales | download |
| Mathematics basics | download |
| Mathematics standard | download |
| Media | download |
| Mizo | download |
| Multi skill foundation course | download |
| National cadet corps (ncc) | download |
| Nepali | download |
| Odia | download |
| Painting | download |
| Persian | download |
| Punjabi | download |
| Rai | download |
| Russian | download |
| Sanskrit | download |
| Science | download |
| Security | download |
| Sherpa | download |
| Sindhi | download |
| Social science | download |
| Spanish | download |
| Tamang | download |
| Tamil | download |
| Tangkhul | download |
| Telug telangana | download |
| Telugu | download |
| Thai | download |
| Tibetan | download |
| Urdu | download |
Question Paper for Class X (Examination 2019)
| Subject name | Download |
| Arabic | download |
| Assamese | download |
| Automobile technology | download |
| Banking and insurance | download |
| Basic agriculture | download |
| Beauty and wellness | download |
| Bengali | download |
| Bhutia | download |
| Bodo | download |
| Carnatic music | download |
| Dynamics of retailing | download |
| Elements of book-keeping and accountancy (commerce) | download |
| Elements of business (commerce) | download |
| English language & literature | download |
| English comm | download |
| E-publishing and e-office(commerce) | download |
| Fit | download |
| Food production | download |
| Foundation of information technology | download |
| French | download |
| Front office operations | download |
| German | download |
| Gujarati | download |
| Gurung | download |
| Health care services | download |
| Hindi a | download |
| Hindi b | download |
| Hindustani music (percussion instrument) | download |
| Hindustani music (vocal) | download |
| Home science | download |
| Information and communication technology (ict) | download |
| Information technology | download |
| Introduction to financial markets | download |
| Introduction to tourism | download |
| Japanese | download |
| Kannada | download |
| Kashmiri | download |
| Lepcha | download |
| Limboo | download |
| Malayalam | download |
| Manipuri | download |
| Marathi | download |
| Marketing and sales | download |
| Mathematics | download |
| Mizo | download |
| National cadet corps (ncc) | download |
| Nepali | download |
| Odia | download |
| Painting | download |
| Persian | download |
| Punjabi | download |
| Rai | download |
| Russian | download |
| Sanskrit | download |
| Science | download |
| Security | download |
| Sherpa | download |
| Sindhi | download |
| Social science | download |
| Spanish | download |
| Tamang | download |
| Tamil | download |
| Tangkhul | download |
| Telugu telangana | download |
| Telugu | download |
| Thai | download |
| Tibetan | download |
| Urdu | download |
Benefits of Solving CBSE Previous Year Question Papers Class 10
While practicing the previous year’s CBSE Class 10 question papers, students not only cover the entire CBSE Class 10 syllabus but also engage in a valuable revision process.
It allows them to gauge the time required for solving specific sections of these papers, which is beneficial for managing time during the actual board exam.
Therefore, it is essential for students to solve at least 5-6 CBSE previous year question papers for each subject to enhance their speed, accuracy, confidence, exam temperament, and time management skills.
Regular assessments in school may sometimes cause students to lose focus on key concepts. In such cases, the previous year’s CBSE Class 10 question papers serve as a handy tool. They help students evaluate their preparation level and ensure that they have covered all the important concepts.
Additionally, the provided solution PDFs for these papers assist students in understanding how to present their answers effectively from an exam perspective. For a broader understanding of question patterns over the years, students can also refer to the 10 Years Question Paper CBSE Class 10.
We hope that students find this information on “class 10 board exam papers” useful for their exam preparation. Keep learning and stay tuned for further updates on CBSE Exams.
Frequently Asked Questions on CBSE Class 10 Previous Year Question Papers
Q 1: Why should I practice Class 10 previous year’s exam papers?
Practicing Class 10 previous year’s exam papers helps you familiarize yourself with the exam pattern, question types, and difficulty level. It provides valuable insights into which topics are frequently asked and enhances your time management and exam-solving skills.
Q 2: How many CBSE Class 10 previous year question papers should I solve for each subject?
It is recommended to solve at least 5-6 previous year question papers for each subject. This will give you a comprehensive understanding of the subject, boost your confidence, and prepare you effectively for the actual board exams.
Q 3: Are the solutions provided with the CBSE Class 10 previous year question papers?
Yes, most websites and study resources provide solution PDFs with the CBSE Class 10 previous year question papers. These solutions assist you in understanding the correct approach to solving problems and writing answers effectively.
Q 4: Can solving previous year’s question papers improve my exam performance?
Absolutely! Solving CBSE Class 10 previous year question papers enhances your exam preparation by giving you exposure to the exam format and frequently asked topics. It boosts your confidence, accuracy, and time management skills, ultimately leading to improved performance in the board exams.
Q 5: Is it beneficial to refer to 10 Years Question Paper CBSE Class 10?
Yes, referring to the 10 Years Question Paper CBSE Class 10 is advantageous. It gives you a broader perspective on the types of questions asked over the years. It helps you identify recurring patterns and allows you to focus on essential topics that are frequently tested in previous exams.
Syllabus Class 10 For CBSE Students – Download Pdf
The CBSE has recently released the Syllabus Class 10 for the 2023-24 academic year, a crucial resource for students preparing for board exams. This syllabus outlines the course structure, unit-wise marks distribution, and allocated periods for each unit. Moreover, it includes information about reference books, projects, assignments, and practicals, providing students with a comprehensive guide to plan their studies effectively.
For easy access, we have provided links to the Syllabus PDF for all major subjects. Familiarizing themselves with this syllabus will enable students to better organize their exam preparation. Additionally, covering the CBSE syllabus for Class 9 will contribute to enhanced academic knowledge and better learning outcomes.

To ensure success in their exams, students are encouraged to thoroughly review the syllabus for each subject before delving into their preparation. By doing so, they will become acquainted with the topics and concepts to be covered in each subject, ensuring they are well-prepared for the board exam, which is designed according to the CBSE 10th syllabus.
CBSE Syllabus Class 10 for all Subjects 2023-24 – Download pdf
It is essential for students to adhere to the CBSE Class 10 Syllabus guidelines while studying. To access the syllabus for all subjects, simply click on the links provided below. In addition to the theory subjects, students will also find the practical syllabus for CBSE Class 10, ensuring comprehensive coverage of the curriculum.
- CBSE Syllabus for Class 10 Maths
- CBSE Syllabus for Class 10 Science
- CBSE Syllabus for Class 10 Social Science
- CBSE Syllabus for Class 10 English
- CBSE Syllabus for Class 10 English Communicative
- CBSE Syllabus for Class 10 Hindi A
- CBSE Syllabus for Class 10 Hindi B
Class 10 marks a pivotal juncture in every student’s life, as it determines the academic path they will pursue for higher studies. During the revision phase, the CBSE Syllabus for Class 10 proves invaluable.
It allows students to monitor their progress on studied topics and identify areas that require further attention. In Class 10, the significance of practicals is on par with theory, emphasizing a well-rounded approach to education.
CBSE Class 10 Scheme of Studies
The following table presents the scheme of studies for Class 10 students. It includes 5 compulsory subjects, 2 optional subjects, and 2 subjects designated for internal assessment.
| Type | Subject Name |
|---|---|
| Compulsory Subjects | Language 1 |
| Language 2 | |
| Science | |
| Mathematics | |
| Social Science | |
| Optional | Skill Subject |
| Language 3 / Any Academic subject other than those opted for above | |
| Subjects of Internal Assessment | Art Education |
| Health and Physical Education |
Students can access the downloadable PDF of the CBSE 10th Syllabus, which includes the marks weightage for each subject. It is recommended that they prepare for the board exam in accordance with the prescribed syllabus.
Benefits of Knowing the CBSE Board Syllabus
Here are the advantages of being familiar with the CBSE Class 10th Syllabus:
1. Provides an overview of the topics covered in the academic session, allowing students to know what to expect.
2. Serves as a helpful guide during Class 10 exam preparation, aiding students in their board exam readiness.
3. The well-structured syllabus progresses from fundamental to more advanced topics, making it easier for students to grasp concepts effectively.
4. Enables students to identify specific areas they need to focus on, streamlining their study efforts.
5. Boosts students’ confidence as they prepare thoroughly for their Class 10 Board exams, knowing they have covered all essential topics.
Objectives of The Syllabus
The CBSE 10th Syllabus is carefully crafted to cultivate the following skills in students:
1. Encourages self-exploration and boosts self-awareness, helping students tap into their inner potential.
2. Instills moral values and nurtures a love for learning, fostering all-round development.
3. Equips students with technological knowledge, empowering them to utilize technology for the betterment of society.
4. Cultivates the ability to showcase talents and skills, promoting individuality and creativity.
5. Emphasizes physical fitness, health, and integrated learning of arts, fostering a holistic approach to education.
Frequently Asked Questions
Q 1: Is 2023 syllabus reduced?
The upcoming board exams for 2023 will be split into two sections, and they will adhere to a reduced syllabus. Below, we have included links to download the revised and rationalized syllabus for all subjects in CBSE Class 10 in PDF format.
Q 2: What is the CBSE Syllabus?
The CBSE Syllabus is a comprehensive outline of the subjects and topics covered in the academic year for Class 10 students. It provides a structured plan for the curriculum, including details about subjects, chapters, and marks distribution.
Q 3: Where can I find the CBSE Class 10 Syllabus?
The CBSE Syllabus is available on the official website of the Central Board of Secondary Education (CBSE). It can also be accessed through various educational portals and school websites.
Q 4: What are the compulsory subjects in Class 10?
The compulsory subjects for Class 10 typically include Mathematics, Science, Social Science, English, and a second language, which could be Hindi, Sanskrit, or another regional language.
Q 5: Can students choose optional subjects in Class 10?
Yes, in addition to the compulsory subjects, Class 10 students may have the option to choose two additional elective subjects. These subjects can vary depending on the school or state board.
NCERT Maths Class 10 Book: Free pdf Download
NCERT Maths Class 10 Book: If you are a Class 10 student under the CBSE Board seeking the best study materials for Maths, look no further than NCERT Textbooks. These books are considered the top choice for their comprehensive content and straightforward approach to understanding concepts.
NCERT Class 10 Maths books are highly recommended for CBSE students as they cover the entire syllabus. They are expertly designed by subject specialists to provide accurate and reliable information for all subjects. At BYJU’S, you can access the free PDF of NCERT Maths Book Class 10, making it easily accessible for everyone.

Preparing from these books will not only help you excel in your board exams but also in competitive exams. The questions in these textbooks are thoughtfully curated to establish a strong foundation for all the covered concepts and topics. The language used in NCERT Class 10 Books for Maths is designed to be understandable and accessible to students of all levels.
For mastering Mathematics, students can rely on NCERT Books for Class 10 Maths, which offer a wide range of illustrative problems and their solutions. Hence, it is highly recommended that all students choose these textbooks for their studies. Additionally, practicing NCERT solutions, sample papers, and previous years’ question papers can significantly enhance problem-solving skills.
Whenever you encounter difficulties in solving Maths problems, the NCERT Books for Class 10 Maths can be your go-to resource. Moreover, revisiting the fundamentals from the NCERT Books of earlier classes is also beneficial for a better understanding of concepts. Embracing these resources will surely pave the way for your academic success.
NCERT Maths Class 10 Book: Free pdf Download
Maths Book in English – Chapter Wise pdf
- Chapter 1 – Real Numbers
- Chapter 2 – Polynomials
- Chapter 3 – Pair of Linear Equations in Two Variables
- Chapter 4 – Quadratic Equations
- Chapter 5 – Arithmetic Progressions
- Chapter 6 – Triangles
- Chapter 7 – Coordinate Geometry
- Chapter 8 – Introduction to Trigonometry
- Chapter 9 – Some Applications of Trigonometry
- Chapter 10 – Circles
- Chapter 11 – Areas Related to Circle
- Chapter 12 – Surface Areas and Volumes
- Chapter 13 – Statistics
- Chapter 14 – Probability
Maths Book in Hindi – Chapter Wise pdf
- अध्याय 1. वास्तविक संख्या
- अध्याय 2. बहुपद
- अध्याय 3. दो चर वाले रैखिक समीकरण युग्म
- अध्याय 4. द्विघात समीकरण
- अध्याय 5. समांतर श्रेढ़िया
- अध्याय 6. त्रिभुज
- अध्याय 7. निर्देशांक ज्यामिति
- अध्याय 8. त्रिकोणमिति का परिचय
- अध्याय 9. त्रिकोणमिति वेफ वुफछ अनुप्रयोग
- अध्याय 10. वृत्त
- अध्याय 11. वृत्तों से संबंध्ति क्षेत्रापफल
- अध्याय 12. पृष्ठीय क्षेत्रापफल और आयतन
- अध्याय 13. सांख्यिकी
- अध्याय 14. प्रायिकता
Why One Should Read NCERT Class 10 Maths Book?
The provided link offers chapter-wise NCERT Maths Books in PDF format, aligning with the NCERT’s objective of aiding students in their board exam preparation. By referring to these NCERT Books for Class 10, students can easily clarify any queries they may have. Utilizing these books is advantageous in achieving high scores in the upcoming board examination, as they simplify complex topics and formulas while addressing any doubts.
These books serve various purposes for students:
1. They provide a comprehensive understanding of the topics covered in Class 10 Maths.
2. During revision time, these books become valuable study resources.
3. Students can assess their knowledge gaps and adjust their preparation accordingly.
4. They enable students to deepen their understanding of key topics and concepts.
5. Homework and assignments are based on the content presented in these books.
In summary, the availability of chapter-wise NCERT Maths Books in PDF format ensures effective board exam preparation and enhances students’ learning experience, supporting them in achieving academic excellence.
Frequently Asked Questions
Q 1: What is NCERT, and why are NCERT Maths books important?
NCERT (National Council of Educational Research and Training) is an autonomous organization in India responsible for formulating and publishing educational resources, including textbooks. NCERT Maths books hold immense significance as they offer a well-structured curriculum, easily comprehensible explanations, and comprehensive coverage of mathematical concepts.
Q 2: Are NCERT Maths books enough to prepare for board exams?
Yes, NCERT Maths books are highly recommended for board exam preparation. They cover the entire syllabus prescribed by the respective education boards and serve as a solid foundation for understanding concepts.
Q 3: Are NCERT Maths books suitable for competitive exams?
NCERT Maths books provide a strong conceptual understanding of mathematics, which is beneficial for competitive exams as well. Nevertheless, certain competitive exams might necessitate supplementary reference books and practice materials.
Q 4: Are there separate NCERT Maths books for different classes?
Yes, NCERT Maths books are available for different classes, from Class 1 to Class 12. Every book is carefully crafted to align with the particular curriculum and mathematical intricacy suitable for its respective grade.
Q 5: Are there any supplementary materials available along with NCERT Maths books?
NCERT often provides supplementary materials, such as exemplar problems, solutions, and question banks, which can help students with additional practice and a deeper understanding of the subject.
Q 6: Are NCERT Maths books available in different languages?
Yes, NCERT Maths books are available in various regional languages, in addition to English and Hindi, to cater to students from different linguistic backgrounds.
Q 7: Can I download NCERT Maths books online?
Yes, NCERT Maths books are available for free download on the official NCERT website. Additionally, some educational platforms may also offer downloadable versions of these books.
Q 8: Are there any video lectures or online resources available for NCERT Maths books?
Yes, several online platforms provide video lectures and supplementary resources that complement the content of NCERT Maths books, making learning more interactive and engaging.
Q 9: How often are NCERT Maths books updated?
NCERT periodically reviews and updates its textbooks to incorporate any necessary changes in the curriculum or to improve the content based on feedback from educators and students.
Q 10: Are NCERT Maths books applicable to all education boards in India?
NCERT Maths books are primarily designed for the CBSE (Central Board of Secondary Education) curriculum. However, many state boards in India also use NCERT textbooks, and the concepts covered in these books are generally applicable across different boards.
Physics for 10th Class Students: Syllabus of NCERT Science
Below is the comprehensive list of chapters and subtopics from the Physics for 10th Class NCERT textbook. By clicking on the links provided, students can explore each subtopic and gain a thorough understanding of all the concepts covered in the subject.
Please note that the list has been curated in accordance with the latest CBSE syllabus. The content presented in each sub-topic has been meticulously crafted by expert physics teachers to facilitate in-depth learning. Feel free to click on the links below to delve into the details of all Class 10 Physics concepts.
Physics for 10th Class Chapters in NCERT Science
In class 10, following are the physics chapter in NCERT science book.
Light-Reflection and Refraction: CHAPTER 10
Following topics are covered in class 10 chapter 10:
- Reflection Of Light
- Spherical Mirrors
- Refraction Of Light
The Human Eye And The Colorful World: Chapter 11
The topics described in chapter 11 are:
- The Human Eye
- Defects of Vision And Their Correction
- Refraction of Light Through A Prism
- Dispersion Of White Light By a Glass Prism
- Atmospheric Refraction
- Scattering of Light
Electricity: Chapter 12
Chapter includes:
- Electric Current and Circuit
- Electric Potential and Potential Difference
- Circuit Diagram
- Ohm’s Law
- Factors on which the Resistance of a Conductor depends
- Resistance Of a System of Resistors
- Heating Effect of Electric Current
- Electric Power
Magnetic Effects Of Electric Current: Chapter 13
In this chapter, students will learn about
- Magnetic Field and Field Lines
- Magnetic Field due to a Current-Carrying Conductor
- Force on a Current-Carrying Conductor in a Magnetic Field
- Electric Motor
- Electromagnetic Induction
- Electric Generator
- Domestic Electric Circuits
Sources Of Energy: Chapter 14
Topics covered in this chapter are:
- What is a good source of energy?
- Conventional Sources of Energy
- Alternative or Non-Conventional Sources of Energy
- Environmental Consequences
- How Long will an Energy Source last us?
Management of Natural Resources: Chapter 16
Chapter have following topics:
- Why Do We Need To Manage Our Resources?
- Forests and WildFire
- Water For All
- Coal and Petroleum
Frequently Asked Questions:
Q 1: How can I study 10th Physics?
Maximize your practice of theorems, formulas, and numerical problems to strengthen your understanding. Pay particular attention to ray diagrams in the light chapter. Solving previous year papers or sample papers is also essential as it is both rewarding and conducive to effective learning with ease.
Q 2: What are the topics in Physics class 10?
- Chapter1: Light – Reflection and Refraction.
- Chapter2: The Human Eye and the Colourful World.
- Chapter3: Electricity.
- Chapter4: Magnetic Effects of Electric Current.
- Chapter5: Sources of Energy.
Q 3: What are main points of physics?
In the realm of physics, the interactions among particles and physical entities like planets, molecules, atoms, or subatomic particles are governed by the fundamental laws of matter, energy, and the forces of nature. These laws dictate the underlying principles that shape the behavior and dynamics of the universe.
Q 4: Which topics are hard in physics?
Heat and Thermodynamics.
Mechanics.
Electrostatics and Magnetism.
Current Electricity.
Optics.
Modern Physics.
Electromagnetic Induction.
Q 5: Which part of physics is easy?
Kinematics, as a branch of physics, focuses on the motion of objects without delving into the forces responsible for that motion. This chapter proves relatively straightforward as it revolves around fundamental concepts such as distance, displacement, speed, and velocity. Understanding kinematics becomes accessible through the aid of diagrams and graphs, which facilitate visualization and comprehension of the subject matter.
Read Also:
- Chemical Reactions and Equations
- Acids, Bases and Salts
- Life Processes
- Control and Coordination
- How Do Organisms Reproduce?
- Heredity and Evolution
- Magnetic Effects of Electric Current
- Our Environment
- 10th Class Physics Textbook English Medium pdf Download
Life Process Notes Class 10 NCERT Science Chapter 5
Life Process Notes Class 10: All living organisms share fundamental characteristics that set them apart from non-living entities. These traits include breathing, growth, the need for nutrition, reproduction, and responsiveness to stimuli. These collective attributes distinguish living beings from the inanimate world.
To maintain a stable internal environment and ensure proper functioning, living organisms undergo vital processes known as life processes. These essential activities persist even during periods of rest or inactivity. Life processes, which encompass nutrition, photosynthesis (for plants), transportation, metabolism, respiration, reproduction, and excretion, are indispensable for all living organisms, spanning both the plant and animal kingdoms.
Throughout the Life Process Notes Class 10, we will delve into the intricacies of these life processes, with a particular focus on how they manifest in plants, animals, and human beings.
Life Process Notes Class 10 NCERT Science Chapter 5
What is Life?
As far as our current knowledge extends, Earth stands as the sole known planet to harbor life. Within this remarkable biosphere, beings are born, live, pass away, and eventually integrate back into nature.
The distinction between living organisms and inanimate entities is discernible through a range of criteria, primarily rooted in the various life processes they undergo.
Life Process
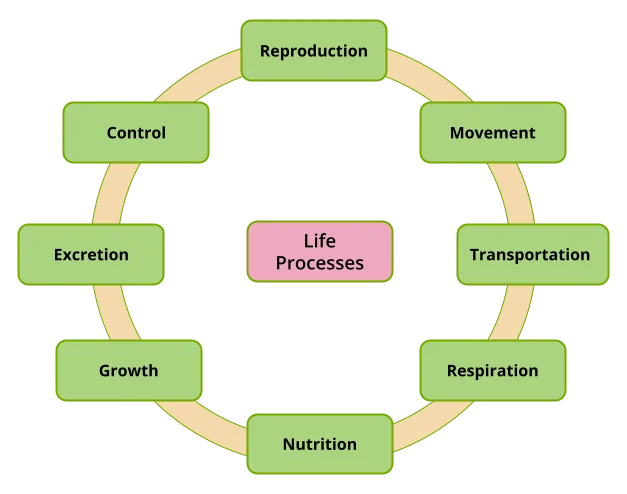
Life Process Notes Class 10 – Image 1
The sustenance of living organisms remains crucial irrespective of whether they are in motion, at rest, or even asleep. The combined processes responsible for maintaining life are known as life processes. Nutrition, respiration, circulation, and excretion are among the fundamental life processes that play a vital role.
In unicellular organisms, a single cell is responsible for performing all these essential processes. On the other hand, multicellular organisms possess well-developed systems specifically designed to carry out these crucial life processes efficiently.
Nutrition: Life Process Notes Class 10
Nutrition refers to the process by which an organism obtains the essential nutrients required for nourishment and sustenance. There are two primary modes of nutrition: autotrophic and heterotrophic.
Autotrophic nutrition is found in plants, algae, and certain bacteria. These organisms synthesize their own food using light energy (photosynthesis) or chemical energy (chemosynthesis).
Heterotrophic nutrition is observed in bacteria, fungi, and animals. They acquire energy from organic compounds by consuming plants or other animals as food.
Heterotrophic nutrition further encompasses subtypes such as holozoic (ingesting solid food), saprophytic (feeding on decaying matter), and parasitic (feeding on a host organism).
Autotrophic Nutrition
When an organism can sustain itself by producing its own food using sunlight or chemicals, this mode of nutrition is referred to as autotrophic nutrition.
Plants exemplify photoautotrophs as they photosynthesize, harnessing light energy to synthesize their food.
On the other hand, some bacteria are chemoautotrophs, utilizing chemical substances to obtain energy for their nourishment.
Photosynthesis
Photosynthesis plays a crucial role in the formation of food. Plants utilize sunlight and water to create nourishment not only for themselves but also for other organisms.
Within the green parts of plants, chlorophyll absorbs light energy. This energy is employed to split water into hydrogen and oxygen. Subsequently, the hydrogen is utilized to reduce carbon dioxide, converting it into carbohydrates, primarily glucose.
Chlorophyll serves as a vital component of photosynthesis, while stomata facilitate the intake of carbon dioxide from the surroundings.
The overall chemical reaction for photosynthesis can be represented as follows:
6CO2 + 6H2O → C6H12O6 + 6O2
Stomata
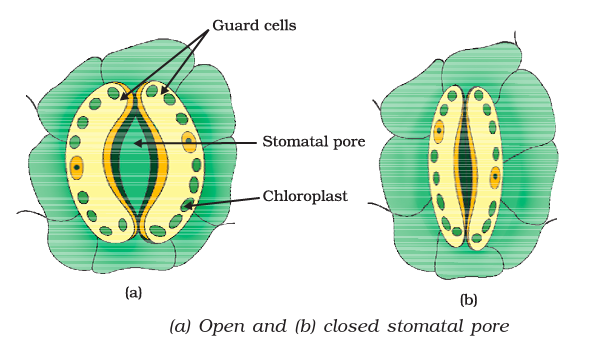
Life Process Notes Class 10 – Image 2
Stomata are small pores present on the leaves that play a vital role in gas exchange.
Primarily located on the underside of the leaf, these pores allow the entry and exit of gases.
Each stoma is flanked by guard cells, responsible for regulating the opening and closing of the pore.
The functioning of the guard cells is influenced by their water content, determining the extent of gas exchange through the stomata.
Saprophytic Nutrition
Saprophytic nutrition refers to the mode of nutrition in which certain organisms feed on dead and decaying organic matter.
Parasitic Nutrition
The parasitic mode of nutrition involves certain organisms feeding at the expense of another organism, causing harm in the process.
These parasites thrive either on or inside the body of a host organism, obtaining their nutrients directly from the host’s body.
For instance, leeches are examples of ectoparasites, while Ascaris represents an endoparasite. Additionally, there are parasitic plants like Cuscuta that exhibit this mode of nutrition.
Nutrition in Amoeba
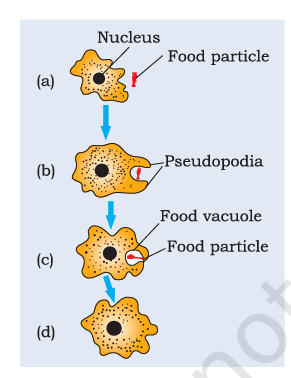
Life Process Notes Class 10 – Image 3
Amoeba adopts the Holozoic mode of nutrition for feeding. Using pseudopodia, it engulfs food particles through a process called phagocytosis. The engulfed food becomes enclosed within a food vacuole.
As the food vacuole moves through the cytoplasm, digestion, absorption, and assimilation occur.
Once the food vacuole opens outside, the undigested food is expelled through egestion.
Nutrition in Paramoecium
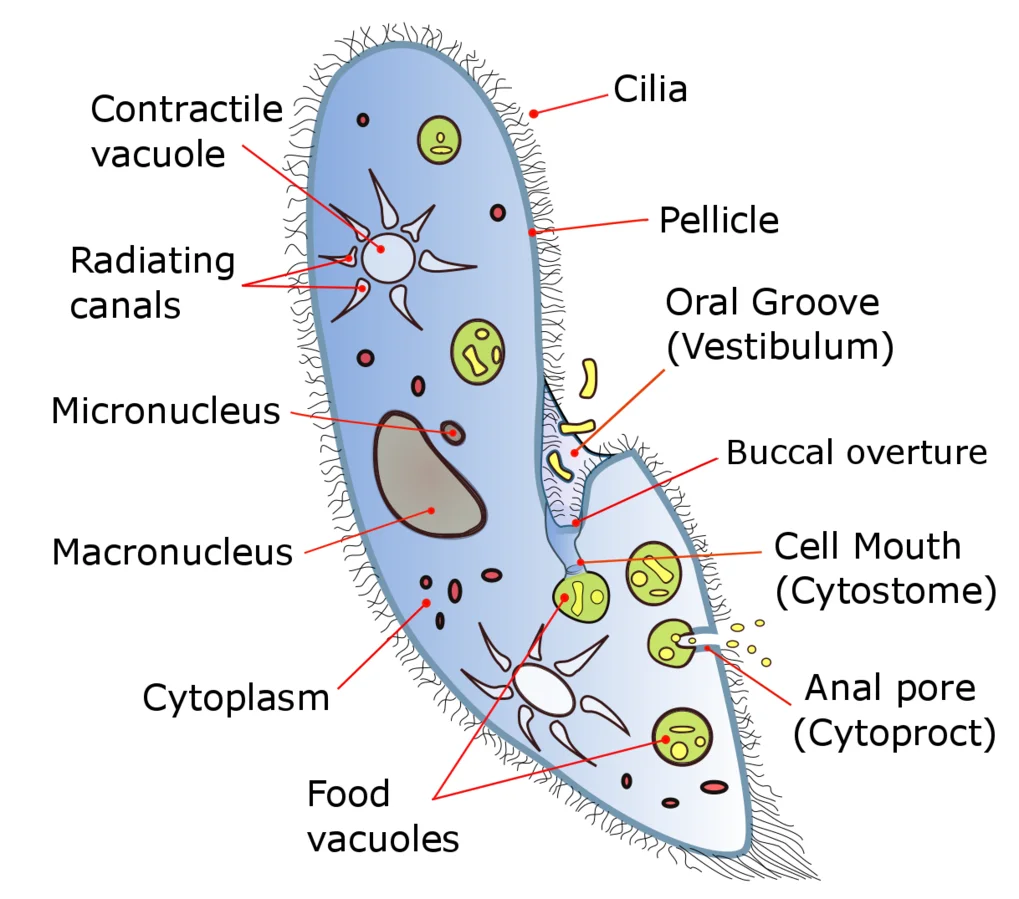
Life Process Notes Class 10 – Image 4
- Paramoecium, like Amoeba, demonstrates holozoic nutrition.
- The presence of cilia aids Paramoecium in engulfing food through the oral groove.
- Upon engulfment, a food vacuole forms, enclosing the ingested food.
- The food vacuole undergoes movement through the cytoplasm in a process known as cyclosis.
- Within the food vacuole, digestion occurs, and the nutrients are absorbed by the cytoplasm.
- Any undigested food is eliminated through a small pore called an anal pore or cytopyge.
Nutrition in Humans: Life Process Notes Class 10
- Humans are classified as omnivores, capable of consuming both plant-based and animal-based foods.
- Due to their higher complexity, humans possess a sophisticated nutrition system.
- The digestive system comprises an alimentary canal and associated digestive glands, working in unison to provide nourishment to the body.
- Human nutrition consists of five stages: Ingestion, Digestion, Absorption, Assimilation, and Egestion.
- Among these stages, ingestion, digestion, absorption, and egestion occur within the alimentary canal, while assimilation takes place throughout the entire body.
Alimentary Canal
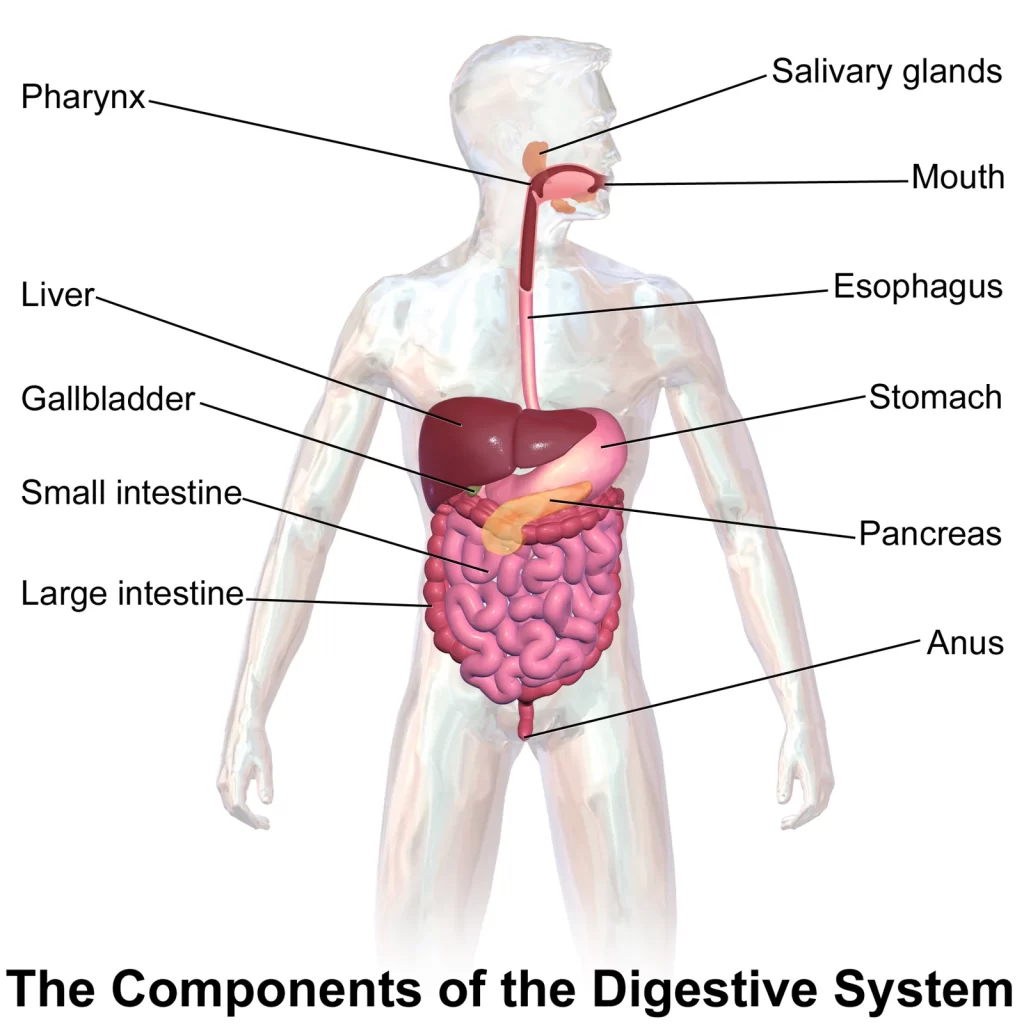
Life Process Notes Class 10 – Image 5
- The human alimentary canal is an elongated tube with varying diameters.
- It begins at the mouth and extends to the anus.
- The parts of the alimentary canal consist of the esophagus, stomach, small intestine, and large intestine.
Mouth
- The mouth serves as the entrance to the alimentary canal, facilitating the ingestion of food.
- Located behind the mouth, the buccal cavity is often referred to as the mouth itself.
- Inside the buccal cavity, teeth aid in the mastication or chewing of food.
- The tongue, equipped with taste buds, contributes to the sensation of taste while eating.
- Additionally, salivary glands open into the buccal cavity, releasing saliva that initiates the process of digestion.
Teeth

Life Process Notes Class 10 – Image 6
- Teeth are rigid structures found in the buccal cavity, serving to cut, shear, and masticate the food we consume.
- A vertical section of a tooth reveals four distinct layers: enamel, dentine, cement, and dental pulp.
- Enamel, the outermost layer, showcases a glossy appearance, high mineral content, and stands as the hardest substance in the human body.
- Dentine forms the bulk of the tooth and comprises approximately 70% inorganic salts.
Cement lines the tooth and the bony socket it rests in. - The dental pulp, residing at the tooth’s core, contains nerve endings, blood vessels, lymph vessels, and connective tissue.
- Humans possess four types of teeth: incisors, canines, molars, and premolars, each fulfilling specific functions.
- Incisors are adept at cutting food, canines excel at tearing it apart, while molars and premolars skillfully crush and grind it.
- In adult humans, the dental formula represents the number of each type of tooth on one-half of the mouth, and it is expressed as 2:1:2:3.
Life Process Notes Class 10
Oesophagus
- Once swallowed, the food proceeds into the esophagus, a muscular tube approximately 25 cm in length, equipped with a sphincter (valve or opening) at each end.
- The primary role of the esophagus is to facilitate the movement of food and fluids, from the mouth to the stomach, after they have been swallowed.
- Peristaltic movements within the esophagus push the food downward, aiding in its smooth transportation.
Stomach
- The stomach is a robust, sack-shaped organ, receiving food from the esophagus at one end and connecting to the small intestine at the other end.
- The inner lining of the stomach produces mucous, hydrochloric acid, and digestive juices.
- Within the stomach, food is mixed and churned, forming a semi-solid mass called chyme.
- Enzymes present in gastric juice actively break down the food particles.
- Hydrochloric acid serves two purposes: aiding in the partial digestion of proteins and neutralizing harmful bacteria.
- To protect its own wall from the action of hydrochloric acid, the stomach secretes a protective layer of mucus.
Small Intestine
- The small intestine, the lengthiest segment of the alimentary canal, measures approximately 20 feet in humans.
- It comprises distinct regions: the duodenum, which follows the stomach; the jejunum, located in the middle part; and the ileum, which extends further into the large intestine.
- The internal lining of the small intestine is structured into finger-like projections known as villi.
- In the duodenum, a common pancreatic duct from both the pancreas and liver opens.
- The majority of chemical digestion and absorption of nutrients occur within the small intestine.
Large Intestine
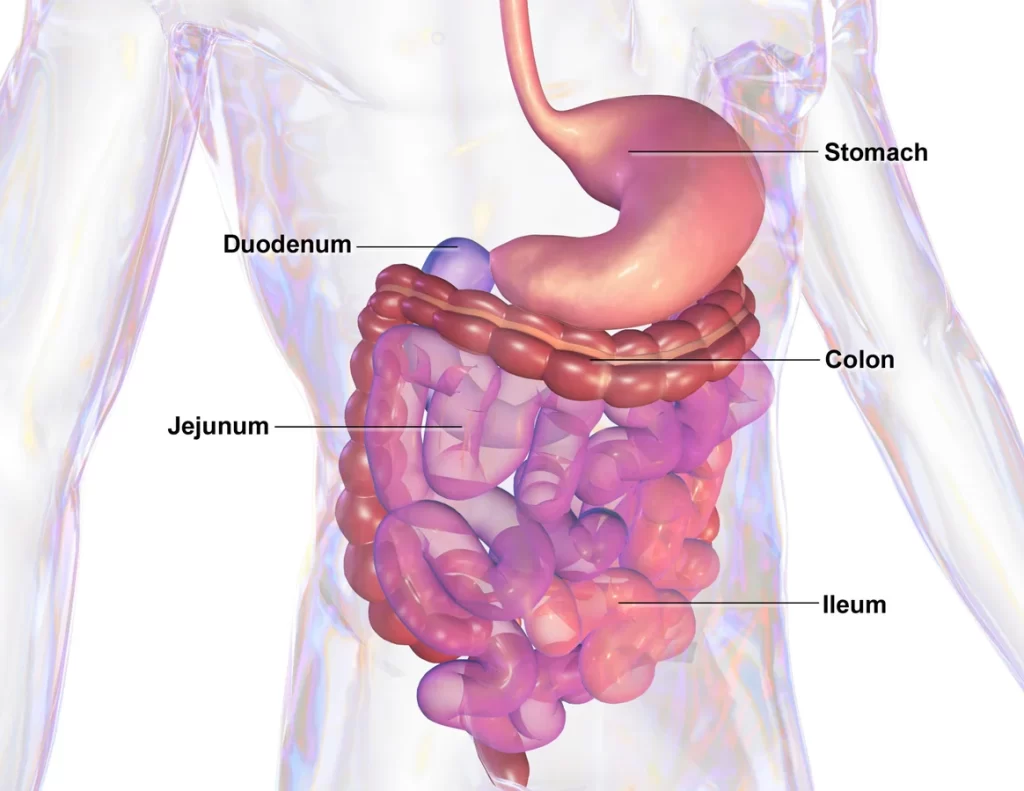
- The human large intestine spans approximately 5 feet in length.
- It consists of two main regions: the colon, which is about 1.5 meters long, and the rectum, measuring around 10 centimeters in adults.
- Following the ileum, the large intestine continues as the colon, which can be further divided into three segments: the ascending colon, transverse colon, and descending colon.
- At the base of the ascending colon, a small finger-like projection called the appendix can be observed. This structure houses beneficial bacteria necessary for food digestion.
- The rectum serves as the terminal part of the large intestine and opens externally through the anus.
- Within the anus, there are internal and external anal sphincters, responsible for controlling bowel movements.
Peristalsis
Peristalsis refers to a continuous, wave-like motion that occurs throughout the alimentary canal, starting from the esophagus and extending to the small intestine.
The muscles found in the alimentary canal’s wall are responsible for generating peristalsis.
This coordinated movement aids in propelling food along the entire length of the alimentary canal.
Digestive Glands
- Numerous glands contribute digestive juices essential for food digestion.
- Among them are the salivary glands, gastric glands, liver, gallbladder, and pancreas.
- The salivary glands produce saliva, which initiates the digestion process in the mouth.
- Gastric glands, located in the stomach wall, secrete hydrochloric acid and the enzyme pepsin.
- The liver secretes bile, stored in the gallbladder, to aid in the digestion of fats.
- The pancreas releases a variety of digestive enzymes, collectively known as pancreatic juice.
- Pancreatic juice contains enzymes such as trypsin, chymotrypsin, lipase, and amylase, crucial for effective food digestion.
Pancreas
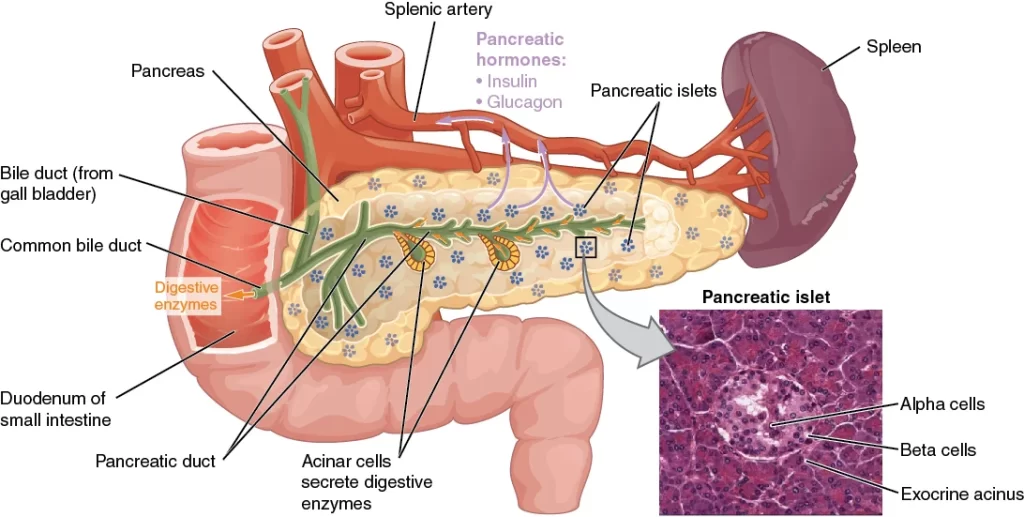
- The pancreas, located behind the stomach in humans, is a lengthy and flat gland.
- This gland plays a significant role as both an endocrine and exocrine organ.
- In its capacity as an endocrine organ, the pancreas releases two hormones, insulin and glucagon, which regulate blood sugar levels.
- As an exocrine gland, the pancreas secretes pancreatic juice, consisting of a combination of various digestive enzymes.
- These digestive enzymes include trypsin and chymotrypsin, which are proteases responsible for breaking down proteins.
- Additionally, the pancreas secretes amylase, which aids in digesting the starch content of food.
- Another essential component of pancreatic enzymes is pancreatic lipases, vital for the digestion of fats.
Holozoic Nutrition
Holozoic nutrition refers to the mode of nutrition in which animals consume their food in its entirety.
In holozoic nutrition, the process involves five distinct steps: ingestion, digestion, absorption, assimilation, and egestion.
Physiology of Digestion
- Mechanical digestion initiates in the buccal cavity, where teeth chew and grind the food, while saliva mixes with it, forming a bolus.
- The digestion of starch commences in the buccal cavity, facilitated by salivary amylase, which converts starch into maltose.
- In the stomach, food is churned through the muscular contraction and relaxation of its wall, breaking it down into simpler substances.
- The digestion of proteins begins in the stomach with the action of pepsin, which breaks proteins into smaller fragments known as peptides.
- The bolus, after mixing with gastric juice, transforms into a fine, soluble form called chyme.
- Upon entering the small intestine, complete digestion occurs, facilitated by various enzymes present in the pancreatic juice, bile, and intestinal juice.
- The digested food is thoroughly absorbed by the villi and microvilli of the small intestine.
- Subsequently, undigested food enters the large intestine.
- In the large intestine, the colon facilitates the absorption of water and salts, while the rectum temporarily stores the undigested food before defecation.
Digestive System in Other Animals
- The structure and function of digestive systems differ across various animal species.
- The design of the digestive system is influenced by the animal’s food habits.
- Herbivores have a lengthy alimentary canal since their plant-based diet contains cellulose, which takes a considerable time to digest.
- Conversely, carnivorous animals possess a relatively shorter alimentary canal as meat is digested more rapidly.
Anatomy of Digestive Tract
- The human alimentary canal, also known as the gastrointestinal tract, stretches approximately 30 feet (9 meters) in length.
- Starting from the mouth, it extends all the way to the anus, serving as a tube with varying diameters.
- The alimentary canal includes several distinct parts: the esophagus, stomach, and small intestine (comprising the duodenum, jejunum, and ileum), as well as the large intestine (consisting of the colon and rectum).
- For the digestion process, salivary glands, pancreas, and liver play vital roles as major digestive glands.
- Additionally, glands present in the walls of the stomach and small intestine also contribute significantly to the digestion of food.
Role of HCl
- The gastric glands present in the stomach wall are responsible for secreting hydrochloric acid.
- The pH of gastric acid typically ranges between 1.5 to 3.5.
- The hydrochloric acid fulfills several important functions:
- It converts inactive pepsinogen and pro-rennin into their active forms, pepsin and rennin, respectively.
- The acidic environment created by the gastric acid facilitates the digestion of proteins.
- It acts as a defense mechanism by killing bacteria that may have entered the body through food, preventing infections.
- By creating an acidic environment, it hinders the putrefaction of food in the stomach.
- To protect itself from the corrosive action of gastric acid, the stomach secretes a thick layer of mucus through its mucous glands.
- Excessive amounts of acid can lead to damage to the gastric mucosa and result in gastric and duodenal ulcers.
Salivary Glands
- Salivary glands, as exocrine glands, secrete saliva, which is then delivered to the mouth through a system of ducts.
- In humans, there are three major pairs of salivary glands: parotid, submandibular, and sublingual.
- Typically, healthy individuals produce around 0.5 to 1.5 liters of saliva per day.
- Saliva serves multiple important functions in the oral cavity:
- It lubricates and safeguards the soft and hard tissues of the oral cavity.
- Saliva provides protection against dental caries.
- It inhibits microbial growth in the oral cavity.
- Saliva promotes soft tissue repair by reducing clotting time and increasing wound contraction.
- Containing the enzyme amylase, saliva initiates the hydrolysis of starch into maltose and dextrin, enabling digestion to begin before food reaches the stomach.
- Saliva acts as a solvent, allowing solid particles to dissolve and interact with the taste buds on the tongue.
Heterotrophic Nutrition
- Heterotrophic mode of nutrition refers to a nutritional strategy in which an organism relies on others for food.
- These organisms depend on autotrophs to fulfill their nutritional needs.
- For instance, animals that consume plants as their food are known as herbivores, while those that feed on other animals are called carnivores.
- Holozoic, saprophytic, and parasitic nutrition are all different types of heterotrophic nutrition.
Glandular Epithelium
- Numerous small glands play a vital role in the digestion of food, primarily located within the inner layer of the stomach and intestine.
- These glands are situated in the epithelial lining of both the stomach and intestine.
- The glands found in different regions of the stomach are known as gastric glands. Their responsibilities include the secretion of mucus, hydrochloric acid, and enzymes like pepsinogen.
- Within the epithelial lining of the small intestine and large intestine, we find the intestinal glands.
- The glands in the small intestine secrete intestinal juice, also referred to as succus entericus. This juice contains hormones, digestive enzymes, alkaline mucus, and substances that neutralize hydrochloric acid coming from the stomach.
- Intestinal juice effectively completes the digestion initiated by pancreatic juice.
- As for the glands in the large intestine, their function involves facilitating the absorption of water and electrolytes.
Villi and Micro Villi
- The small intestine is responsible for the complete digestion and absorption of food.
- The digestion process is completed with the help of pancreatic juice from the pancreas, bile from the liver, and intestinal juice secreted by the intestinal glands.
- To absorb all the digested nutrients efficiently, the small intestine is lined with long finger-like projections known as villi (singular: villus).
- Each villus has its cell membrane on the lumen side, which is further folded into microscopic processes called microvilli, significantly increasing the internal surface area of the intestinal walls for absorption.
- Digested nutrients pass through diffusion into the semipermeable villi.
- Moreover, villi actively participate in the chemical digestion of food by secreting digestive enzymes.
Liver
- The liver serves as the largest and principal digestive gland in humans.
- Positioned in the upper right-hand portion of the abdomen, this organ exhibits a dark reddish-brown color due to its rich blood supply.
- The liver fulfills several significant functions, including:
- Secreting bile, which aids in the digestion process.
- Filtering the blood from the digestive tract before distributing it to the rest of the body.
- Detoxifying various metabolites and acting as an antidote.
- Producing essential proteins for blood clotting and other bodily functions.
- Storing and releasing glucose as required.
- Processing hemoglobin from dead and worn-out red blood cells, specifically for storing iron.
- Facilitating the conversion of harmful ammonia into urea, a less toxic substance, in the liver.
Digestive Juices
- Collectively known as digestive juices, pancreatic juice, bile, and intestinal juice (succus entericus) play essential roles in the digestion process.
- A common duct connects the digestive glands and pours their secretions into the duodenum.
- Upon entering the small intestine, chyme undergoes complete digestion, facilitated by various enzymes.
- In the duodenum, the acidity of chyme is converted to alkalinity by the action of bile from the liver, which is crucial for the activity of pancreatic enzymes.
- Bile also emulsifies fats into smaller globules, aiding in their digestion.
- Pancreatic and intestinal amylases work together to break down carbohydrates into glucose.
- For the breakdown of proteins into amino acids, trypsin and chymotrypsin serve as the proteases responsible.
- Finally, lipase, an essential enzyme, acts on the emulsified fats, breaking them down into glycerol and fatty acids.
Water Absorption in Large Intestine
- The large intestine does not partake in the digestion of food or the absorption of nutrients.
- Its primary role revolves around absorbing water from the remaining indigestible food material, thereby solidifying the stool.
- Additionally, the large intestine aids in the absorption of vitamins produced by bacteria that inhabit this region.
- Moreover, the innermost layer of the large intestine acts as a protective barrier, safeguarding against microbial infections and invasions.
- The rectum temporarily stores undigested food until it is eventually expelled during the process of defecation.
Respiration
Introduction to Respiration
- Respiration broadly means the exchange of gases.
- Animals and plants have different means of exchange of gases.
- At a cellular level, respiration means the burning of food to generate the energy needed for other life processes.
- Cellular respiration may take place in the presence or absence of oxygen.
Respiration in Humans
- The human respiratory system is a sophisticated system responsible for vital processes such as breathing, gas exchange, and cellular respiration.
- A well-organized respiratory system facilitates the smooth process of breathing and gas exchange.
- Breathing involves the inhalation of oxygen and the exhalation of carbon dioxide, a crucial exchange that sustains life.
- This gaseous exchange occurs within the lungs, ensuring that oxygen is distributed to all cells throughout the body.
- Cellular respiration, a fundamental process, takes place in every cell, providing the energy needed for various cellular activities.
Respiratory System
- The human respiratory system comprises various essential components, including the nose, nasal cavities, pharynx, larynx, trachea (windpipe), bronchi, bronchioles, and alveoli.
- A pair of lungs encloses the bronchioles and alveoli.
- To facilitate the inhalation and exhalation of gases, the rib cage, muscles associated with it, and the diaphragm all play vital roles.
- The exchange of gases transpires between the alveolar surface and the surrounding blood vessels.
- Alveoli, with their extensive surface area, serve as crucial sites for the efficient exchange of gases.
Physiology of Respiration
- Breathing in humans is facilitated by the coordinated action of internal intercostal and external intercostal muscles, which attach to the ribs, and the diaphragm.
- During inhalation, the dome-shaped diaphragm contracts, flattening out, and the rib cage expands due to the intercostal muscles’ action. This process increases the lungs’ volume, causing a drop in pressure, and allows air to rush in from the outside.
- To exhale, the diaphragm relaxes and returns to its dome shape, while the chest cavity contracts due to the intercostal muscles’ action. This reduces the volume inside the lungs, increases pressure, and forces air out.
- Inhaled air enriches the oxygen concentration in the alveoli, allowing oxygen to simply diffuse into the surrounding blood vessels.
- Conversely, blood coming from cells carries a higher concentration of carbon dioxide than the outside air, leading carbon dioxide to diffuse out of the blood vessels into the alveoli.
- Therefore, breathing occurs due to the combined action of intercostal muscles and the diaphragm, while the exchange of gases takes place through the simple process of diffusion.
Inhalation and Exhalation
- Inhalation refers to the process of taking in air rich in oxygen, while exhalation involves giving out air rich in carbon dioxide.
- A single breath encompasses both inhalation and exhalation.
- Throughout the day, a person breathes numerous times.
- The frequency of breathing, measured as the number of breaths in one minute, is known as the individual’s breathing rate.
Diffusion
Diffusion is the natural movement of molecules from areas of high concentration to areas of low concentration, occurring spontaneously without the expenditure of any energy.
Cellular Respiration
Cellular respiration is a series of metabolic reactions that occur within cells, converting the biochemical energy obtained from food into a chemical compound known as adenosine triphosphate (ATP).
Metabolism encompasses a range of chemical reactions carried out to sustain the cells’ living state in an organism. These reactions can be categorized into two groups:
1. Catabolism: This involves breaking down larger molecules to release energy.
2. Anabolism: It pertains to the synthesis of various compounds required by the cells.
Respiration is classified as a catabolic process as it breaks down large molecules into smaller ones, releasing energy to fuel cellular activities.
The key processes of cellular respiration include glycolysis, the Krebs cycle, and the electron transport chain.
Aerobic Respiration
Aerobic respiration is a metabolic process that converts glucose, a type of food, into energy in the presence of oxygen.
The overall equation for aerobic respiration is as follows:
Glucose + Oxygen ⇒ Carbon Dioxide + Water + Energy
This vital process occurs in animals, plants, and other living organisms.
Respiration in Lower Animals
- Lower animals do not possess a complex respiratory system like lungs and alveoli. Instead, their respiration occurs through simple exchange mechanisms.
- For instance, animals like earthworms take in gases directly through their skin.
- Fishes have gills that facilitate gaseous exchange in water.
- Insects utilize a tracheal system, which comprises a network of tubes that enable air circulation and gaseous exchange.
- As for frogs, they breathe through their skin while in water and use their lungs for respiration when on land.
Respiration in Muscles
- Muscle respiration can become anaerobic when there is an insufficient supply of oxygen.
- Under such conditions, glucose undergoes breakdown, resulting in the production of carbon dioxide and lactic acid.
- The accumulation of lactic acid leads to muscle soreness.
- This specific form of anaerobic respiration is referred to as lactic acid fermentation.
ATP
- ATP serves as the cell’s energy currency.
- ATP is an abbreviation for Adenosine Tri-Phosphate.
- This molecule is formed as a result of crucial reactions like photosynthesis and respiration.
- The three phosphate bonds in ATP are high-energy bonds, and their breaking releases a significant amount of energy.
- This released energy is subsequently utilized for various other metabolic reactions.
Respiration in Plants
- Plants lack specialized structures for gaseous exchange, unlike animals and humans.
- Instead, they rely on stomata (located in leaves) and lenticels (found in stems) to facilitate gas exchange.
- In comparison to animals, the rate of respiration in plant roots, stems, and leaves is significantly lower.
Transpiration
- Transpiration is a natural biological process whereby water is released in the form of water vapor from the aerial parts of plants.
- This phenomenon primarily takes place through stomata, where gas exchange (oxygen and carbon dioxide) also occurs.
- Transpiration plays a vital role in transporting water from the roots to the upper sections of plants, a phenomenon described by the ‘transpirational pull theory’.
- The loss of water, particularly from leaves, creates a straw-like effect that pulls water upwards from the roots.
- Additionally, transpiration serves as an excretory mechanism in plants, helping to eliminate excess water.
Why Do We Need Lungs?
Gas exchange in unicellular organisms like amoeba occurs through their general body surface via osmosis. In lower animals such as earthworms, gaseous exchange takes place through their moist skin. In these organisms, the oxygen requirement is sufficiently met through these methods.
However, as animals become more complex, like humans, the requirement for oxygen cannot be solely fulfilled by diffusion. Diffusion cannot effectively supply oxygen to the deep-seated cells within the body. This challenge has led to the evolution of a more intricate mechanism of gaseous exchange, which is the development of lungs.
Lungs have alveoli, providing a significantly large surface area that facilitates the necessary gas exchange process. The specialized structure of alveoli enables efficient exchange of gases, meeting the increased oxygen demands of complex organisms like humans.
Transportation in Human Beings
Transportation
Survival of all living organisms relies on essential components such as air, water, and food. Animals ensure their intake through regular processes like breathing, drinking, and eating. To distribute these necessary elements to body cells and tissues, organisms have a transportation system.
In plants, the vascular tissue plays a vital role in transporting substances. It facilitates the movement of water, nutrients, and other essential compounds throughout the plant’s body, ensuring its proper functioning and growth.
Transportation in Humans
The circulatory system is responsible for transportation in humans. It comprises blood, blood vessels, and the heart as its main components. This system plays a crucial role in supplying oxygen and nutrients to various parts of the body while removing carbon dioxide and other excretory products. Additionally, it aids in fighting infections and maintaining overall health.
Heart
The heart is a muscular organ located in the thoracic region, slightly towards the left side of the chest. It serves as the primary pumping organ of the body. The human heart is divided into four chambers that play a crucial role in the circulation of oxygenated and deoxygenated blood. The upper two chambers are known as atria, while the lower two chambers are referred to as ventricles.
Blood Vessels
Blood vessels are responsible for carrying blood throughout the body. There are three main types of blood vessels: arteries, veins, and blood capillaries. Arteries are vessels that carry oxygenated blood, while veins carry deoxygenated blood. The exchange of gases between the blood and body cells takes place at the capillaries.
Blood Pressure
Blood pressure refers to the force exerted by blood as it flows through the blood vessels.
There are two types of blood pressure: systolic and diastolic. Diastolic pressure is the pressure on the arterial walls when the heart is in its relaxation phase, representing the minimum pressure in the arteries. The normal range for diastolic blood pressure is 60 – 80 mm Hg.
On the other hand, systolic pressure is the pressure on the arterial walls when the heart is contracting and pumping blood, representing the maximum pressure in the arteries. The normal range for systolic blood pressure is 90 – 120 mm Hg.
Bleeding
Bleeding occurs when blood vessels rupture, but it is a natural response to injury. The body’s defense mechanism involves platelets, which aid in blood clotting at the site of injury. Blood clotting is a process that forms a gel-like mass to prevent excessive blood loss from the body. This clot is created by the combination of platelets and fiber-like proteins found in the blood.
Double Circulation
In the human body, blood undergoes two rounds of circulation through the heart. The first circulation is during pulmonary circulation, where blood flows between the heart and the lungs. The second circulation is during systemic circulation, where blood is pumped from the heart to the rest of the body and back. This dual circulation in human beings is referred to as double circulation.
Life Process Notes Class 10 – Transportation in Plants
Transportation in Plants
Transportation is a crucial process in plants, ensuring the distribution of water and essential nutrients to all parts of the plant for its survival. Plants have separate transportation systems for food and water. Xylem is responsible for transporting water, while phloem transports food to various parts of the plant.
Phloem
The phloem plays a vital role in transporting nutrients and sugars, such as carbohydrates, from the leaves to metabolically active areas of the plant. This tissue consists of sieve tubes, companion cells, phloem fibres, and phloem parenchyma cells. The flow of materials through the phloem is bidirectional, allowing efficient distribution and utilization of nutrients throughout the plant.
Translocation
Translocation is the process of food transport in plants through the phloem, facilitated by mass flow. It involves the movement of photosynthates, which are sugars and organic molecules like amino acids, organic acids, proteins, and inorganic solutes such as potassium, magnesium, nitrate, calcium, sulfur, and iron.
These substances are transported from source tissues, like mature leaves, to sink cells, which are areas of growth and storage.
During translocation, sucrose is loaded from leaves into the phloem using the energy of ATP. This transfer leads to an increase in osmotic pressure, causing water to move from nearby cells into the phloem tissue, aiding the transport of materials through the phloem.
This osmotically generated pressure difference also enables the transfer of substances from the phloem to tissues where they are needed. In this way, the bulk flow of materials through the phloem occurs, driven by the osmotic pressure difference.
Xylem
Xylem tissue plays a crucial role in transporting water from the roots to all other parts of the plant. It is composed of various cell types, including tracheids, vessels, xylem fibers, and xylem parenchyma.
The flow of water and minerals through the xylem is always unidirectional, moving from the roots upwards to the rest of the plant. This one-way flow ensures efficient distribution of water and nutrients throughout the plant, supporting its growth and survival.
Root Pressure
- The movement of water through the xylem, from the roots to the upper parts of plants, is the result of various forces working in concert.
- One of these crucial forces is known as root pressure, which arises from the osmotic pressure within the cells of the root system.
- This osmotic pressure propels the sap to ascend through the plant stem and reach the leaves.
- Root pressure plays a vital role in the initial transportation of water up the root.
Transport of Water
Water is absorbed by the roots and then transported through the xylem to reach the upper parts of the plant. This upward movement of water, even in the tallest plants, is made possible by the combined action of several forces.
Firstly, imbibition occurs, where water is absorbed by solids, as seen in seeds taking up water when soaked.
Secondly, osmosis plays a vital role. Water moves from areas of lower concentration to areas of higher concentration, and at the roots, cells actively take up ions, resulting in varying ion concentrations. This leads to the movement of water within the root cells through osmosis, forming a continuous water column that gets pushed upwards. This phenomenon is known as root pressure.
Thirdly, transpiration also contributes to the upward movement of water. It creates a straw-like effect, pulling the water column upwards as water is continuously lost from the leaves.
All these forces, imbibition, osmosis, root pressure, and transpiration, work together to ensure the efficient transport of water through the xylem to all parts of the plant.
Life Process Notes Class 10 – Excretion in Humans
Excretion
Excretion refers to the elimination of metabolic waste materials and other non-useful substances from an organism’s body.
While animals possess an advanced and specialized excretory system, plants, on the other hand, lack a well-developed excretory system similar to that of animals.
Plants do not have specific organs dedicated solely to excretion, resulting in a relatively simpler excretion process compared to animals.
Excretion in Unicellular Organisms
Unicellular organisms, such as amoeba and bacteria, eliminate waste products by a straightforward process of diffusion through their general body surface.
In specific unicellular organisms like amoeba and paramecium, the surplus waste is excreted through small structures called contractile vacuoles.
Moreover, undigested food in these unicellular animals is expelled when the food vacuole combines with the general body surface and opens to the external environment.
Excretory System of Humans
The human excretory system comprises the following components:
– Two kidneys
– Two ureters
– A urinary bladder
– A urethra
This system is responsible for producing urine as a waste product.
Kidneys
The main excretory organs in the body are the paired kidneys, acting as the essential filtration units. Each kidney consists of numerous tiny filtration units known as nephrons.
These kidneys play vital roles, including:
– Filtering waste materials, medications, and toxic substances from the blood.
– Regulating the fluid balance (osmolarity) of the body.
– Managing ion concentration within the body.
– Regulating pH levels.
– Controlling the volume of extracellular fluid.
– Secreting hormones that aid in red blood cell production, support bone health, and regulate blood pressure.
Nephron
The nephrons serve as the fundamental structural and functional units of the kidneys.
In each kidney, there are millions of nephrons, which collectively form the essential building blocks of kidney function. Each nephron consists of two main parts: the Malpighian body and the renal tubule.
The Malpighian body comprises a cup-like structure called Bowman’s capsule, which surrounds a cluster of capillaries known as the glomerulus. Together, they function as a filtration system, separating waste materials from many useful substances.
The renal tubule consists of three regions: the proximal convoluted tubule, the Loop of Henle, and the distal convoluted tubule. These regions are responsible for reabsorbing valuable substances back into the blood and filtering out the remaining waste substances.
The final product produced by the nephrons is called urine, which includes the waste materials removed from the body.
Haemodialysis
When the kidneys stop working properly, it causes many problems. To help with this situation, a special method called dialysis has been created. Dialysis involves the use of a machine filter called a dialyzer or artificial kidney.
Its purpose is to eliminate excess water and salt, balance other electrolytes in the body, and remove waste products resulting from metabolism. During dialysis, blood is withdrawn from the body and passes through a series of tubes containing a semipermeable membrane.
On the other side of the membrane, a fluid called dialysate flows, which draws impurities through the membrane, helping to cleanse the blood of toxins and restore balance to the body’s internal environment.
Excretion in Plants
Cellular respiration, photosynthesis, and various metabolic reactions in plants result in the production of numerous excretory products. The major excretory products in plants include carbon dioxide, excess water produced during respiration, and nitrogenous compounds from protein metabolism.
Plants release two gaseous waste products: oxygen during photosynthesis and carbon dioxide during respiration. The elimination of gaseous waste occurs through stomatal pores on the leaves.
A fascinating aspect is that the oxygen released during photosynthesis is used for respiration, while the carbon dioxide released during respiration is utilized for photosynthesis. This demonstrates the interconnected and mutually beneficial nature of these essential processes.
Plants excrete excess water through transpiration. Moreover, they store organic by-products in various forms within different plant parts. For instance, gums, oils, latex, and resins are waste products stored in components like bark, stems, and leaves. Eventually, plants shed these parts as they grow and renew themselves.
A few examples of plant excretory products include oil derived from oranges, eucalyptus, and jasmine, latex from rubber trees and papaya trees, as well as gums from acacia trees. Additionally, at times, plants even excrete waste products directly into the soil.
Frequently Asked Questions on Life Process Notes Class 10
Q: What are life processes?
According to Life Process Notes Class 10, Life processes are the essential activities that living organisms perform to maintain their life and survive. These processes include nutrition, respiration, transport, excretion, regulation, reproduction, and growth.
Q: What is nutrition in life processes?
Nutrition is the process by which living organisms obtain and utilize nutrients from their environment. It involves ingestion, digestion, absorption, assimilation, and egestion of food to provide energy and essential substances for growth and repair.
Q: How does respiration occur in living organisms?
Respiration is the process of breaking down food molecules to release energy. In most living organisms, respiration involves the intake of oxygen and the release of carbon dioxide during the breakdown of glucose or other organic compounds.
Q: What is the significance of transport in life processes?
Transport refers to the movement of substances like nutrients, water, gases, and waste products within living organisms. It is essential for distributing nutrients and oxygen throughout the body and removing waste materials for excretion.
Q: What is excretion, and why is it necessary for living organisms?
Excretion is the process of eliminating metabolic waste products from the body. It is vital to maintain a balance of internal conditions, remove harmful substances, and prevent the accumulation of toxic waste that could be detrimental to the organism’s health.
Q: How do living organisms regulate their internal environment?
Living organisms maintain their internal environment through various mechanisms, such as feedback loops, hormonal regulation, and the nervous system. These processes help in adjusting and stabilizing internal conditions like body temperature, blood pH, and water balance.
Q: What is the role of reproduction in life processes?
Reproduction is the process by which living organisms produce offspring, ensuring the continuation of their species. It is essential for the survival and genetic diversity of the species.
Q: How do living organisms grow?
Growth in living organisms is the result of an increase in the number and size of cells. It occurs through cell division and the accumulation of new materials to develop and increase the overall size of the organism.
Q: Can you give some examples of life processes in plants and animals?
Sure! In plants, examples of life processes include photosynthesis (nutrition), transpiration (excretion), and growth. In animals, examples include breathing (respiration), digestion (nutrition), and reproduction.
Q: How do unicellular organisms carry out life processes?
As single-celled organisms, unicellular organisms carry out all life processes within a single cell.. They take in nutrients, carry out respiration, excrete waste, and reproduce all in a single cell.
Q: Why are life processes essential for the survival of living organisms?
Life processes are essential for living organisms because they enable them to obtain energy, nutrients, and oxygen required for survival, growth, and reproduction. These processes also help in maintaining the internal balance and removing waste products to ensure the proper functioning of the organism.
Read Also:
- Chemical Reactions and Equations
- Acids, Bases and Salts
- Control and Coordination
- How Do Organisms Reproduce?
- Heredity and Evolution
- Magnetic Effects of Electric Current
- Our Environment
- Metals and Non Metals Class 10 NCERT Science Chapter 3 Notes
- Human Eye and the Colourful World Notes Chapter 10 Science
- Complete Class 10 Study Material
- Class 10th Chapter 11 Science Notes for NCERT Students
NCERT Books for Class 10 Science – Download pdf
Welcome to our platform, where we readily provide access to NCERT Books for Class 10 Science, making it easy for students to study. Class 10 marks a crucial stage for most students as it lays the foundation for their future studies.
This year is particularly significant as students face their first board exams. Therefore, mastering all the topics and subjects of Class 10 through thorough study of NCERT books becomes crucial for success in these exams.
These books are thoughtfully crafted by subject experts, with a primary focus on providing detailed, chapter-wise explanations of various topics and concepts as per the Class 10 CBSE Science Syllabus.
The NCERT book for Class 10 Science serves as a valuable resource for students, helping them study and prepare effectively for their board exams. Teachers of Class 10 also utilize these NCERT books to create assignments and homework for their students.
One of the notable strengths of NCERT textbooks is their ability to present even the most complex formulas and methods in a simple and comprehensible manner, making it easier for students to grasp the concepts. As a result, NCERT Books for Class 10 Science play a vital role in helping students achieve their academic goals.

NCERT Books for Class 10 Science – Download pdf
Class 10 Science Chapter-wise pdf in English
Please access the chapter-wise PDFs of the Class 10 CBSE Science book from the table provided for download.
- Chapter 1 – Chemical Reactions and Equations
- Chapter 2 – Acids Bases and Salt
- Chapter 3 – Metals and Non-metals
- Chapter 4 – Carbon and Its Compounds
- Chapter 5 – Life Process
- Chapter 6 – Control and Coordination
- Chapter 7 – How Do Organisms Reproduce?
- Chapter 8 – Heredity and Evolution
- Chapter 9 – Light – Reflection and Refraction
- Chapter 10 – The Human Eye and The Colourful World
- Chapter 11 – Electricity Solutions
- Chapter 12 – Magnetic Effects of Electric Current
- Chapter 13 – Our Environment
Class 10 Science Chapter Wise pdf in Hindi
For obtaining the chapter-wise PDFs of the Class 10 CBSE Science book in the Hindi language, kindly follow the links below and proceed with the download.
- अध्याय 1. रासायनिक अभिक्रियाएँ एवं समीकरण
- अध्याय 2. अम्ल, क्षारक एवं लवण
- अध्याय 3.धातु एवं अधात
- अध्याय 4 .कार्बन एवं उसवेफ यौगिक
- अध्याय 5.जैव प्रक्रम
- अध्याय 6. नियंत्राण एवं समन्वय
- अध्याय 7.जीव जनन कैसे करते है?
- अध्याय 8. आनुवंशिकता एवं जैव विकास
- अध्याय 9. प्रकाश – परावर्तन तथा अपवर्तन
- अध्याय 10. मानव नेत्र तथा रंग बिरंगा संसार
- अध्याय 11. विद्युत
- अध्याय 12. विधुत धारा के चुंबकीय प्रभाव
- अध्याय 13. हमारा पर्यावरण
As students work on the exercise questions, they can enhance their understanding by consulting Class 10 Notes for Science or NCERT Solutions Science Class 10. These solutions offer valuable insights into effective answer-writing techniques, thereby aiding them in achieving higher scores in the board exam.
Why One Should Read NCERT Class 10 Science Book?
The NCERT Class 10 Science Book offers comprehensive information on all the topics and concepts aligned with the CBSE syllabus for Class 10 Science. It serves as a valuable resource for students to resolve any doubts they may encounter while studying this subject.
Here are some benefits of using the NCERT Class 10 Science Book:
1. Enables students to gain a deeper understanding of the Class 10 Science topics.
2. Facilitates thorough knowledge acquisition of Class 10 Science concepts.
3. Provides a convenient reference during revision periods.
4. Allows students to self-assess their performance and adjust their study approach accordingly.
5. Enhances students’ knowledge of key subject matters.
6. Serves as a basis for homework and assignments.
Read Also:
- RD Sharma Class 10 Book Pdf Free Download Without Solutions
- Class 10 Science Book Pdf in Hindi Download for NCERT Students
- Class 10 Notes for Science NCERT
Frequently Asked Questions
What is the importance of NCERT Science books?
NCERT Science books are essential as they provide a comprehensive understanding of scientific concepts according to the CBSE syllabus. They serve as the foundation for various competitive exams and help students to grasp fundamental principles effectively.
Are NCERT books enough for board exam preparation?
Yes, NCERT books are considered highly beneficial for board exam preparation. However, it is recommended to supplement your studies with additional reference materials and practice questions to strengthen your knowledge and problem-solving skills.
Can NCERT Class 10 Science books be used for other competitive exams as well?
Yes, NCERT Class 10 Science books are valuable resources for various competitive exams, including scholarship exams and entrance tests. They provide a solid grounding in scientific concepts, which is advantageous for further studies and exams.
Where can I download the PDF version of NCERT Class 10 Science book?
You can download the PDF version of the NCERT Class 10 Science book from the official NCERT website or other reputable educational websites that offer free access to NCERT materials.
Are the NCERT books available in multiple languages?
Yes, NCERT books are published in several languages, including Hindi and English. This allows students to study in their preferred language and enhances accessibility.
Are NCERT Class 10 Science books sufficient for understanding complex scientific concepts?
While NCERT Class 10 Science books provide a solid foundation, some complex scientific concepts may require further explanation. Students can seek additional guidance from teachers, online resources, or reference books to gain a deeper understanding.
Do NCERT Class 10 Science books contain practical experiments and activities?
Yes, NCERT Class 10 Science books include practical experiments and activities to support the theoretical concepts. These hands-on exercises help students develop a better understanding of scientific principles through practical applications.
Are there any additional study materials available that complement NCERT Class 10 Science books?
Yes, several publishers offer supplementary study materials, guidebooks, and question banks that complement NCERT Class 10 Science books. These resources provide additional practice and help students reinforce their learning.
How can NCERT Class 10 Science books help in day-to-day learning and problem-solving?
NCERT Class 10 Science books provide clear explanations and examples, which help students relate scientific concepts to real-life situations. This aids in day-to-day learning and enables better problem-solving abilities.
Can NCERT books be used by students following other educational boards apart from CBSE?
Yes, NCERT books can be used by students of various educational boards as they cover fundamental scientific principles applicable to most syllabuses. However, it is advisable to cross-check the topics with the respective board’s curriculum.